The role played by miRNAs in respiratory diseases is closely linked to the specific miRNA, as the non-coding genetic material can play a triggering or protective role.
MiRNAs are involved in the genesis of asthma, as a relevant modification in the miRNA generation of airway cells has been described by numerous studies [
113,
114,
115,
116,
117,
118]. An analysis stated that epithelial miRNA-141 controlled the presence of airway mucus in asthma [
118], while different studies implicated epithelial miRNAs in airway eosinophilia; moreover, an evaluation of miRNAs in asthma stated that a group of epithelial miRNAs was reduced in these subjects [
118]. For instance, miRNA-30a-3pa, an miRNA also implicated in the growth and apoptosis of tumour cells [
119,
120], was considerably reduced in the blood of asthmatic subjects and is probably involved in airway eosinophilic inflammation.
Moreover, it was reported that miRNA-3934 concentrations might be employed to differentiate asthma subjects from non-asthmatic subjects. Regarding the mechanism, miRNA-3934 suppressed the increase in AGEs, which provoked an increase in apoptosis in basophils [
123]. These findings suggest that miRNA-3934 can reduce the onset of asthma by targeting the RAGE and probably inhibiting other pathways, such as the TGF-β/Smad signalling pathway.
Even more interesting are the data relating to a possible correlation between miRNAs and HMGB1 in the pathogenesis of asthmatic disease. There are three runt-related transcriptional factor (RUNX) genes, namely
RUNX1,
RUNX2, and
RUNX3, as well as maternal smoking, which have been reported to potentially support the onset of asthma in children by increasing the expression of
RUNX1 [
132]. Moreover, RUNX2 is described as stimulating the gene transcription of a sterile alpha motif (SAM) pointed domain comprising an ETS transcription factor (
SPDEF) and may join the promoter of
HMGB1 [
133,
134]. Wu et al. evaluated miRNA-30a-3p production in asthma patients and non-asthmatic subjects and considered the relationship between miRNA-30a-3p and airway eosinophilia [
135]. They found that miRNA-30a-3p production was significantly reduced in the bronchial brushings of asthma patients compared to normal controls. Epithelial miRNA-30a-3p production was negatively related to several factors indicating airway eosinophilia, such as eosinophils in sputum or bronchial biopsies and exhaled nitric oxide in patients. The authors demonstrated that
RUNX2 is a target of miRNA-30a-3p and augments HMGB1 expression. HMGB1 and RUNX2 production are both increased in the airway epithelium and are related to each other in asthmatic subjects. A reduction in miRNA-30a-3p increased RUNX2 and HMGB1 production, while increasing RUNX2 stimulated HMGB1 in BEAS-2B cells. Interestingly, an airway increases in mmu-miRNA-30a-3p inhibited HMGB1 and RUNX2 expression and reduced airway eosinophilia in an experimental animal model [
135]. Therefore, epithelial miRNA-30a-3p could target the RUNX2/HMGB1 axis to reduce airway eosinophilia in asthma (
Figure 2).
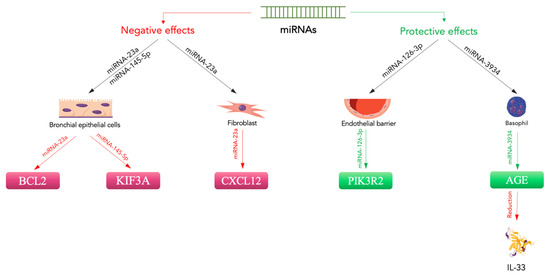
Figure 2. Protective and negative effects of miRNAs on asthma. MiRNA-23a downregulates CXCL12 expression in fibroblasts [
122]. In bronchial epithelial cells, it negatively regulates BLC2 expression, while miRNA-145-5p suppresses KIF3A [
121,
122]. These miRNAs promote bronchial inflammation and disease exacerbation. In contrast, protective effects are related to the action of miRNA-126-3p on the endothelial barrier through PIK3R2 [
127]. Additionally, miRNA-3934 acts to mitigate IL-33 levels [
123]. Magenta indicates downregulation, green indicates upregulation.
3.2. MicroRNAs and Acute Respiratory Distress Syndrome
ALI or its more critical manifestation, ARDS, is a severe acute pulmonary pathology with a significant mortality rate. ALI/ARDS is caused by different pulmonary conditions, such as pneumonia, or indirect extra-pulmonary damage, such as sepsis [136]. Its characteristics include alteration of the alveolar-capillary membrane, increased inflammation and reduced alveolar fluid clearance, with secondary hypoxemia, pulmonary oedema, and abnormal gas exchange [137,138,139].
MiRNAs and HMGB1 could also play a role in the genesis of ARDS. A report stated that the increased expression of miRNA-181b reduced the gene expression of importin-a3 and was able to decrease lung damage and mortality rates in ARDS animals [142]. Furthermore, Rao et al. showed that modification of the cytokine signalling suppressor 1 (SOCS1) in an animal model of ARDS reduced inflammatory cytokine production and inflammatory cell accumulation in miRNA-155 (−/−) animals compared to wild-type mice [143].
Finally, in different experiments, researchers assessed which miRNA could improve ARDS by targeting HMGB1 [144]. In experimental in vitro and in vivo models of LPS-induced ARDS models, they studied the effect of miRNA-574-5p on the production of HMGB1, pro-inflammatory cytokines and inflammasomes. MiRNA-574-5p seemed able to reduce the inflammatory reaction by operating on HMGB1. Stimulating the production of miRNA-574-5p or HMGB1 siRNA silencing reduced the stimulation of the NLRP3 inflammasome. Additionally, increased expression of HMGB1 overturned the anti-inflammatory action of miRNA-574-5p. In vivo, the increased production of miRNA-574-5p reduced interstitial oedema and alveolar leucocyte infiltration in ARDS animals [144].
4. Exosomal MicroRNA, Circular RNA, and Long Non-Coding RNA in Respiratory Diseases
Not only circulating miRNAs but also those contained in exosomes could play a role in respiratory diseases. Endothelial progenitor cells (EPCs) are an encouraging possibility for ALI treatment [
145,
146]. Some studies have demonstrated that EPCs can reduce inflammation and vascular leakage and increase bacterial elimination in ALI and infection-induced pulmonary damage [
147,
148,
149,
150]. Lately, exosomes have appeared as a relevant paracrine system that allows cell-to-cell communication by easing the transport of miRNAs from one cell to another [
151].
The significant alteration of circRNAs has been demonstrated in respiratory diseases. For instance, circ_0007385 presence is greater in non-small cell lung cancer (NSCLC) cells and is correlated with a poor prognosis. Silencing circ_0007385 is possible to reduce cell growth and invasion in A549 and H1975 cells and decrease cisplatin resistance [
158]. Furthermore, circ_0007385 silencing reduced the tumour proliferation of A549 cells in vivo. There was a direct interface between HMGB1, miRNA-519d-3p and circ_0007385. The production of miRNA-519d-3p was reduced in NSCLC in a circ_0007385-related manner, and circ_0007385 could secondarily control HMGB1 through miRNA-519d-3p. Thus, by both reducing miRNA-519d-3p and re-establishing HMGB1, it is possible to reverse the inhibitory action of circ_0007385 reduction in cell growth and invasion [
158].
The significant alteration of circRNAs has been demonstrated in respiratory diseases. For instance, circ_0007385 presence is greater in non-small cell lung cancer (NSCLC) cells and is correlated with a poor prognosis. Silencing circ_0007385 is possible to reduce cell growth and invasion in A549 and H1975 cells and decrease cisplatin resistance [
158]. Furthermore, circ_0007385 silencing reduced the tumour proliferation of A549 cells in vivo. There was a direct interface between HMGB1, miRNA-519d-3p and circ_0007385. The production of miRNA-519d-3p was reduced in NSCLC in a circ_0007385-related manner, and circ_0007385 could secondarily control HMGB1 through miRNA-519d-3p. Thus, by both reducing miRNA-519d-3p and re-establishing HMGB1, it is possible to reverse the inhibitory action of circ_0007385 reduction in cell growth and invasion [
158].