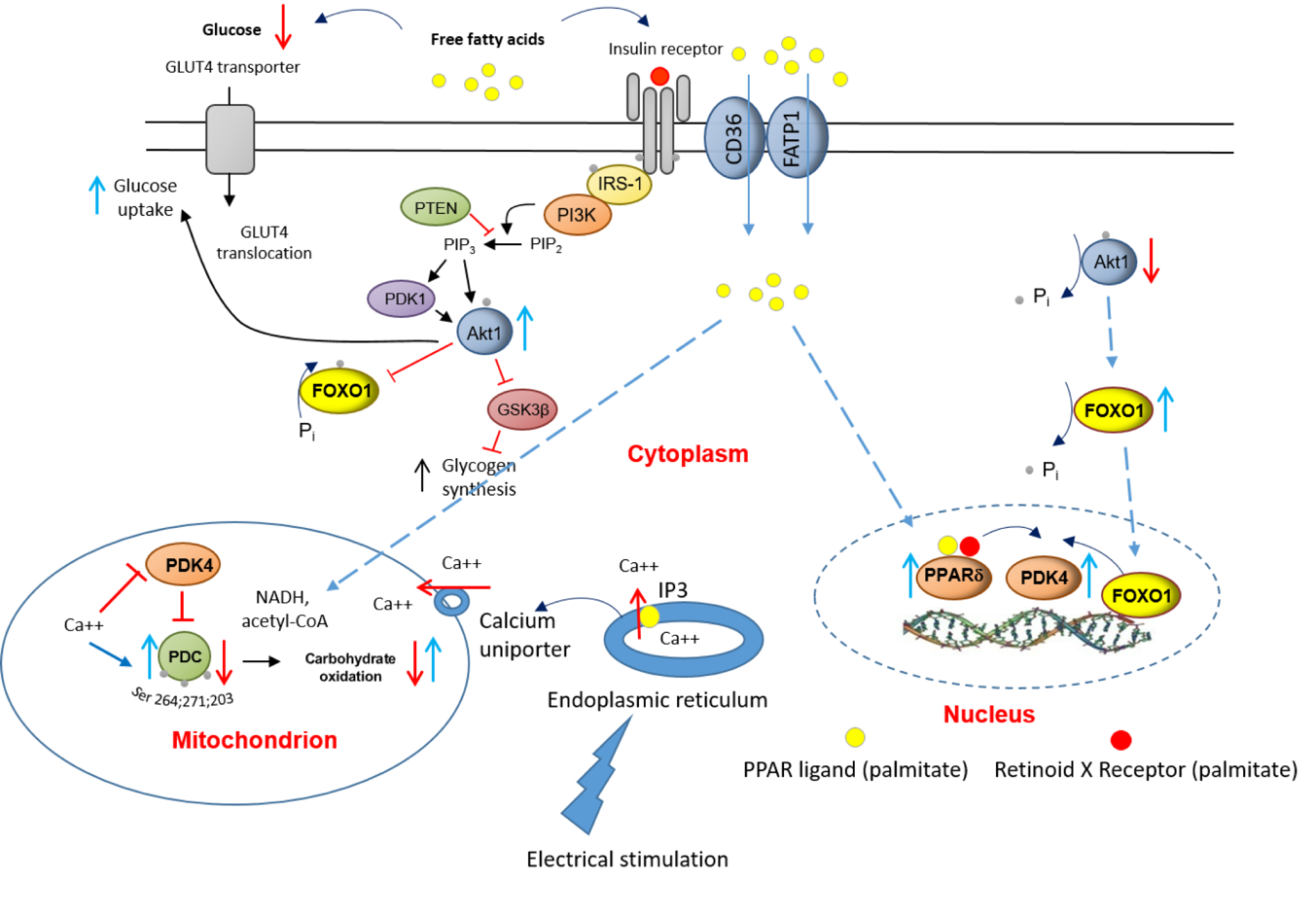
The scheme points to potentially important roles for PPAR delta and FOXO1 in the palmitate-induced increase in myotube PDK4 protein expression and linked reductions in cellular glucose uptake, PDC activity and flux, and rates of CHO derived oxidative ATP generation. Activation of PPAR delta with natural (free fatty acids) or synthetic ligands have been associated with increases in muscle PDK4 mRNA and protein expression, which would thereby inhibit PDC activity. In the case of FOXO1, dephosphorylation (activation) results in its translocation from the cytosol to the nucleus, where it can bind directly to the promoter region of the PDK4 gene. Moreover, FOXO1 dephosphorylation appears to be coupled via a signalling cascade to changes in circulatory FFA and/or insulin availability, thereby tying muscle PDK4 gene transcription (amongst others) to substrate availability. Furthermore, the finding that 16 hrs of EPS calcium release rescued this palmitate-induced dysregulation of cellular CHO metabolism via modulation of the same molecular and cellular events, alludes to the mechanisms by which chronic physical activity will protect skeletal muscle against lipid-induced muscle metabolic inflexibility and IR.
- fuel selection
- PPAR delta
- FOXO1
- PDK4
- PDC activity
Introduction
A schematic illustration of the underlying mechanism by which palmitate inhibits the carbohydrate (CHO)/glucose-derived pyruvate mitochondrial ATP production through activation of PPARδ- and FOXO1-mediated upregulation of PDK4 protein. Blue upright arrow denotes upregulation and red downward arrow denotes downregulation. CD36, fatty acid translocase; FATP1 insulin-sensitive fatty acid transporter; PDK1, 3-phosphoinositide-dependent kinase-1; GLUT4, glucose transporter isoform 4; IGF, insulin-like growth factor; FFA, free fatty acid; Akt1, serine/threonine-protein kinase 1; PTEN, phosphatase and tensin homolog; PI3K, phosphoinositol 3-kinase; GSK3β, glycogen synthase kinase; IRS-1, insulin receptor substrate 1.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21165942