Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
COVID-19 is an infectious disease declared as a pandemic on 11 March 2020 by the World Health Organization (WHO). As the pandemic evolved, so did the search for potential prophylactic and therapeutic agents. Several therapies have been developed, including vaccines, antivirals, monoclonal antibodies, and others.
- antiretroviral drugs
- inclusion complexes
- COVID-19
- SARS-CoV-2
1. COVID-19 Etiopathology
COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which belongs to a diverse group of coronaviruses characterized by enveloped, single-stranded, positive sense ribonucleic acid (RNA) viruses with a long-range tropism, which gives them the ability to cause overwhelming diseases [1].
Viruses are known to enter the host cell with the help of receptors that mediate endocytosis. In the case of SARS-CoV-2, it has been reported that the spike protein is responsible for binding to the angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell surface, which is the entry point for the virus. However, SARS-CoV-2 entry is not only dependent on the binding of the spike protein to the ACE-2 receptor, but it also requires the priming of the spike protein by the serine-2 transmembrane protease (TMPRSS2), which is crucial for the fusion of the virus to the host cell membrane. This synergy between the ACE-2 receptor and TMPRSS2 is necessary for the virus to enter into the host. The expression of TMPRSS2 is much higher than the ACE-2 receptor, suggesting that the latter is the limiting factor for SARS-CoV-2 during the early stage of infection [2][3].
Evidence has shown that the first target of the virus is the respiratory system. However, it can cause alterations in different body organs. Although some patients are asymptomatic or have mild to moderate symptoms, a percentage can develop severe illness [4]. The most common complications are acute respiratory failure syndrome (ARDS), septic shock, and sepsis. Risk factors such as age and comorbidities such as chronic diseases are related to severe illness and mortality [5].
Several symptoms have been associated with this disease, such as fever, cough, difficulty in breathing, headache, fatigue, sore throat, rhinorrhea, anorexia, myalgias, diarrhea, and in severe cases, pneumonia. The primary transmission mode is from person to person, through inhalation of the droplets released when coughing or sneezing. In general, symptomatic people are more contagious. However, transmission is also possible through fomites [5].
Moreover, it is valuable to mention that viruses are susceptible to mutations leading to the potential development of new variants. In response to the emergence of new SARS-CoV-2 variants, WHO has classified the variants according to the Greek alphabet (e.g., Alpha, Beta, Gamma, and Delta).
The strains of interest present mutations on the spike protein, which often results in altered virus comportment and may lead to immune escape [1][5][6].
Therefore, comprehensive knowledge of the current prophylactic and treatment schemes for COVID-19 is of the utmost importance to preparing efforts to fight disease dissemination.
2. COVID-19 Prevention and Treatment Approaches
As the pandemic evolved, so did the search for potential prophylactic and therapeutic agents (Figure 1).
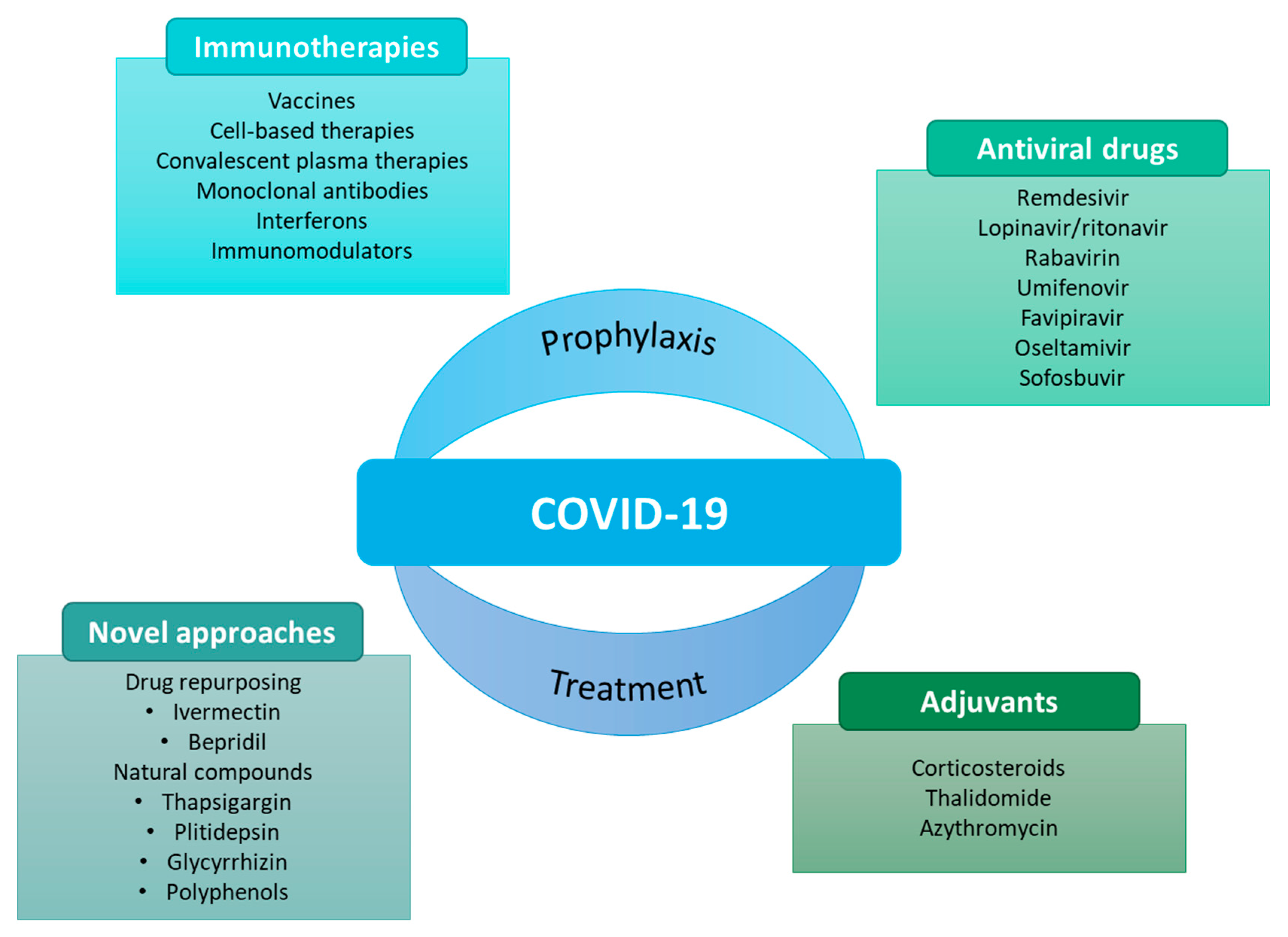
Figure 1. Some examples of prophylactic and therapeutic approaches against COVID-19 [7].
Vaccines have undoubtedly conquered the research space to prevent SARS-CoV-2 because of their advantages in the prophylaxis of COVID-19. Besides that, some therapies have also been considered against COVID-19. Many of these therapies have emerged from drug repurposing. Repurposing of a drug consists of using an existing medicine with a new therapeutic purpose beyond its primary indications [1][7][8][9][10].
Table 1 summarizes the currently available therapies against COVID-19 and their main underlying action mechanisms.
Table 1. Potential therapeutic agents used in the treatment of COVID-19.
| Drug | Drug Class | Mechanism of Action | Reference |
|---|---|---|---|
| Chloroquine and Hydroxychloroquine | Antimalarials | Increasing endosomal pH; interfering with the glycosylation of cellular receptors of SARS-CoV-2; immunomodulator. | [11] |
| Remdesivir | Antivirals | Interfering with the viral replication; inhibiting the viral RNA-dependent RNA polymerase (RdRp). |
[1] |
| Favipiravir | Antivirals | Binds to the viral RdRp and reduces its reproduction. | [11] |
| Lopinavir and Rotinavir | Protease inhibitors | Could act by inhibiting SARS-CoV-2 protease for protein cleavage; interfering with virus replication. | [11] |
| Darunavir | Protease inhibitors | Could act by inhibiting SARS-CoV-2 protease for proteins cleavage; interfering with virus replication. | [11] |
| Niclosamide | Anthelmintics | Inhibiting replication and 3CL protease enzyme inhibition. |
[9] |
| Ivermectin | Anthelmintics | Inhibits IMPα/β1 associated nuclear import of proteins of the virus. | [11] |
| Convalescent Plasma Therapy | Immunoglobulins | Non-neutralizing antibodies bind to the pathogen and contribute to prophylaxis and recovery. | [9] |
| Mesenchymal Stem Cell Therapy | Pluripotent stem cells | Prevent the release of cytokines. | [9] |
| Glycyrrhizin | Prenol lipids | Inhibits replication, adsorption, and penetration of the virus. | [9] |
| Cinanserin | 5-HT 2A and 5-HT 2C receptor antagonist | Inhibition of the protease enzyme. | [9] |
| Dexamethasone | Corticosteroids | Reduces inflammation-induced lung damage and, consequently, inhibits the progression to respiratory failure. | [12] |
| IFN-β | Immunomodulators | Increases the production of anti-inflammatory cytokines and downregulates the production of pro-inflammatory cytokines. | [13] |
| Baricitinib | Janus kinase (JAK) inhibitors | Interfering with viral entry by inhibiting one of the endocytosis regulators and can prevent the activation of STAT. |
[11] |
| Tocilizumab Bamlanivimab Etesevimab Lenzilumab Risankizumab CR3022 |
Monoclonal Antibodies | Neutralizing antibodies can block the entry of the virus into host cells and recruit host effector pathways to destroy virus-infected cells. | [1][11] |
| Camostat Mesylate | Transmembrane protease, serine 2 (TMPRSS2) inhibitor | Interfering with viral entry. | [11] |
2.1. Vaccines
The spread of COVID-19 has mobilized research and development (R&D) efforts. Therefore, several approaches for vaccine development against COVID-19 have been tested, such as inactivated virus, live attenuated, recombinant protein, adenovirus vector, influenza virus vector, as well as mRNA and DNA vaccines. As a revolutionary innovation, mRNA vaccine technology has uniquely controlled the COVID-19 pandemic [14].
Briefly, mRNA vaccines are composed of a vehicle, particularly lipid nanoparticles, that enables the delivery of a nucleic acid molecule encoding the antigen of interest. In the case of SARS-CoV-2, the spike protein is delivered into the target cell in the human host, allowing the host cell to produce the target protein and express the antigen to elicit an immune response [14].
Currently, two mRNA-based vaccines are approved against COVID-19: Comirnaty (BNT162b2) and Spikevax (mRNA-1273) [15]. According to the literature, mRNA technology is desirable as it works as a template for protein translation and does not require bioreactors. It reduces the risk of bacterial contamination and makes scaling up less challenging. Moreover, mRNA vaccines reduce the risk of immunogenicity compared to other viral vector-based modalities. However, the dependency on cold-chain storage and transport may hamper their global applications [15].
2.2. Antiviral Drugs
Given the clinical picture presented by patients with SARS-CoV-2, another potential therapy is antiviral drugs such as remdesivir (REM), favipiravir (FPV), and lopinavir/ritonavir, which will be described in more detail later in the manuscript.
In brief, these drugs can inhibit the entry of the virus by targeting the type-II transmembrane serine protease (TMPRSS2) and the ACE-2 receptor. They may also interfere with endocytosis or with the action of RNA-dependent RNA polymerase (RdRp) and the SARS-CoV-2 3-chymotrypsin-like protease (3CLpro) through fusion inhibitors.
Despite the promising prospects for this therapeutic class, some groups of antiviral drugs remain to be explored to treat COVID-19 [16][17].
2.3. Convalescent Plasma
The convalescent plasma of patients who have recovered from COVID-19 presents neutralizing antibodies in its constitution, which can fight infection by minimizing the inflammatory response [12]. The reduction in the inflammatory response may happen due to viremia suppression contributing to prophylaxis and recovery.
The administration of passive antibodies may be an option to achieve rapid immunity [9].. In theory, the administration of convalescent plasma should be completed at an early stage for superior efficacy [18]. However, its application continues to be controversial [19].
2.4. Monoclonal Antibodies
Monoclonal antibodies (mAbs) have effectively prevented and treated various viral infections [20]. Currently, potent neutralizing mAbs have been investigated against COVID-19, by targeting the receptor-binding domain (RBD) of the spike glycoprotein of SARS-CoV-2, blocking the binding between the S protein and the host receptor, ACE2 [21][22]. Moreover, other neutralizing mAbs can mediate viral activity by targeting nonblocking epitopes of the RBD or N-terminal domain (NTD) of the spike protein [23][24]. Some neutralizing antibodies studied against COVID-19 have been reviewed previously [25].
However, the emergence of new virus strains with mutations in the protein epitopes may hinder the application of these selective immunotherapies.
Therefore, to overcome mutational virus escape, cocktail therapies aiming at administering antibodies targeting multiple epitopes on the spike protein have been investigated. Nevertheless, these treatment approaches may be challenging and considerably increase manufacturing costs [26].
Recently, the emergence of bispecific mAbs (bsAbs) has gained interest for the treatment of COVID-19 as one molecule can target two different antigen-binding sites [26]. However, the use of these approaches remains to be fully explored.
Although applying mAb-based interventions against SARS-CoV-2 may require periodic updates due to the shifting antigenic landscape, the potential passive immunization in persons with a high risk of ineffective responses is a significant leap forward in the fight against viral evolution [27].
2.5. Interferons
Interferons (IFNs) induce the encoding of several proteins that can inhibit viral replication by decreasing cellular metabolism, interfering with the membrane formation necessary for virus replication, and inducing the release of cytokines that promote adaptive immunity. There are three families of IFNs, but only type I and type III are produced when the immune system detects the presence of viral nucleic acids. IFN-α belongs to type I, as well as INF-β, and fights coronaviruses by inhibiting virus replication [12][20].
According to Sodeifian et al. [28], it is paramount to establish the best time window to prescribe this type of treatment, as evidence has revealed that the administration of INF before the viral peak and the inflammatory phase of the illness could offer a highly protective effect. On the contrary, the administration of IFN during the inflammatory and severe phase of the disease may cause immunopathology and long-lasting harm for patients [28].
2.6. Corticosteroids
Corticosteroids are readily available agents extensively used as anti-inflammatory agents against respiratory infections. However, evidence has suggested that no clear benefits have been observed regarding their application in SARS and MERS patients. Therefore, their application in the initial phase of the COVID-19 pandemic was not recommended [29].
Later, due to preliminary data demonstrating lower mortality in patients with COVID-19 treated with corticosteroids, the use of corticosteroids for treating patients with severe or critical COVID-19 has been recommended [30].
Corticosteroids may play a pleiotropic role in different pathophysiological components in severe COVID-19 [31]. One of the studied drugs is dexamethasone (DEX), a synthetic glucocorticoid [12].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24032974
References
- Forchette, L.; Sebastian, W.; Liu, T. A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics. Curr. Med. Sci. 2021, 41, 1037–1051.
- Salian, V.S.; Wright, J.A.; Vedell, P.T.; Nair, S.; Li, C.; Kandimalla, M.; Tang, X.; Carmona Porquera, E.M.; Kalari, K.R.; Kandimalla, K.K. COVID-19 Transmission, Current Treatment, and Future Therapeutic Strategies. Mol. Pharm. 2021, 18, 754–771.
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115.
- Salasc, F.; Lahlali, T.; Laurent, E.; Rosa-Calatrava, M.; Pizzorno, A. Treatments for COVID-19: Lessons from 2020 and new therapeutic options. Curr. Opin. Pharmacol. 2022, 62, 43–59.
- Rehman, S.U.; Rehman, S.U.; Yoo, H.H. COVID-19 challenges and its therapeutics. Biomed. Pharmacother. 2021, 142, 112015.
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424.
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Alhakamy, N.A.; Md, S.; Nair, A.B.; Deb, P.K. Combating the Pandemic COVID-19: Clinical Trials, Therapies and Perspectives. Front. Mol. Biosci. 2020, 7, 336.
- Alam, S.; Kamal, T.B.; Sarker, M.M.R.; Zhou, J.R.; Rahman, S.M.A.; Mohamed, I.N. Therapeutic Effectiveness and Safety of Repurposing Drugs for the Treatment of COVID-19: Position Standing in 2021. Front. Pharmacol. 2021, 12, 659577.
- Bhandari, R.; Khanna, G.; Kuhad, A. Pharmacological insight into potential therapeutic agents for the deadly COVID-19 pandemic. Eur. J. Pharmacol. 2021, 890, 173643.
- Santos, J.C.; Ribeiro, M.L.; Gambero, A. The Impact of Polyphenols-Based Diet on the Inflammatory Profile in COVID-19 Elderly and Obese Patients. Front. Physiol. 2021, 11, 1783.
- Alanagreh, L.; Alzoughool, F.; Atoum, M. The Human Coronavirus Disease COVID-19: Its Origin, Characteristics, and Insights into Potential Drugs and Its Mechanisms. Pathogens 2020, 9, 331.
- Khani, E.; Khiali, S.; Entezari-Maleki, T. Potential COVID-19 Therapeutic Agents and Vaccines: An Evidence-Based Review. J. Clin. Pharmacol. 2021, 61, 429–460.
- Angel, M. Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus Miguel. Antimicrob. Agents Chemother. 2020, 64, e00399-20.
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94.
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854.
- Hidayati, H.B.; Octavia, E.; Srisetyaningrum, C.T. Antiviral therapy for COVID-2019. Anaesth. Pain Intensive Care 2021, 25, 387–392.
- Negrut, N.; Codrean, A.; Hodisan, I.; Bungau, S.; Tit, D.; Marin, R.; Behl, T.; Banica, F.; Diaconu, C.; Nistor-Cseppento, D. Efficiency of antiviral treatment in COVID-19. Exp. Ther. Med. 2021, 21, 648.
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Korompoki, E.; Fotiou, D.; Migkou, M.; Tzanninis, I.G.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Emerging treatment strategies for COVID-19 infection. Clin. Exp. Med. 2021, 21, 167–179.
- Cao, H.; Ming, L.; Chen, L.; Zhu, X.; Shi, Y. The Effectiveness of Convalescent Plasma for the Treatment of Novel Corona Virus Disease 2019: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 1618.
- Tao, K.; Jagannathan, P.; Shafer, W. SARS-CoV-2 Antiviral Therapy. Clin. Microbiol. Rev. 2021, 34, e00109-21.
- Wu, Y.; Wang, F.; Shen, C.; Peng, W.; Li, D.; Zhao, C.; Li, Z.; Li, S.; Bi, Y.; Yang, Y.; et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020, 368, 1274–1278.
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schäfer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020, 584, 443–449.
- Chi, X.; Yan, R.; Zhang, J.; Zhang, G.; Zhang, Y.; Hao, M.; Zhang, Z.; Fan, P.; Dong, Y.; Yang, Y.; et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science 2020, 369, 650–655.
- Liu, L.; Wang, P.; Nair, M.S.; Yu, J.; Rapp, M.; Wang, Q.; Luo, Y.; Chan, J.F.W.; Sahi, V.; Figueroa, A.; et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 2020, 584, 450–456.
- Taylor, P.C.; Adams, A.C.; Hufford, M.M.; de la Torre, I.; Winthrop, K.; Gottlieb, R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021, 21, 382–393.
- Li, Z.; Li, S.; Zhang, G.; Peng, W.; Chang, Z.; Zhang, X.; Fan, Z.; Chai, Y.; Wang, F.; Zhao, X.; et al. An engineered bispecific human monoclonal antibody against SARS-CoV-2. Nat. Immunol. 2022, 23, 423–430.
- Abraham, J. Monoclonal Antibodies with Extended Half-Life to Prevent COVID-19. N. Engl. J. Med. 2022, 386, 2236–2238.
- Sodeifian, F.; Nikfarjam, M.; Kian, N.; Mohamed, K.; Rezaei, N. The role of type I interferon in the treatment of COVID-19. J. Med. Virol. 2022, 94, 63–81.
- Spagnuolo, V.; Guffanti, M.; Galli, L.; Poli, A.; Querini, P.R.; Ripa, M.; Clementi, M.; Scarpellini, P.; Lazzarin, A.; Tresoldi, M.; et al. Viral clearance after early corticosteroid treatment in patients with moderate or severe COVID-19. Sci. Rep. 2020, 10, 21291.
- Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; Cavalcanti, A.B.; et al. Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-analysis. JAMA-J. Am. Med. Assoc. 2020, 324, 1330–1341.
- van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.; Pickkers, P.; Derde, L.; Leavis, H.; van Crevel, R.; Engel, J.J.; Wiersinga, W.J.; Vlaar, A.P.J.; Shankar-Hari, M.; et al. A guide to immunotherapy for COVID-19. Nat. Med. 2022, 28, 39–50.
This entry is offline, you can click here to edit this entry!
