Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Burn injury induces a complex inflammatory response, both locally and systemically, and is not yet completely unravelled and understood. In order to enable the development of accurate treatment options, it is of paramount importance to fully understand post-burn immunology. Persistent activity of complement, acute phase proteins and pro- and anti-inflammatory mediators, changes in lymphocyte activity, activation of the stress response and infiltration of immune cells have all been related to post-burn local and systemic pathology.
- burn
- immune response
- inflammation
- complexity
1. Systemic Complications
The effect of burn injury on the human body does not end at the margins of the wound and/or by the time the wounds are closed. In fact, acute burn injury could have a severe systemic impact for years or even longer. Severe burn injuries induce a systemic response that is described as the systemic inflammatory response syndrome (SIRS) and is caused by an “over-exuberant” acute phase inflammation [1][2][3][4]. The excessive systemic release of pro-inflammatory cytokines, chemokines, lipids and vasoactive mediators leads to distant organ damage and multiple organ dysfunction syndrome (MODS). Not only does severe burn triggers secondary pathology, but even relatively minor burn injuries can also cause adverse systemic effects [5][6]. A prolonged existence of SIRS can lead to severe muscle protein catabolism, described as persistent inflammation, immunosuppression, and catabolism syndrome, and is associated with an increased risk of multi-organ failure and death [3][7][8].
1.1. Altered Endogenous Steroid Biosynthesis after Burn
Burn injury is followed by a persistent hypermetabolic response for up to two years after the burn, resulting in changes in the endogenous production of besides inflammatory mediators, steroids as well [9][10]. Plasma catecholamines (i.e., adrenalin, noradrenalin and dopamine), glucagon, and cortisol can be elevated by up to 50-fold, which leads to whole-body catabolism, elevated resting energy expenditures and multiorgan dysfunction [9]. Moreover, gender-related differences in the endogenous production of adrenal and gonadal steroids have been reported [11]. Decreased testosterone concentrations and elevated oestrone concentrations were found up to 21 days post-burn. In addition, glucocorticoids’, progestogens’, and androgen precursors’ concentrations positively correlated with the % TBSA burned [11].
Interventions in order to modulate (somewhat) the profound hypermetabolic response after burn, which resulted in significantly decreased morbidity, include, e.g., early excision and grafting of burns, thermoregulation, control of infection, early and continuous enteral nutrition and pharmacologic treatments [9].
1.2. Haemodynamic Failure
The first hours post-burn form a critical phase in which several serious systemic complications can develop in the burn patient, with the risk of mortality. In the first place, there is an acute danger of developing a shock state after severe burn trauma, which could develop within hours post-burn [12]. Patients reach a hypodynamic state directly after burn trauma, which is characterised by increased vascular permeability as a result of high thermal energy and an increase in, amongst others, pro-inflammatory mediators, nitric oxide and prostaglandins. This increase in vascular permeability could cause massive volume depletion via the extravasation of blood plasma to the interstitial tissue. The extravasated plasma proteins lead to an altered osmotic gradient, which further enhances tissue oedema and loss of blood volume [13]. However, mortality due to haemodynamic failure in the acute phase post-burn is rare since clinical interventions have greatly improved over time [14][15]. However, serious morbidity could develop in the early phase post-burn when fluid resuscitation is inappropriate. Adequate and large-volume resuscitation is required to preserve adequate perfusion of all organs, specifically the kidneys, to prevent early acute kidney injury. Also, fluid overload can be detrimental and compromise end-organ function [7][12]. In a multicentre study, it was found that when fluid resuscitation exceeded the calculated needed volume by 25%, there was a higher chance of mortality [16]. These findings illustrate that adequate fluid resuscitation is of great importance in the outcome post-burn.
1.3. Sepsis
Burn-induced disruption of the skin barrier considerably increases the risk of infection, which could lead to sepsis post-burn. Septic patients have a systemic infection to which the immune system strongly reacts, and this overwhelming response could affect multiple organs [17]. Moreover, sepsis could further enhance the immunosuppression that is observed after burn injury since this systemic disease induces apoptosis of immune cells [18]. Thus, septic patients are in a hyperinflammatory state leading to an immunosuppressive state [19]. When burn patients develop sepsis, this is usually observed several days after injury, at the time that circulatory problems often have stabilised [15]. Factors that further contribute to the occurrence of sepsis are likely related to the immune response post-burn. For example, the hyperactive macrophage phenotype seems to have an important role in the development of sepsis by the release of increased amounts of inflammatory cytokines. High concentrations of these inflammatory mediators could subsequently lead to alterations in the immune system, such as the lymphocyte response, with an impaired immune function as an outcome [20]. As such, higher levels of the anti-inflammatory cytokine IL-10, were found in septic burn patients than in non-septic burn patients [21].
It is generally known that early diagnosis of sepsis in a burn patient, with (still) negative blood cultures, is difficult since severe burn injury typically results in a hypermetabolic and highly inflammatory state. Therefore, burn patients often present with sepsis-related criteria, irrespective of systemic infection [9][22][23][24][25].
Still, a better prediction system for early sepsis diagnosis in burn patients could enable improved, earlier treatment of the septic burn patient. Biomarkers might give additional predictive value for sepsis in burn patients, such as certain systemic or local cytokine levels [26][27][28]. In this respect, procalcitonin (PCT), a 116-amino acid polypeptide prohormone of calcitonin, has become an important biomarker to aid in the diagnosis of bacterial sepsis. It has a high potential to improve the clinical assessment of patients [29]. In a normal situation, a very low concentration of procalcitonin is present in the blood. However, production can be stimulated in almost every organ by inflammatory cytokines and especially bacterial endotoxins, for example, in sepsis, which causes large amounts of PCT to be released into the blood. As a result, the amount of PCT could be seen as a potential biomarker of, for example, sepsis. The higher the PCT concentration, the more likely systemic infection and sepsis would be [30]. However, it is very difficult to accurately diagnose sepsis based on biomarkers only [31]; even positive blood cultures in sepsis cohorts are found in around 40% in prospective studies [32][33]. Moreover, sepsis diagnosis in burn patients is based on the clinical situation, i.e., increased fluid requirements, low platelet counts and declining pulmonary and/or renal function.
1.4. Acute Impact on Multiple Organ Systems
Severe burn trauma could damage multiple remote organs in the acute phase, which in the end, can lead to the loss of organ function (Figure 1A). This multiple organ dysfunction syndrome (MODS) is often observed in combination with sepsis in burn patients; however, the enormous inflammatory response to burn-induced tissue damage could also lead to MODS without sepsis, correlated to both the burn wound size and depth of burn [34][35].
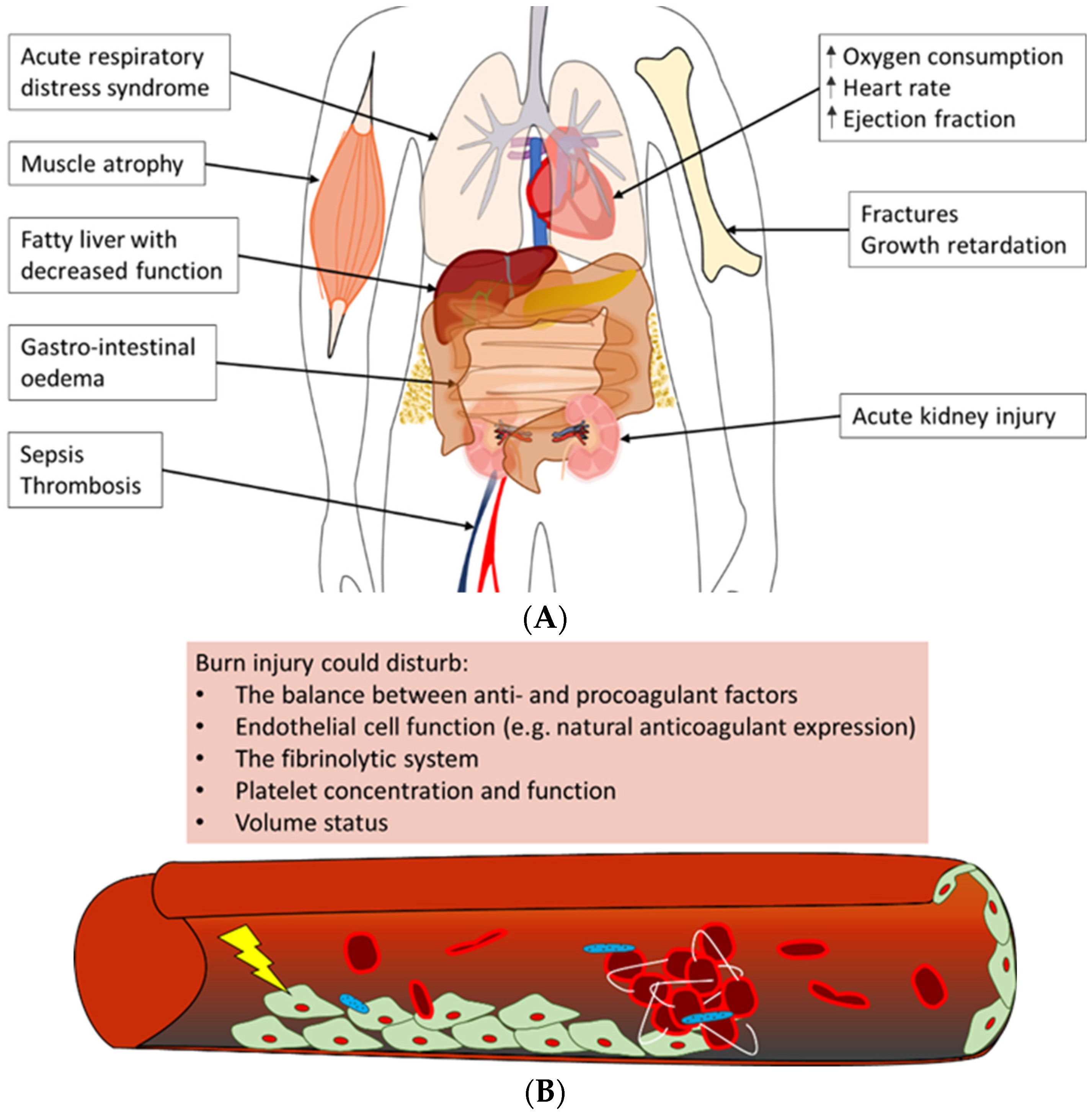
Figure 1. Overview of often observed systemic complications of burn injury [17][35][36][37][38]. (A) Burn patients could develop a variety of complications post-burn. Summarised are possible adverse outcomes of (severe) burn injury in the acute phase, e.g., acute respiratory distress syndrome and acute kidney injury, as well as long-term negative effects, such as growth retardation and a fatty liver. (B) The main trigger of coagulopathy is the inflammatory response post-burn. This response could lead to disturbances in factors that contribute to haemostasis, e.g., pro- and anticoagulant factors, platelet concentration and endothelial cell function, which could result in thrombotic events in burn patients.
One of the most often affected organs in the acute phase post-burn is the kidneys [34][35][39]. Early acute kidney injury occurs in the first days post-burn and is mostly due to a combination of hypovolemia, cardiac dysfunction and denatured proteins that exert a toxic effect on the kidneys. Multiple factors contribute to late acute kidney injury as well; among these is inflammation-induced damage to renal tubular epithelium and renal arteries [39]. Next to the kidneys, the respiratory system is often involved in MODS. Burn patients are at risk of developing acute respiratory distress syndrome (ARDS), which is characterised by pulmonary fluid infiltrates and hypoxemia [36][40]. Some important factors that have been shown to correlate with the development of ARDS post-burn are larger burn wound size and larger full-thickness burn wound area, higher age, too-aggressive fluid resuscitation, and the presence of pneumonia or acute kidney injury [41]. The exact pathophysiology of ARDS is unknown, but largely contributing is the burn-induced inflammatory response that is characterised by infiltrating neutrophils and increased concentrations of pro-inflammatory cytokines. This inflammatory response leads to damage to the endothelium of lung microvasculature and the epithelium of alveoli, resulting in the extravasation of fluid from the vasculature into the alveoli [40]. In addition, acute kidney injury likely enhances respiratory inflammation post-burn [42].
In addition, often observed in the early phase post-burn are haematologic alterations. In short, burn trauma causes alterations in the balance between pro- and anticoagulant factors and damage to the endothelium of the local and systemic vasculature. This could lead to a hypercoagulable state in the burn patient [37][38]. Also, changes in platelet concentrations post-burn, namely early thrombocytopenia, followed by thrombocytosis, could contribute to coagulopathy (Figure 1B) [43][44]. Clinically, these systemic changes could have a major impact on the patient’s prognosis. For example, low platelet concentrations might enhance the risk of bleeding during surgical interventions post-burn. In contrast, the hypercoagulable state of burn patients could increase the risk of deep venous thrombosis (DVT) or pulmonary embolism.
Other organ systems that could be affected in the early phase post-burn include the liver, the heart, the gastrointestinal tract and the central nervous system. In general, contributing to early post-burn dysfunction of these organ systems are the hypodynamic state and factors that stimulate oedema formation in organs, such as inflammation and fluid resuscitation [9]. For example, the impaired production of constitutional liver proteins often occurs as a consequence of liver oedema upon burn trauma. Although the precise cause is unknown, burn patients often suffer from increased abdominal pressure as well, which could result not only in hypoperfusion of the visceral organs but also in cardiac and respiratory failure [45]. Furthermore, activation of the stress response directly impacts the physiology of the heart by increasing, amongst others, the heart rate, ejection fraction and oxygen needs [46]. However, the latter, in turn, causes cardiac depression due to high cytokine release.
1.5. Long-Term Impact on Multiple Organ Systems
In the years following burn trauma, it is hypothesised that the systemic immune response to burn frequently has a negative impact on multiple organ systems. Recent studies show that patients with a history of burn trauma are potentially at increased risk of developing cardiovascular diseases, metabolic syndrome, diabetes, musculoskeletal problems, infectious diseases and cancer [47][48]. For example, several studies have shown a higher rate of hospital admissions for cardiovascular disease among burn patients [49][50][51]. From a pathophysiological point of view, increased concentrations of inflammatory mediators, including IL-1β, IL-6 and TNF-α, result in, amongst others, decreased cardiac contractility [52][53], which may be the biological mechanism through which increased cardiovascular disease risk may arise.
Loss of muscle strength and endurance post-burn also has a major impact on burn patients. Years after burn trauma, patients have reported fatigue, swelling and pain in joints and weakness of the limbs. Biological mechanisms that underlie the loss of skeletal muscle mass post-burn include the effects of the stress hormone catecholamine, burn-induced insulin resistance, and increased need for amino acids for wound healing and the immune system. Also, inflammatory cytokines mediate a catabolic state post-burn [54].
Besides the loss of muscle mass, post-burn changes in endocrine and metabolic pathways can induce alterations in bone tissue. Remodelling of bone tissue is a normal physiological process. However, changes in the activity of the bone-resorbing osteoclasts and the mineralising osteocytes in burn patients could lead to decreased bone mineral density and bone mass. Involved in these changes in bone remodelling are, amongst others, the adrenal stress hormone glucocorticoid and the cytokines IL-1β and IL-6 [55].
2. Prediction of the Clinical Outcome
2.1. The Cytokine Network as Clinical Predictor
Burn injury elicits a massive inflammatory response that results in detectable plasma levels of inflammatory cytokines, which typically peak in concentration within the first week post-burn. Several research groups have questioned whether the blood concentrations of cytokines or other inflammatory proteins might be valuable as predictors of clinical outcomes post-burn [27][56][57][58][59]. As such, cytokine profiles were identified that correlated with outcomes in the first month after injury in adult burn patients with mild to severe injuries [58]. On the day of burn trauma, plasma levels of IL-6, IL-8, IL-10 and CCL2 were significantly higher in non-survivors than in survivors. Furthermore, this cytokine profile of the early phase post-burn correlated with Sequential Organ Failure Assessment (SOFA) scores, a scoring system that often is used to determine the severity of MODS which is based on the functionality of six vital organ systems (i.e., the respiratory, haematologic, hepatic, cardiovascular, neurologic and renal system). Although IL-6, IL-8, IL-10 and monocyte chemoattractant protein 1 (MCP-1) levels correlated to the size of injury 24–48 h post-burn, these cytokines seemed not very dependable clinical markers of outcome compared to demographic data, e.g., age, size of the injury and inhalation injury, with the exception of IL-8 and MCP-1 levels on admission in predicting death [56]. Besides, metalloproteinases (MMPs) were also studied for their association with outcome. Although MMP-8 and -9 were higher in burn patients than in healthy controls, they did not correlate with % TBSA and were not associated with clinical severity or outcome measures. TIMP-1, in contrast, which was also higher in patients than in healthy controls, was independently associated with 90-day mortality, correlated with % TBSA burned, fluid and noradrenaline requirement and SOFA score [60]. Therefore TIMP-1 may serve as a potential biomarker in the outcome of burn patients. However, further research is necessary in order to reveal the biological background for the outcome association.
Overall, several studies point to the importance of cytokines as indicators for the severity of the disease. However, due to the integrative character of the post-burn systemic immune response, with the involvement of many inflammatory factors, it seems impossible to attribute individual cytokine levels to a specific patient prognosis. Therefore it is necessary to use cytokine profiles.
2.2. Molecular Markers as Clinical Predictors
In addition to markers of the post-burn inflammatory response, other markers, including molecular markers, e.g., genomic DNA markers, mRNA/miRNAs/lncRNAs/circRNAs markers, epigenetic markers, proteomics, and metabolics, could serve as potential biomarkers in the outcome of burn patients [61]. The advantage of these non-inflammatory response markers could be that they can predict, for instance, sepsis in burn patients, as markers of the post-burn inflammatory response do not reflect the severity of the infection.
Analyses of changes in genetic processes in the skin during burns revealed three potential novel diagnostic markers in blood from burn patients. Three Hub genes, MCEMP1, MMP9, and S100A12, were shown to be significantly elevated in burn patients and were suggested as key blood biomarkers that can be used to identify skin damage in burn patients [62].
Furthermore, analyses of miRNAs that are potentially involved in early burn-response gene regulation have shown that miRNA-497-5p, which regulates skin cell regeneration, was downregulated in tissue and dermal interstitial fluid (dISF) of burned skin. Therefore, further examination of miRNA-497-5p as a biomarker for the severity of burns is suggested [63].
This entry is adapted from the peer-reviewed paper 10.3390/cells12030345
References
- Dahiya, P. Burns as a model of SIRS. Front. Biosci. (Landmark Ed.) 2009, 14, 4962–4967.
- Gille, J.; Dietz, A.; Taha, H.; Sablotzki, A. A sirs-based automated alarm system for the diagnosis of sepsis after burn injury. Ann. Burn. Fire Disasters 2017, 30, 177–184.
- Toliver-Kinsky, T.; Kobayashi, M.; Suzuki, F.; Sherwood, E.R. 19—The Systemic Inflammatory Response Syndrome. In Total Burn Care, 4th ed.; Herndon, D.N., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 205–220.e204.
- Jeschke, M.G. Postburn Hypermetabolism: Past, Present, and Future. J. Burn Care Res. 2016, 37, 86–96.
- Korkmaz, H.I.; Ulrich, M.M.W.; van Wieringen, W.N.; Vlig, M.; Emmens, R.W.; Meyer, K.W.; Sinnige, P.; Krijnen, P.A.J.; van Zuijlen, P.P.M.; Niessen, H.W.M. The Local and Systemic Inflammatory Response in a Pig Burn Wound Model With a Pivotal Role for Complement. J. Burn Care Res. 2017, 38, e796–e806.
- Sarginson, J.H.; Hollén, L.; Emond, A.; Mackie, I.; Young, A.E. Multicentre observational study describing the systemic response to small-area burns in children. Burns 2021, 47, 560–568.
- Malbrain, M.; Van Regenmortel, N.; Saugel, B.; De Tavernier, B.; Van Gaal, P.J.; Joannes-Boyau, O.; Teboul, J.L.; Rice, T.W.; Mythen, M.; Monnet, X. Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensive Care 2018, 8, 66.
- Mira, J.C.; Brakenridge, S.C.; Moldawer, L.L.; Moore, F.A. Persistent Inflammation, Immunosuppression and Catabolism Syndrome. Crit. Care Clin. 2017, 33, 245–258.
- Williams, F.N.; Herndon, D.N. Metabolic and Endocrine Considerations After Burn Injury. Clin. Plast. Surg. 2017, 44, 541–553.
- Williams, F.N.; Herndon, D.N.; Jeschke, M.G. The hypermetabolic response to burn injury and interventions to modify this response. Clin. Plast. Surg. 2009, 36, 583–596.
- Bergquist, M.; Huss, F.; Hedenstierna, F.F.G.; Hästbacka, J.; Rockwood, A.L.; Kushnir, M.M.; Bergquist, J. Altered adrenal and gonadal steroids biosynthesis in patients with burn injury. Clin. Mass Spectrom. 2016, 1, 19–26.
- Nielson, C.B.; Duethman, N.C.; Howard, J.M.; Moncure, M.; Wood, J.G. Burns: Pathophysiology of Systemic Complications and Current Management. J. Burn Care Res. 2017, 38, e469–e481.
- Wolfe, R.R. Review: Acute versus chronic response to burn injury. Circ. Shock 1981, 8, 105–115.
- Brusselaers, N.; Monstrey, S.; Vogelaers, D.; Hoste, E.; Blot, S. Severe burn injury in europe: A systematic review of the incidence, etiology, morbidity, and mortality. Crit. Care 2010, 14, R188.
- Dokter, J.; Felix, M.; Krijnen, P.; Vloemans, J.F.P.M.; Baar, M.E.V.; Tuinebreijer, W.E.; Breederveld, R.S. Mortality and causes of death of Dutch burn patients during the period 2006–2011. Burns 2015, 41, 235–240.
- Klein, M.B.; Hayden, D.; Elson, C.; Nathens, A.B.; Gamelli, R.L.; Gibran, N.S.; Herndon, D.N.; Arnoldo, B.; Silver, G.; Schoenfeld, D.; et al. The association between fluid administration and outcome following major burn: A multicenter study. Ann. Surg. 2007, 245, 622–628.
- Manning, J. Sepsis in the Burn Patient. Crit. Care Nurs. Clin. N. Am. 2018, 30, 423–430.
- Cao, C.; Yu, M.; Chai, Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis. 2019, 10, 782.
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nature reviews. Immunology 2013, 13, 862–874.
- Schwacha, M.G. Macrophages and post-burn immune dysfunction. Burns 2003, 29, 1–14.
- Csontos, C.; Foldi, V.; Pálinkas, L.; Bogar, L.; Röth, E.; Weber, G.; Lantos, J. Time course of pro- and anti-inflammatory cytokine levels in patients with burns--prognostic value of interleukin-10. Burns 2010, 36, 483–494.
- Jeschke, M.G.; Chinkes, D.L.; Finnerty, C.C.; Kulp, G.; Suman, O.E.; Norbury, W.B.; Branski, L.K.; Gauglitz, G.G.; Mlcak, R.P.; Herndon, D.N. Pathophysiologic response to severe burn injury. Ann. Surg. 2008, 248, 387–401.
- Mann-Salinas, E.A.; Baun, M.M.; Meininger, J.C.; Murray, C.K.; Aden, J.K.; Wolf, S.E.; Wade, C.E. Novel predictors of sepsis outperform the American Burn Association sepsis criteria in the burn intensive care unit patient. J. Burn Care Res. 2013, 34, 31–43.
- Stanojcic, M.; Vinaik, R.; Jeschke, M.G. Status and Challenges of Predicting and Diagnosing Sepsis in Burn Patients. Surg. Infect. 2018, 19, 168–175.
- Yan, J.; Hill, W.F.; Rehou, S.; Pinto, R.; Shahrokhi, S.; Jeschke, M.G. Sepsis criteria versus clinical diagnosis of sepsis in burn patients: A validation of current sepsis scores. Surgery 2018, 164, 1241–1245.
- Chen, X.-L.; Xia, Z.-F.; Wei, D.; Han, S.; Ben, D.-F.; Wang, G.-Q. Role of p38 mitogen-activated protein kinase in Kupffer cell secretion of the proinflammatory cytokines after burn trauma. Burns 2003, 29, 533–539.
- Kraft, R.; Herndon, D.N.; Finnerty, C.C.; Cox, R.A.; Song, J.; Jeschke, M.G. Predictive Value of IL-8 for Sepsis and Severe Infections After Burn Injury: A Clinical Study. Shock 2015, 43, 222–227.
- Widgerow, A.D.; King, K.; Tocco-Tussardi, I.; Banyard, D.A.; Chiang, R.; Awad, A.; Afzel, H.; Bhatnager, S.; Melkumyan, S.; Wirth, G.; et al. The burn wound exudate-an under-utilized resource. Burns 2015, 41, 11–17.
- Schuetz, P.; Beishuizen, A.; Broyles, M.; Ferrer, R.; Gavazzi, G.; Gluck, E.H.; Del Castillo, J.G.; Jensen, J.U.; Kanizsai, P.L.; Kwa, A.L.H.; et al. Procalcitonin (PCT)-guided antibiotic stewardship: An international experts consensus on optimized clinical use. Clin. Chem Lab. Med. 2019, 57, 1308–1318.
- Becker, K.L.; Snider, R.; Nylen, E.S. Procalcitonin assay in systemic inflammation, infection, and sepsis: Clinical utility and limitations. Crit. Care Med. 2008, 36, 941–952.
- Li, A.T.; Moussa, A.; Gus, E.; Paul, E.; Yii, E.; Romero, L.; Lin, Z.C.; Padiglione, A.; Lo, C.H.; Cleland, H.; et al. Biomarkers for the Early Diagnosis of Sepsis in Burns: Systematic Review and Meta-analysis. Ann. Surg. 2022, 275, 654–662.
- Jouffroy, R.; Vivien, B. Positive cultures and clinical outcomes in septic patients: Be aware of the influence from patient selection and the in-hospital confounders. Crit. Care 2019, 23, 332.
- Nannan Panday, R.S.; Lammers, E.M.J.; Alam, N.; Nanayakkara, P.W.B. An overview of positive cultures and clinical outcomes in septic patients: A sub-analysis of the Prehospital Antibiotics Against Sepsis (PHANTASi) trial. Crit. Care 2019, 23, 182.
- Kallinen, O.; Maisniemi, K.; Böhling, T.; Tukiainen, E.; Koljonen, V. Multiple organ failure as a cause of death in patients with severe burns. J. Burn Care Res. 2012, 33, 206–211.
- Nguyen, L.N.; Nguyen, T.G. Characteristics and outcomes of multiple organ dysfunction syndrome among severe-burn patients. Burns 2009, 35, 937–941.
- Cumming, J.; Purdue, G.F.; Hunt, J.L.; O’Keefe, G.E. Objective estimates of the incidence and consequences of multiple organ dysfunction and sepsis after burn trauma. J. Trauma 2001, 50, 510–515.
- King, D.R.; Namias, N.; Andrews, D.M. Coagulation abnormalities following thermal injury. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2010, 21, 666–669.
- Kowal-Vern, A.; Gamelli, R.L.; Walenga, J.M.; Hoppensteadt, D.; Sharp-Pucci, M.; Schumacher, H.R. The effect of burn wound size on hemostasis: A correlation of the hemostatic changes to the clinical state. J. Trauma 1992, 33, 50–56, Discussion 56–57.
- Clark, A.; Neyra, J.A.; Madni, T.; Imran, J.; Phelan, H.; Arnoldo, B.; Wolf, S.E. Acute kidney injury after burn. Burns 2017, 43, 898–908.
- Cartotto, R. Acute Respiratory Distress Syndrome in the Burn Patient. In Burns, Infections and Wound Management; Shiffman, M.A., Low, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 111–122.
- Sine, C.R.; Belenkiy, S.M.; Buel, A.R.; Waters, J.A.; Lundy, J.B.; Henderson, J.L.; Stewart, I.J.; Aden, J.K.; Liu, N.T.; Batchinsky, A.; et al. Acute Respiratory Distress Syndrome in Burn Patients: A Comparison of the Berlin and American-European Definitions. J. Burn Care Res. 2016, 37, e461–e469.
- Clemens, M.S.; Stewart, I.J.; Sosnov, J.A.; Howard, J.T.; Belenkiy, S.M.; Sine, C.R.; Henderson, J.L.; Buel, A.R.; Batchinsky, A.I.; Cancio, L.C.; et al. Reciprocal Risk of Acute Kidney Injury and Acute Respiratory Distress Syndrome in Critically Ill Burn Patients. Crit. Care Med. 2016, 44, e915–e922.
- Marck, R.E.; Montagne, H.L.; Tuinebreijer, W.E.; Breederveld, R.S. Time course of thrombocytes in burn patients and its predictive value for outcome. Burns 2013, 39, 714–722.
- Warner, P.; Fields, A.L.; Braun, L.C.; James, L.E.; Bailey, J.K.; Yakuboff, K.P.; Kagan, R.J. Thrombocytopenia in the pediatric burn patient. J. Burn Care Res. 2011, 32, 410–414.
- Kirkpatrick, A.W.; Ball, C.G.; Nickerson, D.; D’Amours, S.K. Intraabdominal Hypertension and the Abdominal Compartment Syndrome in Burn Patients. World J. Surg. 2009, 33, 1142–1149.
- Williams, F.N.; Herndon, D.N.; Suman, O.E.; Lee, J.O.; Norbury, W.B.; Branski, L.K.; Mlcak, R.P.; Jeschke, M.G. Changes in cardiac physiology after severe burn injury. J. Burn Care Res. 2011, 32, 269–274.
- Barrett, L.W.; Fear, V.S.; Waithman, J.C.; Wood, F.M.; Fear, M.W. Understanding acute burn injury as a chronic disease. Burn. Trauma 2019, 7, 23.
- Duke, J.M.; Randall, S.M.; Wood, F.M.; Boyd, J.H.; Fear, M.W. Burns and long-term infectious disease morbidity: A population-based study. Burns 2017, 43, 273–281.
- Duke, J.M.; Randall, S.M.; Fear, M.W.; Boyd, J.H.; Rea, S.; Wood, F.M. Understanding the long-term impacts of burn on the cardiovascular system. Burns 2016, 42, 366–374.
- Duke, J.M.; Randall, S.M.; Fear, M.W.; O’Halloran, E.; Boyd, J.H.; Rea, S.; Wood, F.M. Long term cardiovascular impacts after burn and non-burn trauma: A comparative population-based study. Burns 2017, 43, 1662–1672.
- O’Halloran, E.; Shah, A.; Dembo, L.; Hool, L.; Viola, H.; Grey, C.; Boyd, J.; O’Neill, T.; Wood, F.; Duke, J.; et al. The impact of non-severe burn injury on cardiac function and long-term cardiovascular pathology. Sci. Rep. 2016, 6, 34650.
- Garner, L.B.; Willis, M.S.; Carlson, D.L.; DiMaio, J.M.; White, M.D.; White, D.J.; Adams, G.A.T.; Horton, J.W.; Giroir, B.P. Macrophage migration inhibitory factor is a cardiac-derived myocardial depressant factor. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2500–H2509.
- Maass, D.L.; White, J.; Horton, J.W. IL-1beta and IL-6 act synergistically with TNF-alpha to alter cardiac contractile function after burn trauma. Shock 2002, 18, 360–366.
- Holavanahalli, R.K.; Helm, P.A.; Kowalske, K.J. Long-Term Outcomes in Patients Surviving Large Burns: The Musculoskeletal System. J. Burn Care Res. 2016, 37, 243–254.
- Klein, G.L. Disruption of bone and skeletal muscle in severe burns. Bone Res. 2015, 3, 15002.
- Bergquist, M.; Hästbacka, J.; Glaumann, C.; Freden, F.; Huss, F.; Lipcsey, M. The time-course of the inflammatory response to major burn injury and its relation to organ failure and outcome. Burns 2019, 45, 354–363.
- Hur, J.; Yang, H.T.; Chun, W.; Kim, J.-H.; Shin, S.-H.; Kang, H.J.; Kim, H.S. Inflammatory cytokines and their prognostic ability in cases of major burn injury. Ann. Lab. Med. 2015, 35, 105–110.
- Matsuura, H.; Matsumoto, H.; Osuka, A.; Ogura, H.; Shimizu, K.; Kang, S.; Tanaka, T.; Ueyama, M.; Shimazu, T. Clinical Importance of a Cytokine Network in Major Burns. Shock 2019, 51, 185–193.
- Finnerty, C.C.; Jeschke, M.G.; Qian, W.J.; Kaushal, A.; Xiao, W.; Liu, T.; Gritsenko, M.A.; Moore, R.J.; Camp, D.G., 2nd; Moldawer, L.L.; et al. Determination of burn patient outcome by large-scale quantitative discovery proteomics. Crit. Care Med. 2013, 41, 1421–1434.
- Hästbacka, J.; Fredén, F.; Hult, M.; Bergquist, M.; Wilkman, E.; Vuola, J.; Sorsa, T.; Tervahartiala, T.; Huss, F. Matrix metalloproteinases -8 and -9 and tissue inhibitor of metalloproteinase-1 in burn patients. A prospective observational study. PLoS ONE 2015, 10, e0125918.
- Muñoz, B.; Suárez-Sánchez, R.; Hernández-Hernández, O.; Franco-Cendejas, R.; Cortés, H.; Magaña, J.J. From traditional biochemical signals to molecular markers for detection of sepsis after burn injuries. Burns 2019, 45, 16–31.
- Yang, R.; Wang, Z.; Li, J.; Pi, X.; Wang, X.; Xu, Y.; Shi, Y.; Zhou, S. Identification and Verification of Five Potential Biomarkers Related to Skin and Thermal Injury Using Weighted Gene Co-Expression Network Analysis. Front. Genet. 2021, 12, 781589.
- Foessl, I.; Haudum, C.W.; Vidakovic, I.; Prassl, R.; Franz, J.; Mautner, S.I.; Kainz, S.; Hofmann, E.; Obermayer-Pietsch, B.; Birngruber, T.; et al. miRNAs as Regulators of the Early Local Response to Burn Injuries. Int. J. Mol. Sci. 2021, 22, 9209.
This entry is offline, you can click here to edit this entry!
