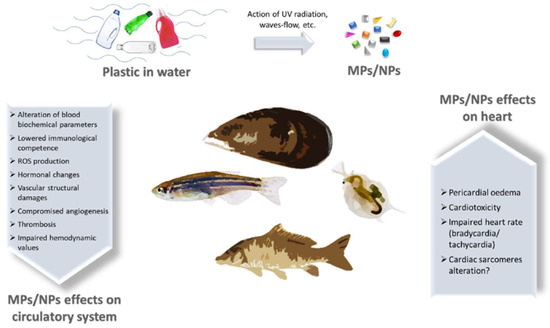
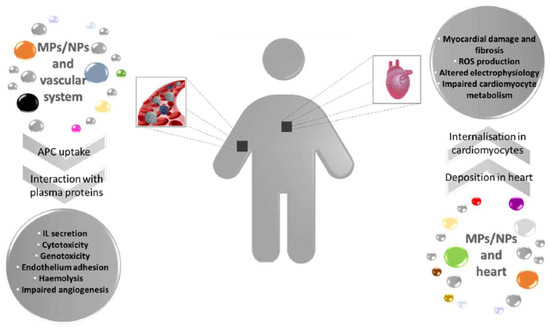
Plastic abandoned in the environment is prone to segregation, leading to the generation of microplastics (MPs) and nanoplastics (NPs), which can reach aquatic and terrestrial organisms. MPs/NPs in water can access fish’s bodies through the gills, triggering an inflammatory response in loco. Furthermore, from the gills, plastic fragments can be transported within the circulatory system altering blood biochemical parameters and hormone levels and leading to compromised immunocompetence and angiogenesis. In addition, it was also possible to observe an unbalanced ROS production, damage in vascular structure, and enhanced thrombosis. MPs/NPs led to cardiotoxicity, pericardial oedema, and impaired heart rate in fish cardiac tissue. MPs/NPs effects on aquatic organisms pose serious health hazards and ecological consequences because they constitute the food chain for humans. Once present in the mammalian body, plastic particles can interact with circulating cells, eliciting an inflammatory response, with genotoxicity and cytotoxicity of immune cells, enhanced haemolysis, and endothelium adhesion.
1. Introduction
Plastic demands raised enormously during the last few years. Plastic products bring several benefits to human beings and the community, playing an even more fundamental part in the food industry, constituting a sustainable and safe option. This widespread plastic use has inevitably produced plastic waste. In 2020, nearly 30 million tonnes of plastic waste were collected in European countries, of which only 34.6% undertook the recycling route (
https://plasticseurope.org/, 17 January 2023). Unfortunately, a still-too-high percentage of post-consumer plastic waste is sent to landfill or misprocessed, contributing to ecosystem pollution. The persistence of current production and waste management practices will predictably lead to 12 billion tonnes of plastic waste in the natural environment by 2050 [
1]. In addition, only in 2010, up to 12.7 million metric tonnes of plastic were released into the oceans [
1], leading to the accumulation of over 250,000 tonnes of plastics abandoned in oceans [
2]. In this scenario, it has been foreshadowed that plastic waste in the environment will constitute a stratigraphic marker for the Anthropocene [
3]. Plastic waste persists in the natural environment not only for its vast problematic presence itself but also for its persistence and slow degradation. These environmental pollutants pose severe threats to different animal species, constituting physical traps, barriers to the food supply or congestion and ultimately causing death [
4,
5].
Plastic pollutants which enter the ecosystem in small pieces, typically less than 5 mm in size, are called microplastics (MPs) and are generally industrially produced. In such cases, where plastic particles of this precise size are intentionally made, pollutants are called primary MPs [
6]. The small particles generated by degradation processes such as thermo-oxidation or mechanical segregation on larger plastic pieces are called secondary MPs [
7]. Additionally, plastic waste can present as particles of less than 1 μm, called nanoplastics (NPs) [
8,
9]. MPs and NPs are chemically inert compounds which are able to penetrate and indefinitely persist in both terrestrial and marine environments and freshwaters [
10,
11,
12], concurring to a recently described ecotoxocity [
13,
14]. They were recently reported as health-hazardous compounds [
15] due to their ability to act as vectors for chemical contaminants such as heavy metals [
16,
17] as well as pathogens [
18,
19].
Due to their small size, they directly enter the food chain by the gastrointestinal route, as demonstrated by the ingestion of contaminated algae by planktonic crustacean
Daphnia magna, then to a secondary consumer fish, and finally reaching an end consumer fish [
20,
21,
22].
Additionally, it has been speculated that MPs and NPs might act as carriers for pollutants to living organisms, thus leading to their bioaccumulation [
23,
24]. Regardless of the model under study, it is clear that MPs and NPs can be ingested and accumulated in larger marine fauna by direct transfer from prey to predator, including big predators like humans. Ingestion is the main route via which plastics enter the human body [
25].
Sources of human-ingested MPs and NPs are mussels [
26], commonly consumed fish [
27], commercial salt [
28], sugar and honey [
29], tea bags [
30] and drinking water [
31,
32]. Unsurprisingly, contaminants can also be found in plastic water containers and bottled water [
33,
34] and, massively, on plates during meals after settling of dust particles [
35]. In vivo murine models showed that MPs, upon intestinal internalisation, reach the bloodstream within 15 min after ingestion, mainly accumulating in the liver [
36,
37]. Exposure of hepatocytes to MP compromises cell membrane integrity in a dose-dependent manner, as demonstrated in the HepG2 cell model [
38]. Using a gastric adenocarcinoma cell line, it was also possible to observe changes in cell shape and the triggering of an inflammatory response which may ultimately lead to cell death [
39]. Furthermore, recently it has been described that NPs can enter the human and livestock food chain through plant consumption [
40]. Toxicity might be directly caused by plastic debris by their accession to plant cells. In fact, it has been demonstrated that plant cells can take up particles of less than 0.1 μm, thus entering the food chain and representing a serious threat to higher organisms. Moreover, due to the compounds and pollutants adsorbed on the surface of MPs/NPs, they could inhibit plant growth and imbalance plant symbiosis with fungi and microbial species [
40].
Another known route of exposure to MPs is inhalation, as they are consistently found in the air. It was recently shown cytotoxicity, ROS production, and inflammation in an in vitro lung cell model [
41]. In a study including healthy and asthmatic mice treated with MPs via inhalation, the authors demonstrated severe lung inflammation and worsening respiratory failure in the latter [
42]. Additionally, dose-dependent pulmonary fibrosis has been recently observed in murine models, activating oxidative stress markers upon polystyrene administration [
43].
Regardless of the different routes via which MPs and NPs may access the human body, they undertake internalisation through active or passive influx, and they reach distant organs using the circulatory system. This review provides a picture of the latest pieces of evidence of the effects on the vascular system and cardiac tissue exerted by MPs and NPs in aquatic fauna and humans (schematically summarised in Figure 1 and Figure 2).
Figure 1. Scheme of MPs and NPs effects on cardiovascular system of aquatic organisms. Plastic wastes in water are prone to segregation due to the concerted action of UV-radiation, waves and water flows, leading to the generation of MPs and NPs. These particles reach aquatic fauna and enter the body, where they exert a plethora of detrimental effects on circulatory system and heart.
Figure 2. MPs and NPs effects on vascular system and heart in mammals. Plastic fragments enter the human body through different routes and are internalised within the cells of vascular system where they can trigger several cellular responses. Additionally, MPs/NPs reach the cardiac tissue causing structural and metabolic damages. IL = Interleukin.
2. MP/NP Effects on Aquatic Fauna Vascular System
Whenever plastic materials reach the aquatic environment, they become prone to disintegration through the mechanical action of waves and/or water flows, water animals, and UV radiation. Water is, therefore, the vector for pollutants, namely plastics, and the way of access to the aquatic vertebrates and invertebrates’ cardiovascular systems.
Fishes have a closed cardiocirculatory system, where blood circulates within vessels of various sizes and does not fill any cavities. It is characterised by the presence of one heart, is very simple in structure, and is generally localised behind the gills. Oxygenated and nutrient-rich blood is pumped by the heart in all parts of a fish body within arteries; from peripheric tissues, deoxygenated and nutrient-poor blood is transported through veins towards the different components of the heart and reaches the gills again. In this context, gas exchanges are facilitated, incorporating oxygen from water and eliminating carbon dioxide from the body.
In 2016, Lu and collaborators found polystyrene (PS) microparticles sized 5 μm and 20 μm (exposure concentration 20 mg/L)deposited in gill filaments of
Danio rerio, which can then possibly be transferred to the capillaries [
44]. Consistent with these findings, Ding et al. not only confirmed the presence of PS-MPs in the gills of red tilapia (
Oreochromis niloticus) at all dispersed concentrations of 1, 10 and 100 mg/L, but this organ showed the highest bio-accumulation [
45]. PS-MPs deposition in gills was 18.4, 49.4 and 71.7 × 10
4 μg/kg for nominal oral exposures of 1, 10 and 100 μg/L. Furthermore, the same study observed the distribution of these particles in the circulatory system and the consequent presence in other peripheral organs, such as the gut (concentrations of 25.5, 89.9 and 171.1 × 10
4 μg/kg), liver (12.8, 28.7, 36.6 × 10
4 μg/kg) and brain (10.6, 32.5 and 40.5 × 10
4 μg/kg) [
45].
Previous evidence in a type of Mytilus reported that ingested 0.51 g/L PS-MPs could pass from the gastrointestinal cavity to the haemolymph and subsequently be distributed to other tissues through the circulatory system [
46]. Molluscs present with an open circulatory system where blood is pumped by the heart into the body cavities, and tissues are surrounded by blood so MPs can be transported directly to all major organs. In mussels, it seems that the accumulation mechanism is similar, regardless of the size of plastic particles, possibly via translocation. In two experiments, it was possible to detect them directly in the haemolymph and inside haemocytes after a 3-days exposure time [
46,
47]. Particle size appears to influence the capacity of PS to translocate from the intestinal tract to the haemolymph, with the majority of smaller particles found in the circulation [
46]. These studies showed that the persistence of MPs in fishes’ circulatory systems has some major implications for predators and end-consumers species, including humans.
In common carp (
Cyprinus carpio), exposure to MPs alters blood biochemical parameters, i.e., reduced plasma levels of acetylcholinesterase (AChE) and gamma glutamyl-transferase (GGT) and increased aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) [
48]. Additionally, in immunological tests, specifically lysozyme and alternative complement (ACH50) activities, total immunoglobulins and complement C3 and C4 factors are lowered [
48]. MPs (and NPs) are known to interact with heavy metals, and 30-day exposure to a mixture of plastic debris, mainly polyethylene (PE) and cadmium, further enhanced the detrimental effects on the biochemical and immunological assets in carp [
48]. Importantly, these effects were observed for both concentrations tested (250 and 500 μg/L) when combined with the heavy metal, with the only exception of LDH, which significantly changed at the highest quantity tested. These results suggest a perturbation in cellular homeostasis due to the changes in biochemical parameters, and the altered immune system components might predispose to infectious diseases. To note, this work used a mixture of plastic fragments, mainly represented by PE, the most common type of characterised plastic in water, followed by polypropylene (PP) and PS [
7].
In addition, PS orally administered accumulated in the gill with ROS production and histopathologic changes in loco in both 20 and 200 mg/L treated groups. Concentrations of MPs found in gills were 39.06, 73 and 175 item/individual for 2, 20 and 200 mg/L treated groups. This bioaccumulation was similar to what was found in the intestine. Structural damages were also observed in other distant organs demonstrating the circulatory system’s crucial role in transporting MP [
49].
Recently, Sun et al. investigated the toxic cardiovascular effect of NPs in zebrafish embryos. The fluorescence images demonstrated that the NPs could inhibit sub-intestinal angiogenesis, which can be directly related to impaired cardiovascular formation and development and ultimately suggest that NPs can damage the vascular endothelium in these organisms [
50]. Thrombotic effects were also evaluated, and a hypercoagulable state was found in the caudal vein, where authors observed erythrocytes aggregation and neutrophils recruitment and an incidence of thrombosis markedly elevated after NPs exposure [
50]. From a hemodynamic perspective, decreased carbon monoxide levels and blood flow rate in treated groups were also dose-dependent, clearly detectable at concentration exposures of 100 and 200 μg/mL [
50]. Vascular resistance increases after thrombosis, leading to elevation in blood pressure directly and the contraction process of the myocardium, thus affecting carbon monoxide levels. Therefore, hemodynamic changes further aggravate the thrombotic process. The same study also found ROS production and a generalised inflammatory response for 100 and 200 μg/mL concentrations. Researchers concluded that NPs trigger a pro-inflammatory and pro-coagulant state through the vascular endothelial cell layer, ultimately promoting thrombosis in vivo [
50]. Analysis of haemocytes of the marine bivalve
Mytilus galloprovincialis revealed blood toxicity of increasing concentrations of PS-NPs (1, 5 and 50 μg/mL) [
51]. In the treated group, induction in cellular apoptosis was detected, with a rapid lysosomal destabilisation evidenced by an enhanced lysosomal enzyme release. The latter effect was more pronounced at the highest concentration of 50 μg/mL, accompanied by decreased phagocytic activity, indicating that the immunocompetence is compromised with PS-NPs [
51]. Interestingly, 5 and 50 mg/mL administration induced an immediate lysozyme release, whereas a comparable increase was observed at all the concentrations tested for longer incubation times [
51]. These results undoubtedly demonstrate that NPs compromise the immune function in marine invertebrates.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11020264