Antibiotic resistance is a global health challenge nowadays, and methicillin-sensitive Staphylococcus aureus (MSSA) is a skin infection presents as pimples, boils, abscesses, or infected cuts and is not resistant to certain antibiotics. MSSA affects people of all ages and is known to cause epidemics among sports teams, families, prison inmates, and people who live and work in close quarters and is a global health challenge nowadays, creating problems in antibiotic therapy.
- Vancomycin
- MSSA
- Staphylococcus aureus
- Prevalence
1. Introduction
Nepal is a federal democratic republic nation (Complete territory: 147,181 sq. km) lies in South Asia between China in the north and India in the East, South, and West. The populace is 21.8 million (Male: 10.90 million also, Female: 10.93 million)[1]. The average life expectancy at birth is 57.52 years, and the unrefined birth rate, the rough demise rate, and the baby death rates are 36.9, 11.6, and 97, separately [2].
In Nepal, particularly in the distant districts, the utilization of antimicrobial operators has been insignificant due to their inaccessibility or the nonappearance of clinical consideration; accordingly, protection from antimicrobial specialists has been delayed to create in numerous microorganisms related to clinical contaminations.
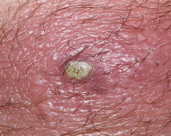
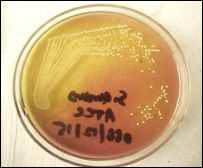
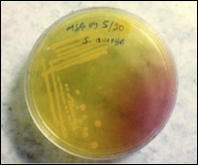
MSSA, or methicillin-susceptible Staphylococcus aureus (Figure 1), is an infection caused by a type of Staph bacteria commonly found on the skin, nose, mouth, and throat infections[3]. Treatment for staph infections generally requires antibiotics, and the bacteria Staphylococcus aureus is transmitted through skin-to-skin contact and resistant to all β-lactam antibiotics[4].
Figure 1 S. aureus Infected skin showing pus
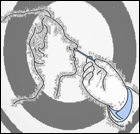
Figure 2. Technique used for swab collection from an infected person
Staphylococcus aureus, a Gram-positive coccus, produces catalase enzyme and coagulase enzyme, which coagulates blood by responding with prothrombin, which changes fibrinogen over to fibrin [5]. S. aureus causes a wide scope of diseases, for example, skin contaminations, including abscesses, impetigo, and necrotizing fasciitis; tissue contaminations, including osteomyelitis and endocarditis; and toxinoses, including poisonous stun disorder, when the insusceptibility of the staphylococcal nasal transporter is smothered[6][7].
If MRSA colonized in nares of a sound individual, a 29% potential danger shows up for MRSA infections [8]. While antibiotics, for example, methicillin are utilized as often as possible in patients, anti-infection safe strains may create. After penicillin use had gotten far and wide to treat diseases, penicillin-safe S. aureus strains emerged. A couple a long time in the wake of following the utilization of penicillin, penicillin-safe S. aureus strains had emerged, and penicillinase-safe methicillin utilization had presented for the treatment of penicillin-safe S. aureus strains. After methicillin use presented in 1961, MRSA strains that were likewise multidrug-safe emerged inside a year. Methicillin that has been giving far and wide MRSA and turns into a futile medication, has not been utilized lately [7].
Methicillin-susceptible Staphylococcus aureus (MSSA) colonizes the skin, causing no side effects and without causing infection, however then may later prompt infection. The infection spreads through direct skin-to-skin contact and may spread through contact with contaminated things or surfaces. The sharing of contaminated individual things with somebody who has MSSA towels, sheets, razors, garments, or athletic gear improves the probability of spreading the infection. Affirmation of MSSA contamination is generally done by refined the infected tissue. S. aureus stays omnipresent in the climate and sound greenery of creatures. It is a commensal just as pathogenic bacterium [1]. It is referred to happen as sound vegetation in the skin of an expected 20% of the total population without causing any unsafe impacts and constantly conveyed.
Additionally, 60% of the population conveys it once in a while during their lifetime [2][3]. Nonetheless, it has considered being a pioneering pathogen for people and creatures on the off chance that it gets an occasion to enter the circulation system and tissue [1][2]. For the most part, it becomes irresistible just when it can go into the skin or mucous film penetrated by entering objects [4]. It is found to cause a scope of ailment from minor skin infections, for example, pimples, impetigo, bubbles, cellulitis, singed skin condition, folliculitis, furuncles, carbuncles, and abscesses, to hazardous sicknesses, for example, pneumonia, osteomyelitis, meningitis, Toxic Shock Syndrome, endocarditis and septicemia [5].
Recent investigations propose that the infection because MRSA isn't just clinic, gained however network procured also. MRSA currently speaks to a worldwide issue. Some huge episodes have been detailed from various pieces of the world, where it had caused serious infections, including septicemia, endocarditis, and meningitis. The infection brought about by MRSA can be costly as far as anti-toxin treatment, seclusion offices and materials, and length of clinic remain. As indicated by a World Wellbeing Association writing, the worldwide budgetary weight as a result of MRSA contamination has worked out to be $20,000 to $ 114,000 for episodes and from $28,000 to $1600,000 for endemic diseases every year [8]. The development and the spread of multidrug-opposition S. aureus microscopic organisms are a worldwide danger to the restorative administration of staphylococcal diseases. Along these lines, the current examination assesses the level of MRSA strains and researches their anti-infection opposition profiles with clinical infections in Everest hospital, in the midtown of Kathmandu Nepal. Additionally, since this locale gets countless travelers consistently, this investigation is more amazing given the limit of MRSA to spread.
2. Materials and methods
2.1. Materials
All the chemicals, kits, antibiotic discs, and microbiological media were purchased from HiMedia Pvt Ltd Co., Mumbai, India.
2.2. Collection of samples
We collected samples (Figure 2) from outdoor and indoor patients from March 2018 to September 2018 at Everest Hospital, New Baneshwar, Kathmandu Nepal. We brought to the laboratory of the Department of Microbiology, Trichandra Multiple Campus, Saraswathi Sadan, Kathmandu, Nepal, for further analysis. EMCH has 300 beds, and 20–30 patients get admitted to different departments every day. The hospital serves patients from Central Kathmandu and the surrounding districts as Lalitpur, Bhaktapur. The microbiology laboratory in this teaching hospital receives up to 20 samples of culture daily from the Out-Patient Department (OPD) and wards/In-Patient Department (IPD). Thus, a total of 526 various clinical specimens viz. blood, urine, stool, pus swab from an ear, eye, throat, vaginal, and body parts where burn or wound occurred were collected from both in-patients and outpatients. Only those patients who were not taking any medication and referred by a clinician included in the study (Figure 1). Samples stored in a sterile, leak-proof, screw-capped container with a proper label (Lab Id no., age, gender, date, time).
2.3. Laboratory Assessment
2.3.1. Isolation of S. aureus
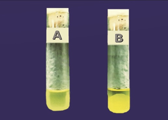
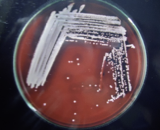
The environment swabs were improved in M-Staph broth and brooded for 48 hours in anaerobic conditions. The dark precipitate was straightforwardly vaccinated in MSA agar for 24 hours. Mannitol maturing settlements (yellow provinces) from MSA were sub-cultured on supplement agar and hatched at 37°C for 24 hours. Brilliant on supplement agar having a round, curved, murky, and smooth-sparkling surface with a measurement of around 2-3mm were demonstrative of S. aureus. Further phenotypic distinguishing proof of the S. aureus made by Gram recoloring, catalase test, oxidase test, coagulase test (slide and cylinder test), and oxidative/fermentative test (Figures 3 and 4).
Figure 3 Photograph of tube coagulase test results
Figure 4 Pure culture of S. aureus in Blood agar
2.3.2. Macroscopic examination
Colony characteristics were examined on MSA and BA medium for the identification of all the isolates. The β-hemolytic activity was detected in the BA medium (Figure 5).
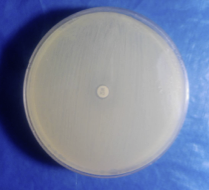
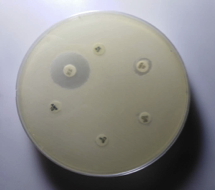
2.3.3. Antibiotic susceptibility testing by disc diffusion method
All identified MRSA and MSSA isolates from various clinical specimens were subjected to in vitro antibiotic susceptibility test by the Kirby-Bauer disc diffusion method following Clinical and Laboratory Standards Institutes (CLSI-2015) guidelines (Figure 5, 6, 7 & 8). The antimicrobial susceptibilities of S. aureus isolate determined using disks of 8 antimicrobials on Mueller-Hinton agar media by modified Kirby Bauer's disc diffusion method as described in the guidelines of CLSI (2015).
Figure 5 Pure culture of S. aureus in Mannitol salt agar
Figure 6 Pure culture of S. aureus in Mannitol salt agar
CX-Cefoxitin (30µg)-R
Figure 7 Antibiotic susceptibility testing of S. aureus.
AMP-Ampicillin(10µg) CIP-Ciprofloxacin(10µg), VA- Vancomycin (30µg) GEN-Gentamycin(10µg), CN-Cephalexin(30µg) COT-Cotrimoxazole(25µg)
Figure 8 Antibiotic susceptibility testing of methicillin-resistant S. aureus
The following 8 antibiotics at the indicated concentrations as ciprofloxacin (10 μg), Gentamicin (10 μg), cotrimoxazole (25 μg), ampicillin (10 μg), cloxacillin (10 μg), Cephalexin (30 μg), Cefoxitin (30 μg), and Vancomycin (30 μg) tested against all the strains. For the identification of MRSA, cefoxitin disc (30 μ) was utilized. A zone of inhibition of under 22 mm or any noticeable development inside the zone of hindrance by S. aureus against Cefoxitin in Muller Hinton agar (MHA) plate was demonstrative of methicillin obstruction. S. aureus ATCC 25923 utilized as a standard control strain. Methicillin opposition was tried for all the S. aureus disengages by the agar screening technique utilizing MHA [20] enhanced with 4% NaCl against cefoxitin disc (Figures 4 and 5). For ICR identification, clindamycin and erythromycin drugs kept separated 15–26 mm in MHA, and the D-shaped inhibition zone was observed around clindamycin.
At the point when pure culture got, a loopful of bacteria was taken from a colony and was moved to the cylinder containing 5 ml typical saline and blended tenderly to acquire homogenous suspension-the turbidity of the suspension, at that point changed by a thickness of McFarland 0.5 to normalize inoculum size. A sterile cotton swab then dipped into the suspension, and the abundance was eliminated by a delicate pivot of the swab against the outside of the cylinder. The swab at that point used to appropriate the bacteria uniformly over the whole surface of Muller Hinton agar.
The inoculated plates left for 3-5 minutes at room temperature and with the aid of sterile forceps the following concentration of antibiotics discs put on the surface of Muller Hinton Agar 50 μg glucose 6-phosphate and were incubated for 16-18 hrs at 37o C. Zone of inhibition after incubation observed and the diameter of inhibitory zones measured in millimeters (mm). The results of the measurement are interpreted as sensitive, intermediate, or resistant according to CLSI guidelines (CLSI 2015). Isolates having intermediate susceptibility added in the total resistant isolates to each antibiotic tested.
2.3.4. Statistical analysis
Microsoft Excel 365 was used to record the laboratory data, and statistical analysis software (IMB-SPSS V21.0) used to calculate a p-value by using the Pearson Chi-Square test. P> 0.05 was considered statistically significant at 95% CI.
3. Results
This study has conducted among patients visiting Everest Hospital, Kathmandu, Nepal. A total of 526 non-repeated, clinical samples subjected to culture from both Inpatient and Outpatient for 6 months include generating data. From the total clinical samples cultured, the highest number of samples, i.e., 177 (33.6%) shows significant growth, 60 (33.8%) showed positive S. aureus growth.
3.1. Age and gender-wise distribution of total patients
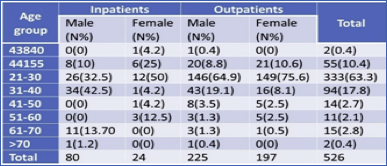
In this experiment, the maximum number of samples collected from male outpatients 225 (52.3%) followed by female outpatients 197 (46.7%) in which male in-patients found 80 (77%) and female in-patients 24 (23%). A maximum number of samples i. e. 149 (75.6%) were from female outpatients of age 21-30, followed by male outpatients of the same age group i. e. 146(64.9%) (Table 1).
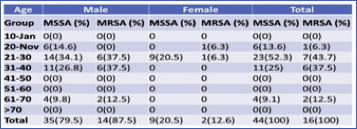
Table 1 Age and Gender-wise distribution of MSSA and MRSA
3.2. Age and Gender wise distribution of MSSA and MRSA
Concerning age and gender-wise distribution, a maximum number of MRSA i. e 7 (43.7%) and MSSA 23 (52.3%) obtained from the age group 21-30. In male maximum MRSA, 6 (37.5%) was obtained from the age group 21-30 and 31-40, whereas the maximum MSSA is 14(34.1%) has obtained from the age group 21-30. Similarly, in the female, 1 (6.3%) MRSA was obtained from the age group 11-20 and 21-30, respectively. The other age group showed no growth, while MSSA 9 (20.5%) has obtained from the age group 21-30, and S. aureus has not observed from other age groups in both male and female patients. The association between gender and prevalence of MRSA and MSSA found insignificant (p>0.05) (Table 2).
Table 2 Age and gender-wise distribution of total patients
3.3. Distribution of MRSA in outpatients and in-patient and among gender
Distribution of MRSA in both out- and in-patient were studied. Out of a total of 526 patients, 422 (80.2%) were obtained from Outpatient, and 104 (19.8%) samples were obtained from In-patients. From a total of 305 male patients, 26.2% of samples were collected from in-patient and 73.7% from Outpatient. Similarly, of 221 female patients, 10.8% from in-patient, and 89.1% from Outpatient. Of the total 16 MRSA, Maximum samples were obtained from in-patient 9 (56.2%) whereas in outpatient 7 (43.7%). The MSSA samples obtained more from outpatient 23 (52.2%) and in-patient 21(47.7%). Thus, the prevalence of MRSA was found to be highest in in-patient, and the prevalence of MSSA was highest in outpatients. There was no significant association between isolates and the type of patients (p>0.05) (Table 3).
Table 3 Distribution of MRSA in outpatients and inpatient
3.4. Distribution of MRSA, MSSA, and Other Organisms
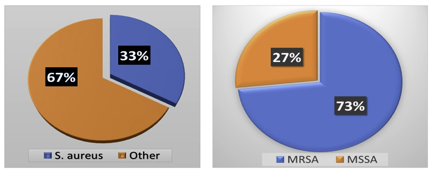
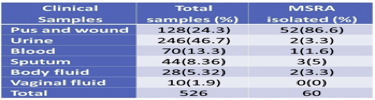
Out of 526 clinical samples (Figure 9), a positive growth of different organisms has seen in 177 (33.6%) samples S. aureus was isolated from 60 (33.8%) different clinical samples in which 67.6% includes bacterial isolate other than S. aureus. Among 60 isolated S. aureus from different clinical samples, 16 (26.6%) of S. aureus were found to be methicillin-resistant S. aureus (MRSA). However, the remaining 44 (73.3%) found to be methicillin-sensitive S. aureus (MSSA).
Figure 9 Distribution of MRSA, MSSA, and Other Organisms during clinical examination of MRSA & MSSA
3.5. Distribution of total isolates in clinical specimens
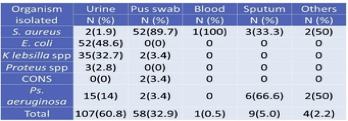
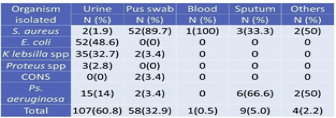
Among the investigated total of 526 clinical specimens (Table 4), 177 (33.7%) specimens showed +ve growth of organisms. Of the 177 positive growths, S. aureus isolated from 60 (33.8%) clinical specimens followed by 89.7% were isolated from pus (Table 2). In total, 107 (60.8%) growth in urine, 52(48.6%) Escherichia coli found.
Table 4 Distribution of total isolates in clinical specimens
3.6. Isolation of MRSA from different clinical samples
Maximum S. aureus 52(86.6%) isolates obtained from pus swab followed by sputum 3(5%). The majority of specimens comprised of urine, but only 2(3.3%) S. aureus was obtained from the specimen, which is similar to body fluids. Whereas 1(1.9%) from blood, there are no isolates from vaginal fluids (Table 5).
Table 5 Isolation of MRSA from different clinical samples
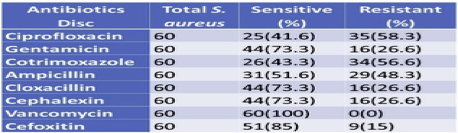
3.7. Antibiotics susceptibility pattern of S. aureus
All S. aureus isolates were found to be sensitive to Vancomycin and were least sensitive to ciprofloxacin (41.6%). Isolates showed 85% sensitivity to Cefoxitin and 73.3% sensitivity towards Gentamicin, cloxacillin, and Cephalexin. Of the total 60 S. aureus isolated, 16 were found to be resistant to Cefoxitin (Table 6).
Table 6 Antibiotics susceptibility pattern of S.aureus
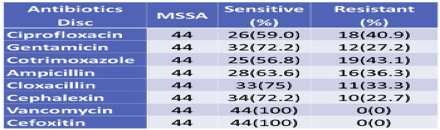
3.8. Antibiotics susceptibility pattern by MSSA
Since MSSA showed less resistance to antibiotics used as compared to MRSA, MSSA showed the highest resistance to cotrimoxazole (43.1%), and the least resistance with Cephalexin (22.7%) followed by Gentamicin (27.2%). Both MRSA and MSSA were found to be 100% Sensitive to Vancomycin (Table 7).
Table 7 Antibiotics susceptibility pattern by MSSA
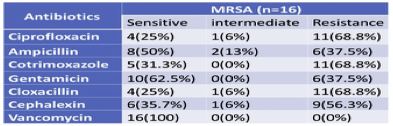
3.9. Antibiotics susceptibility pattern from MRSA
MRSA was found to be highly and equally resistant to cotrimoxazole (68.8%), ciprofloxacin (68.8%), cloxacillin (68.8%), followed by Cephalexin (56.3%). They were found resistant to Gentamicin (37.5%) and ampicillin (37.5%). All MRSA was found to be 100% sensitive to Vancomycin (Table 8). S. aureus obtained from the specimen, which is like body fluids and from the blood, while there are no isolates from vaginal fluids.
Table 8 Antibiotics susceptibility pattern from MRSA
4. Discussion
Methicillin-resistant S. aureus (MRSA) strains have procured a gene that makes them impervious to practically all beta-lactam antibiotics and is an opportunistic pathogen regularly conveyed asymptomatically on the human body. Resistance to other antibiotics is also collective, especially in hospital-associated MRSA [9]. These living beings are serious nosocomial pathogens, and finding a viable treatment can be testing. Network related MRSA strains, which started outside hospitals, are likewise pervasive in certain territories. Human healthcare workers overall are at expanded danger for colonization with MRSA due to occupational exposure [10]. Carriage rates in this population are assessed to be around 2-5% generally speaking, however, range from 1% to 15%, and contrast between regions. In everybody, MRSA carriage rates are assessed to be < 1% to 5%, although they can be higher in certain populations. Schools and childcare focus give off an impression of being normal locales for the dispersal of network-related heredities. Most transmission of these life forms happens inside house-holds because of continuous close contact [11]. Scanty data regarding MRSA is available from Nepal, to the best of our knowledge.
The prevalence rate of MRSA infection in our study was found to be 26.14%, which is following the reports by Udaya et al. (20%) and Mehta et al. (32.8%) from India [12]. In our experiment, a study was conducted among the patients of Everest Hospital, Kathmandu, Nepal, and a total of 526 non-repeated, clinical samples subjected to culture from both Inpatient and Outpatient recorded. From the total clinical samples cultured, the highest number of samples, i.e., 177 (33.6%) shows significant growth, 60 (33.8%) showed positive S. aureus growth. There are three published studies on MRSA pervasiveness from various regions of this nation. The soonest study announced a pervasiveness of 29% in 1990 when there were fewer medical services organizations and less admittance to antibiotics [13]. The subsequent examination reports a commonness of 15.4% from far off western Nepal, where antibiotics are not effectively accessible. At long last, the third investigation reports MRSA commonness of 26.14%, and the authors characteristic this low rate to successful disease control practice in their hospital [14].
Vancomycin is by all accounts the main antimicrobial agent that demonstrated 100% affectability thus might be utilized as the medication of decision for treating multidrug-safe MRSA infections. Vancomycin is anything but a regularly endorsed drug, which is more likely than not because of the more exorbitant cost of the antibiotic and its inaccessibility in numerous pieces of the nation. Nepal. The discoveries of this obstruction in our investigation are generally not exactly those of Khanal et al. [15] and Mukhiya et al. [16]. Likewise, the discoveries in this report are liable to at any rate of five impediments. To start with, infusion drug use status in medical records is conceivably misclassified, which could bring about an under-or overestimation of the level of MRSA diseases in infusion drug clients. Second, rates depended on public evaluations of both intrusive MRSA case checks and the number of inhabitants in people who infuse drugs that probably won't be precise. Third, the rates depend on 2011 information since this is the main year for which populace gauges for the number of people who infuse drugs are accessible. This may be a belittle if current infusion drug use rehearses are in higher danger. Fourth, site-explicit tallies of people who infuse drugs were not accessible, blocking the count of site-explicit rates. At last, obtrusive methicillin-sensitive Staphylococcus aureus observation started in 2016 and couldn't be remembered for this report to describe the effect of the opioid epidemic on these infections [17].
Pandey et al. [18] reported the higher isolation of S. aureus from pus and wound (78.37%) in comparison to blood (17.11%) and urine (4.50%) out of 111 S. aureus-containing clinical samples [18]. The current study also shows a parallel result, where 60.8% growth found in the urine in which 48.6% Escherichia coli and S. aureus isolated from 60 (33.8%) clinical specimens followed by 89.7% were isolated from pus. Our result is following Rajbhandari et al. in Nepal [19]. They also reported the higher prevalence of S. aureus among the in-patient setting, accounting for 86 (62.3%) as compared with 52 (37.7%) in outpatients. Another study carried out in Kathmandu valley by Shrestha et al. [20] reported 44.9% of MRSA from nosocomial S. aureus. Sanjana et al. [21] also reported 348 (39.6%) MRSA isolates at the College of Medical Sciences-Teaching Hospital. Meanwhile, Rijal et al. [22] in Pokhara valley reported 75.5% MRSA isolate, and in a similar report, Tiwari et al. [23]found 69.1% MRSA isolate in Western parts of Nepal.
MRSA was generally isolated from pus specimens in our investigation, which may be because of the detachment of more S. aureus from pus contrasted with other clinical specimens, which is like the findings of different investigations as well. It may be because of the high commonness of MRSA in profound situated injuries contrasted with superficial infections. Our studies show a high predominance of MRSA among OPD patients contrasted with in-patients, which stands out from findings of different past research findings led somewhere else in the world. It may have prompted the advancement of medication obstruction by microorganisms to different normally utilized antibiotics, particularly showing network procured MRSA. This finding makes the researchers alert for directing network put together research concentrate likewise for MRSA and different drug-resistant bacteria [24].
Further, our finding is additionally conflicting with the findings of different studies in Nepal, where network obtained MRSA infection is high. Nonetheless, because of the inconsistent number of tests taken to look at, we can't state the MRSA disengagement rate is high among OPD patients contrasted with in-patients, and this turns into the constraint of our study. Our investigation demonstrated that all MRSA isolates were sensitive to Vancomycin and resistant to Cephalexin, erythromycin, and ciprofloxacin, which resembles the discoveries of different research reports. It demonstrates Vancomycin remains the best option against MRSA infections with 100% treatment adequacy to date.
Infections brought about by MRSA are primarily nosocomial and are progressively announced in numerous nations around the world. The commonness of MRSA shifts in various pieces of the globe going from 1-30% in Europe, 5-40% in Asian nations, 10-half in the USA, and the UK. The pervasiveness pace of MRSA in Nepal detailed being 15-69% from various territories in the nation [25].
5. Conclusion
Antibiotic resistance is a global health challenge, and Methicillin-resistant Staphylococcus aureus (MRSA), is one of the biggest problems throughout the world, including Nepal. This study was conducted among patients visiting Everest Hospital, Kathmandu, Nepal, with a total of 526 non-repeated clinical samples subjected to culture from both Inpatient and Outpatient. From the total clinical samples cultured, the highest number of samples, i.e., 177 (33.6%) shows significant growth, 60 (33.8%) showed positive S. aureus growth. Our study demonstrated that there is a high pervasiveness of MRSA among clinical specimens, particularly pus, and MRSA are 100% delicate to Vancomycin to date. These findings prescribe utilizing legitimate antibiotics to lessen MRSA infections in Nepal, which incorporates; conversation with doctors and people in general about the effect of MRSA, reconnaissance, and location of MRSA, exacting requirement of infection control procedures in emergency clinics, and reasonable utilization of Vancomycin for treatment of MRSA related infections.
References
- Ansari S, Nepal HP, Gautam R, Rayamajhi N, Shrestha S, Upadhyay G, et al. Threat of drug-resistant Staphylococcus aureus to health in Nepal. BMC Infect Dis. 2014;14(157):1471-2334.
- Mishra SK, Rijal BP, Pokhrel BM. Emerging threat of multidrug-resistant bugs Acinetobacter calcoaceticus baumannii complicated and methicillin-resistant Staphylococcus aureus. BMC Res Notes. 2013; 6:98.
- Raut S, Gokhale S, Adhikari B. Prevalence of extended-spectrum beta-lactamases among Escherichia Coli and Klebsiella Isolates in Manipal Teaching Hospital, Pokhara, Nepal. J Microbiol Infect Dis. 2015;2(5):69–75.
- Acharya A, Khanal A, Kanungo R, Mohapatra T. Characterization and susceptibility patterns of clinically significant Enterococcus species in eastern Nepal. Nepal Med Coll J. 2007;9(4):250–254.
- Easow JM, Joseph NM, Shankar PR, Rajamony AP, Dhungel BA, Shivananda G. Streptococcus pneumonia infections in western Nepal. Southeast Asian J Trop Med Public Health. 2011;42(4):912-919.
- Kumari N, Mohapatra TM, Singh YI. Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in a Tertiary-Care Hospital in Eastern Nepal. JNMA J Nepal Med Assoc. 2008;47(170):53-56.
- Shrestha B, Rana SS. D test: a simple test with significant implications for Staphylococcus aureus macrolide-lincosamide-streptogramin B resistance pattern. Nepal Med Coll J. 2014;16(1):88–94.
- Khanal LK, Jha BK. Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) among skin infection cases at a hospital in Chitwan, Nepal. Nepal Med Coll J. 2010;12(4):224–228.
- Shakya B, Shrestha S, Mitra T. Nasal carriage rate of methicillin-resistant Staphylococcus aureus among at National Medical College Teaching Hospital, Birgunj, Nepal. Nepal Med Coll J. 2010;12(1):26–29.
- Joshi S, Ray P, Manchanda V. Methicillin-resistant Staphylococcus aureus (MRSA) in India: Prevalence and susceptibility pattern. Indian J Med Res. 2013; 137(2): 363-9.
- Bhatt CP, Karki BMS, Baral B, Gautam S, Shah A, Chaudhary A. Antibiotic susceptibility pattern of S. aureus and methicillin-resistant S. aureus in a tertiary care hospital. J Pathol Nepal. 2014; 4: 548-51.
- Tiwari HK, Sapkota D, Das AK, Sen MR. Assessment of different tests to detect methicillin-resistant S. aureus. Southeast Asian J Trop Med Public Health. 2009; 40:801
- Kshetry AO, Pant ND, Bhandari R et al. Minimum inhibitory concentration of Vancomycin to MRSA isolated from different clinical samples at a tertiary care hospital in Nepal. Antimicrob Resist Infect Control. 2016; 5: 27.
- Rijal KR, Pahari N, Shrestha B. Prevalence of MRSA in school children in Pokhara. Nepal Med Coll J. 2008; 10:192-5.
- Khanal R, Sah P, Lamichhane P, Lamsal A, Upadhaya S, Pahwa VK. Nasal carriage of MRSA among health care workers at a tertiary care hospital in Western Nepal. Antimicrob Resist Infect Control. 2015; 4: 39.
- Mukhiya RK, Shrestha A, Rai SK. Prevalence of MRSA in hospitals of Kathmandu Valley. Nepal Journal of Science and Technology. 2012; 13(2): 185-90.
- Shrestha B, Pokhrel BM, Mahopatra TM. Study of nosocomial isolates of S. aureus with special reference to MRSA in a tertiary care hospital in Nepal. Nepal Med Coll J. 2009; 11: 123-6.
- Pandey S, Raza, MS, Bhatta CP. Prevalence and antibiotic sensitivity pattern of methicillin-resistant-Staphylococcus aureus in Kathmandu medical college–teaching hospital. JIOM34(11), 13–17 (2012).
- Sanjana RK, Singh R, Chaudhary N. Prevalence and antimicrobial susceptibility pattern of MRSA in CMS Teaching Hospital. Journal of College of Medical Sciences-Nepal. 2010; 6: 1-6.
- Shrestha B, Pokhrel BM, Mohapatra TM. Phenotypic characterization of nosocomial isolates of Staphylococcus aureus with reference to MRSA. Infect. Dev. Ctries.3(7), 554–560 (2009).
- Rijal KR, Shrestha N, Pahari N. et al. Methicillin-resistant Staphylococcus aureus in patients visiting the western regional hospital, Pokhara. JIOM30(1), 21–25 (2008).
- Shakya B, Shrestha S, Mitra T. Nasal carriage rate of MRSA at NMC Birgunj, Nepal. Nepal Med Coll J. 2010; 12: 26-9.
- Tiwari HK, Das AK, Sapkota D, Sivarajan K, Pahwa VK. Methicillin-resistant Staphylococcus aureus: prevalence and antibiogram in a tertiary care hospital in western Nepal. Infect. Dev. Ctries.3(9), 681–684 (2009).
- Rai SK, Tuladhar NR, Shrestha HG. Methicillin-resistant S. aureus in a tertiary medical care center, Nepal. Indian J Med Microbiol. 1990; 8: 108-9.
- Rajbhandari R, Manandhar SP, Shrestha J. Comparative study of MRSA and its antibiotic susceptibility pattern in indoor and outdoor patients in Bir Hospital, Nepal. Nepalese J Microbiol. 2003; 1: 62-5.