1. Nanomaterials
In the case of particles, platelets, or fibers, the surface area per unit volume is inversely proportional to the material’s diameter D. The surface/volume ratio R is 6/D. The smaller the diameter, the larger the surface area per unit volume.
Typical nano-fillers under investigation include, nanoparticles, nanotubes, nanofibers, fullerenes, and nanowires, where are classified into three classes by their geometries, such as one-, two-, or three-dimensional nanoscale materials [
5,
6].
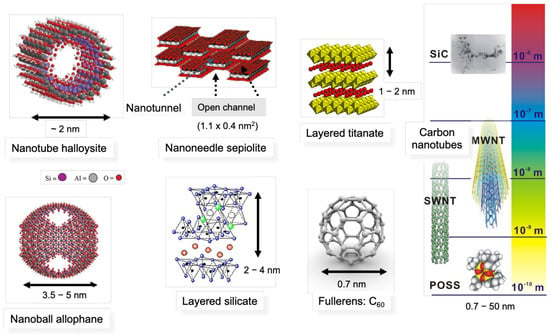
Figure 1 shows the schematic illustration of several nano-fillers with dimensions. Silica, nano-silicon carbide (
n-SiC), carbon black, fullerens, polyhedral oligomeric sislesquioxanes (POSS), allophane nanoball are classified as nanoparticle reinforcing agents. Carbon nanofibers, carbon nanotubes and nanofibers (halloysite, nickel nanostrand: NiNs) are fibrous materials. In the case of the filler having a nanometer thickness and a high aspect ratio (50–1000) platelet structure, it is classified as a layered nanomaterial, such as an organically modified layered silicate (
Figure 1) [
1,
3,
5].
Figure 1. Schematic illustration of several nano-fillers (nanomaterials) with dimension. Polyhedral oligomeric sislesquioxanes (POSS), carbon nanotubes (single-wall nanotube (SWNT) and multi-wall nanotube (MWNT)), nanofiber (halloysite), nanoneedle (sepiolite), nanoballs (fullerens, allophane), layered nanomaterials (layered silicate, layered titanate), and silicon carbide (SiC).
2. Multifunctional Properties
Polymer nanocomposites have interphases that dominate the composite properties due to the very small size of the nano-filler component. For nano-fillers, at least one dimension is on the nanometer scale, as shown in
Figure 1. The polymers are thermoplastic, thermoset, or rubber and elastomer. The polymer–nano-filler interface is the dominant factor for the nanocomposite properties. The interaction between the polymer molecule is entirely a molecular-level interaction. The surface chemistry and bulk mixing dynamics have affected the incorporation of nanoparticles into the polymer matrix. Since the invention of polymer nanocomposite in 1993 [
7], polymer nanocomposites have become an important class of materials that offer superior properties as compared with those of conventional macrocomposites. A new property is the multifunctional properties that extend their scope of application to new areas.
This new class of materials is now being introduced in structural applications, such as gas barrier film, flame retardant product, and other load-bearing applications. The polymer nanocomposites exhibit other property enhancement such as thermal (stability and conductivity) ablation, electrical, optical, tribological, chemical resistance. These multifunctional properties of polymer nanocomposites are discussed in detail in the literature [
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18]. It is a very complex matter to understand the reason why the property enhancement takes place in polymer nanocomposites.
The researchers of Toyota Central Research & Development (TCRD) reported work based on Nylon-6-clay nanocomposites [
7]. The resulting composite with a loading of only 4.2 wt% organically modified layered silicate possessed a double modulus, a 50% enhanced strength, and an increase in heat distortion temperature of 80 °C, as compared with the neat Nylon-6 [
19]. In recent years, for nanocomposite fabrication, organically modified layered silicate (organoclay) has been used in various polymer systems including epoxy, polyurethanes, and so on [
1].
The US Army research laboratory investigated the ballistic impact strength of polycarbonate-layered silicate nanocomposites [
6,
20]. Nanocomposites showed an important role in longer-range missiles. Koo and colleague investigated the nanocomposites for high-temperature application by cyanate ester, epoxy, phenolic, nylon-11, etc., and the feasibility of using these materials for fire retardant coating, rocket production insulation, rocket nozzle ablative materials, damage tolerant performance [
21]. Nanoclay plays an important role in reducing the flammability of coating system for solid rocket exhaust plumes (3600 °C) at very high velocity. Flammability enhancement by organoclay is an important issue for many applications as compared to pure polymer systems [
1].
NASA Langley Center developed transparent nanocomposites with organically modified layered silicate. Those are lightweight and durable and suitable for aerospace applications [
6,
22]. Using nanoclays in carbon fiber/epoxy reinforced composites cryogenic storage system has been developed [
23]. Both mechanical and thermal expansion characteristics were improved to avoid micro cracking and thermal cycling because of the temperature range from –196 to 125 °C for space application.
3. Structure and Characterization Techniques for Nanocomposites
Nanomaterials provide reinforcing efficiency with their high aspect ratio. The properties of a nanocomposite are greatly affected by the size scale of the component phases and the degree of mixing between the two phases. Depending on the nature of the components used (layered silicate or nanofiber) and the method of preparation, the obtained properties may be different [
1].
Of particular interest has been recently developed nanocomposites consisting of a polymer and layered silicate because they often exhibit remarkably improved mechanical and various other properties [
1,
3,
5,
8], as compared with pure polymer or conventional macrocomposites. Layered silicates have a layer thickness in the order of 1 nm and very high aspect ratio (e.g., 10–1000). Therefore, a few weight percent of layered silicate properly dispersed throughout the polymer matrix provides a much larger surface area for polymer/filler interaction than conventional composites. Depending on the strength of interfacial interaction between polymer matrix and layered silicate (modified or not), three different types of polymer-layered silicate (PLS) nanocomposites are thermodynamically achievable.
(1) Intercalated nanocomposites: in an intercalated nanocomposite, the insertion of polymer matrix into the layered silicate structure occurs in a crystallographically regular fashion, regardless of the silicate layer (clay) to polymer ratio. Properties of the composites typically resemble those of ceramic materials.
(2) Flocculated nanocomposites: conceptually this is same with intercalated nanocomposites; however, silicate layers are sometimes flocculated due to hydroxylated edge–edge interaction of the silicate layers. The length of the oriented collections in the range of 300–800 nm is far larger than the original silicate layer (mean diameter 150 nm) [
24]. Such flocculation presumably is governed by an interfacial energy between polymer matrix and organoclays and controlled by ammonium cation-matrix polymer interaction. The polarity of the matrix polymer is of fundamental importance in controlling the nanoscale structure.
(3) Exfoliated nanocomposites: in exfoliated nanocomposites, the individual silicate layers are separated in a continuous polymer matrix by an average distance that is entirely dependent on the layered silicate loading. Usually, the clay content of an exfoliated nanocomposite is much lower than that of intercalated nanocomposites.
The preparative methods are broadly classified into three main categories.
- (1)
-
Intercalation of polymer or pre-polymer from solution
This is based on a solvent system in which polymer or pre-polymer is soluble and the silicate layers are swellable. The layered silicate is first swollen in a solvent, such as water, chloroform or toluene, etc. When the polymer and layered silicate solutions are mixed, the polymer chains intercalate and displace the solvent within the interlayer of the silicate. Upon solvent removal, the intercalated structure remains, resulting in polymer-layered silicate (PLS) nanocomposites.
- (2)
-
In situ intercalative polymerization method
In this method, the organically modified layered silicate is swollen within the liquid monomer or a monomer solution so as the polymer formation can occur in between the intercalated sheets. Polymerization can be initiated either by heat or radiation, by the diffusion of a suitable initiator or by an organic initiator or catalyst fixed through cation exchange inside the interlayer before the swelling step by the monomer.
- (3)
-
Melt intercalation method
This method involves annealing, statically or under shear, a mixture of the polymer and organically modified layered silicate above the softening point of the polymer. This method has great advantages over either in situ intercalative polymerization or polymer solution intercalation. Firstly, this method is environmentally benign due to the absence of organic solvents. Secondly, it is compatible with current industrial processes, such as extrusion and injection molding. Melt intercalation method allows the use of polymers which were previously not suitable for in situ polymerization or solution intercalation method. Other possibilities are exfoliation adsorption, and template synthesis [
1,
13]. Nowadays, this solvent-free method is much preferred for practical industrial material production by its high efficiency and possibility of avoiding environmental hazards.
Analogously, in fibrous or particle-reinforced polymer nanocomposites, dispersion of the nanoparticle and adhesion at the particle–matrix interface play pivotal roles in determining the mechanical properties of the nanocomposite. Without proper dispersion, the nanomaterial will not offer improved mechanical properties over that of conventional composites, in fact, a poorly dispersed nanomaterial may degrade the mechanical properties [
1]. Graphite and graphene platelets have a similar geometry (layered sheet structure) with clay; therefore, a clay polymer reinforcement concept is also applicable. Additionally, optimizing the interfacial bond between the particle and the matrix, one can tailor the properties of the overall composite, similar to what is performed on macrocomposites. For example, good adhesion at the interface will improve properties such as interlaminar shear strength, delamination resistance, fatigue, and corrosion resistance.
Various techniques for nanocomposite characterization have been employed. The structure of the nanocomposites has typically been established using a Wide-angle X-ray diffraction (WAXD), small-angle X-ray scattering (SAXS) analysis, scanning electron microscopy (SEM), and transmission electron microscope (TEM) observation [
1,
16]. The structure of the PLS nanocomposites has typically been established using WAXD analysis and TEM observation. Due to its easiness and availability WAXD is most commonly used to probe the PLS nanocomposite structure and sometimes to study the kinetics of the polymer melt intercalation [
1,
16]. By monitoring the position, shape, and intensity of the basal reflections from the distributed silicate layers, the nanocomposite structure either intercalated or exfoliated may be identified. For example, in case of exfoliated nanocomposites, the extensive layer separation associated with the delamination of the original silicate layers in the polymer matrix results in the eventual disappearance of any coherent X-ray diffraction from the distributed silicate layers.
On the other hand, for intercalated nanocomposites, the finite layer expansion associated with the polymer intercalation results in the appearance of a new basal reflection corresponding to the larger gallery height. Although, WAXD offers a convenient method to determine the interlayer spacing of the silicate layers in the original layered silicates and in the intercalated nanocomposites (within 1–4 nm); however, little can be said about the spatial distribution of the silicate layers or any structural in-homogeneities in the PLS nanocomposites.
Additionally, some layered silicates initially do not exhibit well-defined basal reflection. Thus, peak broadening and intensity decreases are very difficult to study systematically. Therefore, conclusions concerning the mechanism of nanocomposites formation and their structure based solely on WAXD patterns are only tentative. On the other hand, TEM allows a qualitative understanding of the internal structure, spatial distribution of the various phases, and defect structure through direct visualization. However, special care must be exercised to guarantee a representative cross section of the sample.
With SEM, researchers can obtain images of surface features associated with a sample. In addition, two other microscopies, scanning probe microscopy (SPM) and scanning tunneling microscopy (STM) are useful to analyze nanotube research [
16]. The SPM uses the interaction between a sharp tip and a surface to obtain an image. For STM, a sharp conducting tip is held sufficiently close to a surface (typically ~0.5 nm), such that electrons can ‘tunnel’ across the gap [
16]. This method provides surface structural and electronic information at atomic level. The invention of the STM inspired the development of other ‘scanning probe’ microscopes, such as atomic force microscope (AFM) [
16]. The AFM uses a sharp tip to scan the entire sample. Raman spectroscopy has also proven to be a useful probe for carbon-based material properties [
16].
This entry is adapted from the peer-reviewed paper 10.3390/eng4010028