The Earth’s magnetic field is one of the basic abiotic factors in all environments, and organisms had to adapt to it during evolution. On some occasions, organisms can be confronted with a significant reduction in a magnetic field, termed a “hypomagnetic field—HMF”, for example, in buildings with steel reinforcement or during interplanetary flight. HMFs can modify cell signalling by affecting the contents of ions (e.g., calcium) or the reactive oxygen species (ROS) level, which participate in cell signal transduction. Additionally, HMFs have different effects on the growth or functions of organ systems in different organisms, but negative effects on embryonal development have been shown. Embryonal development is strictly regulated to avoid developmental abnormalities, which have often been observed when exposed to a HMF. Only a few studies have addressed the effects of HMFs on the survival of microorganisms. Studying the magnetoreception of microorganisms could be useful to understand the physical aspects of the magnetoreception of the HMF.
- hypomagnetic field
- magnetic zero
- magnetoreception
1. Introduction
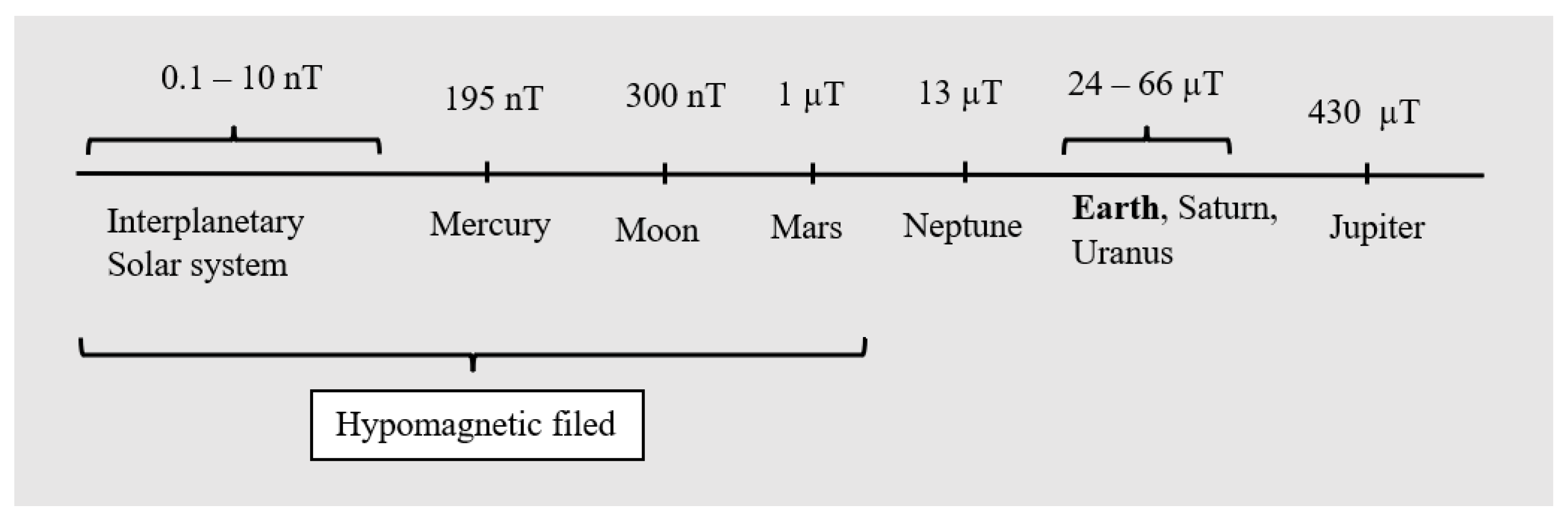
2. Mechanisms of Magnetoreception of HMFs
- 1.
-
Radical pair mechanism
- 2.
-
Universal physical mechanism
- 3.
-
Molecular gyroscope mechanism
This entry is adapted from the peer-reviewed paper 10.3390/pr11010282
References
- Monteil, C.L.; Lefevre, C.T. Magnetoreception in Microorganisms. Trends Microbiol. 2019, 28, 266–275.
- Binhi, V.N.; Prato, F.S. Biological effects of the hypomagnetic field: An analytical review of experiments and theories. PLoS ONE 2017, 12, e0179340.
- Mo, W.; Liu, Y.; He, R. Hypomagnetic field, an ignorable environmental factor in space? Sci. China Life Sci. 2014, 57, 726–728.
- Kivelson, M.G.; Bagenal, F. Planetary magnetospheres. In Encyclopedia of the Solar System, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 7; pp. 137–157.
- Zhang, Z.; Xue, Y.; Yang, J.; Shang, P.; Yuan, X. Biological Effects of Hypomagnetic Field: Ground-Based Data for Space Exploration. Bioelectromagnetics 2021, 42, 516–531.
- Pavlík, M. Compare of shielding effectiveness for building materials. Prz. Elektrotechniczny 2019, 95, 137–140.
- Guo, C.; Liu, D. Quantitative Analyses of Magnetic Field Distributions for Buildings of Steel Structure. In Proceedings of the 2012 Sixth International Conference on Electromagnetic Field Problems and Applications, Dalian, China, 19–21 June 2012.
- Erdmann, W.; Kmita, H.; Kosicki, J.Z.; Kaczmarek, Ł. How the Geomagnetic Field Influences Life on Earth—An Integrated Approach to Geomagnetobiology. Space Life Sci. 2021, 51, 231–257.
- Wajnberg, E.; Acosta-Avalos, D.; Alves, O.C.; de Oliveira, J.F.; Srygley, R.B.; Esquivel, D.M.S. Magnetoreception in eusocial insects: An update. J. R. Soc. Interface 2010, 7, S207–S225.
- Binhi, V.N.; Savin, A.V. Molecular gyroscopes and biological effects of weak extremely low-frequency magnetic fields. Phys. Rev. E 2002, 65, 051912.
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95.
- Gauron, C.; Rampon, C.; Bouzaffour, M.; Ipendey, E.; Teillon, J.; Volovitch, M.; Vriz, S. Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci. Rep. 2013, 3, srep02084.
- Van Huizen, A.V.; Morton, J.M.; Kinsey, L.J.; Von Kannon, D.G.; Saad, M.A.; Birkholz, T.R.; Czajka, J.M.; Cyrus, J.; Barnes, F.S.; Beane, W.S. Weak magnetic fields alter stem cell–mediated growth. Sci. Adv. 2019, 5, eaau7201.
- Adams, B.; Sinayskiy, I.; Petruccione, F. An open quantum system approach to the radical pair mechanism. Sci. Rep. 2018, 8, 15719.
- Hore, P.J.; Mouritsen, H. The Radical-Pair Mechanism of Magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344.
- Ruiz-Gómez, M.J.; Sendra-Portero, F.; Martínez-Morillo, M. Effect of 2.45 mT sinusoidal 50 Hz magnetic field on Saccharomyces cerevisiae strains deficient in DNA strand breaks repair. Int. J. Radiat. Biol. 2010, 86, 602–611.
- Barnes, F.; Greenenbaum, B. Some Effects of Weak Magnetic Fields on Biological Systems: RF fields can change radical concentrations and cancer cell growth rates. IEEE Power Electron. Mag. 2016, 3, 60–68.
- Brocklehurst, B.; Mclauchlan, K.A. Free radical mechanism for the effects of environmental electromagnetic fields on biological systems. Int. J. Radiat. Biol. 1996, 69, 3–24.
- Binhi, V.N.; Prato, F.S. A physical mechanism of magnetoreception: Extension and analysis. Bioelectromagnetics 2016, 38, 41–52.
- Otsuka, H.; Mitsui, H.; Miura, K.; Okano, K.; Imamoto, Y.; Okano, T. Rapid Oxidation Following Photoreduction in the Avian Cryptochrome4 Photocycle. Biochemistry 2020, 59, 3615–3625.
- Novikov, V.V.; Yablokova, E.V.; Fesenko, E.E. The Effect of a “Zero” Magnetic Field on the Production of Reactive Oxygen Species in Neutrophils. Biophysics 2018, 63, 365–368.
- Yan, M.-M.; Zhang, L.; Cheng, Y.-X.; Sappington, T.W.; Pan, W.-D.; Jiang, X.-F. Effect of a near-zero magnetic field on development and flight of oriental armyworm (Mythimna separata). J. Integr. Agric. 2021, 20, 1336–1345.
- Zhang, B.; Wang, L.; Zhan, A.; Wang, M.; Tian, L.; Guo, W.; Pan, Y. Long-term exposure to a hypomagnetic field attenuates adult hippocampal neurogenesis and cognition. Nat. Commun. 2021, 12, 1174.
- Gupta, A.; Stait-Gardner, T.; Price, W.S. Is It Time to Forgo the Use of the Terms “Spin–Lattice” and “Spin–Spin” Relaxation in NMR and MRI? J. Phys. Chem. Lett. 2021, 12, 6305–6312.
- Zangi, R.; Hagen, M.; Berne, B.J. Effect of Ions on the Hydrophobic Interaction between Two Plates. J. Am. Chem. Soc. 2007, 129, 4678–4686.
- Zhao, V.; Jacobs, W.M.; Shakhnovich, E.I. Effect of Protein Structure on Evolution of Cotranslational Folding. Biophys. J. 2020, 119, 1123–1134.
