Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The current state of Targeted Alpha Therapy (TAT) in prostate cancer, particularly in mCRPCT (metastatic castration-resistant prostate cancer). The widely used Radium-223 and the novel trend in the TAT field with a special focus on prostate-specific membrane antigen (PSMA)-based alpha therapy.
- prostate cancer
- targeted alpha therapy
- SPECT/CT
- PET/CT
1. Introduction
1.1. Prostate Cancer: Incidence and Survival
Prostate cancer (PCa) is the fourth most common malignancy worldwide in 2020, with 1,414,259 new cases (7.3% of all cancers) after breast cancer (11.7%), lung cancer (11.4%) and colorectum cancer (10%) [1]. It represents the third leading cause of cancer-related death in men. In fact, prostate cancer is not the primary cause of decease, since it is characterized by a slow growth and usually death occurs because of metastases spreading in the pelvic and retroperitoneal lymph nodes, spinal cord, bladder, rectum, bone, and brain. To confirm that researchers can observe the survival as a function of the stage, localized PCa is indolent and has a 5-year survival rate of nearly 100%, while it decreases to 30% in metastatic PCa [2].
1.2. Androgen Deprivation Therapy
Around 80–90% of PCa is androgen-dependent, so androgen deprivation therapy (ADT) is generally the first therapeutic choice whose aim is suppressing serum testosterone to “castrate” levels (defined as <50 ng/mL) and inhibiting androgen receptors (AR) [2][3]. ADT finds application in intermediate and high-risk PCa as neoadjuvant and adjuvant therapy in association with radiotherapy or in a salvage setting, as adjuvant treatment for nodal metastases after prostatectomy in patients with biochemical recurrence and short doubling times and, thirdly, in metastatic PCa [4]. ADT can be obtained with different medical approaches. First of all, GnRH agonists (leuprolide, goserelin, buserelin, triptorelin) and antagonists (degarelix, relugolix) inhibit the pituitary and reduce the androgen production via the HPG axis. Since 10% of androgen is converted in the adrenal gland from androgen precursors such as pregnenolone and DHEA, Abiraterone acetate is used to inhibit the conversion of precursors in adrenal gland as well as in testes and prostate-tumor tissues. In locally advanced and metastatic prostate cancer, antiandrogens such as Bicalutamide and Flutamide are used to interfere with the signaling of AR and can be combined with ADT in a therapeutic asset known as Combined Androgen Blockade (CAB) [5].
1.3. Epithelial to Mesenchymal Transition and Anoikis
The cytoskeleton plays a pivotal role in epithelial to mesenchymal transition (EMT) and so in progression. During the EMT process, the cells acquire a more fibroblastic appearance, becoming more invasive and motile. Given the crucial role of EMT, Anoikis-inducing agents have been proposed as potential therapy since they can stabilize the cytoskeleton, restrict the movements of cancer cells, and inhibit the intracellular signals involved in tumoral survival [6][7].
1.4. Metastatic Prostate Cancer’s Therapy
Unfortunately, in 20–30% of cases, the tumor goes toward progression even with hematic testosterone level below 20 ng/dL [2] and become castration-resistant PCa (CRPCa). Researchers demonstrated that the resistance to ADT occurs after approximately 10–15 months [8]. However, the overall survival in these patients has improved over time. In fact, the reactivation of AR signaling is seen in CRPCa. AR is targeted by androgen synthesis inhibitors (abiraterone) and AR-ligand inhibitors (enzalutamide, apalutamide, and daroglutamide). However, when a tumor becomes highly glycolytic and responsive to 18F-fluorodoxyglucose (18F-FDG), resistance to these agents and progression to aggressive disease can be observed. Another mechanism of resistance concerns the differentiation of AR-indifferent carcinoma such as neuroendocrine, which has a significant 18F-FDG uptake and leads to the loss of AR signaling [9]. Moreover, some studies demonstrated how androgen ablation itself can reactivate AR signaling pathways. Finally, the AR heterogeneity expression that pre-exists in treatment-naive primary tumors could explain the resistance to AR signaling inhibitors (ARSI) [10]. These are the basis that explains patients’ relapse and therapeutic failure. The therapeutic resistance can be overcome using cytotoxic agents targeting the microtubules as a first-line chemotherapy. For metastatic castration-resistant PCa (mCRPC), Docetaxel, a taxane chemotherapy, was the first drug to be approved in 2004, which was followed by another taxane chemotherapy, Cabazitaxel. In 2010, the FDA approved in the United States Sipuleucel-T, a vaccine therapy targeting prostatic acid phosphatase (PAP), for asymptomatic and minimally symptomatic patients with mCRPC. The FDA authorized the anti-PD1 immune checkpoint inhibitor pembrolizumab in 2017 for the treatment of unresectable or metastatic solid cancers that have progressed after receiving conventional therapy and have microsatellite instability (MSI-H) or a defect in mismatch repair (dMMR) [11][12]. In 2020, Olaparib and rucaparib, which target PARP, were approved by the FDA for mCRPC with genomic mutations on homologous recombination (HR) DNA repair genes which are present in up to 20% of mCRPC patients [4]. PTEN loss is present in 40–60% of mCRPC tumors and leads to hyperactivation of the PI3K–Akt–mTOR signaling pathway. Since there has been evidence of a cross-talk between the PI3K–Akt–mTOR pathway and AR signaling, the Phase 3 IPATential150 study with first-line mCRPC patients examined the combination of abiraterone with the Akt inhibitor ipatasertib. Patients taking abiraterone with ipatasertib saw a significant radiological PFS advantage, according to the research [13].
1.5. Nuclear Medicine Therapy and Prostate Cancer: What Is Known
For the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive mCRPC who have already had androgen receptor (AR) pathway inhibition and taxane-based chemotherapy, the FDA has authorized PLUVICTOTM (Lutetium 177Lu vipivotide [14]. Additionally, the FDA has authorized the use of Locametz® (East Hanover, NJ, USA) (gallium 68Ga gozetotide), a diagnostic imaging agent, to detect PSMA-positive lesions using positron emission tomography (PET). In this perspective, the two radiopharmaceuticals are employed according to the so-called theranostic approach, consisting of the sequential administration of two molecules with identical or very similar characteristics: the first one is bound to a radionuclide emitting photons or positrons for diagnostic purposes, while the second is conjugated with a radionuclide emitting particles to exert an anti-tumor effect, as shown in Figure 1 [15]. Targeted alpha therapy (TAT) represents an optimal weapon against cancer cells because it can selectively deliver a high burden of radiation to cancer cells and spares normal surrounding tissue thanks to the short range of alpha particles [16][17]. The great energy deposition results in the possibility of obtaining irreparable damage in DNA which is oxygen independent and hence eliminates the major mechanism of therapeutic resistance [18]. In the landscape of targeted alpha therapy, Radium-223 (223Ra) is a cornerstone. The double-stranded DNA breaks caused by this alpha emitter in cancer cells are incorporated into osteoblastic metastatic lesions. For mCRPC patients with symptomatic bone metastases but no visceral metastases, the FDA authorized it in 2013 [19]. New agents have been proposed such as Thorium 227 (227Th). It has been conjugated to BAY2315497, a human anti-PSMA antibody, which demonstrated a preferential tumor uptake. The combination with AR antagonist increased the sensibility to growth inhibition. This seemed to be due to the improved PSMA expression induced by AR antagonist [20]. Actinium-225 PSMA (225Ac-PSMA), indeed, has been proposed for “superscan” patients because the short tissue penetration range of its alpha particle could result in a more favorable microdosimetry in case of red marrow infiltration [21]. Another alpha emitter example is Astatine-211 (211At) linked with PSMA, which was recently investigated for the treatment of PCa [18].
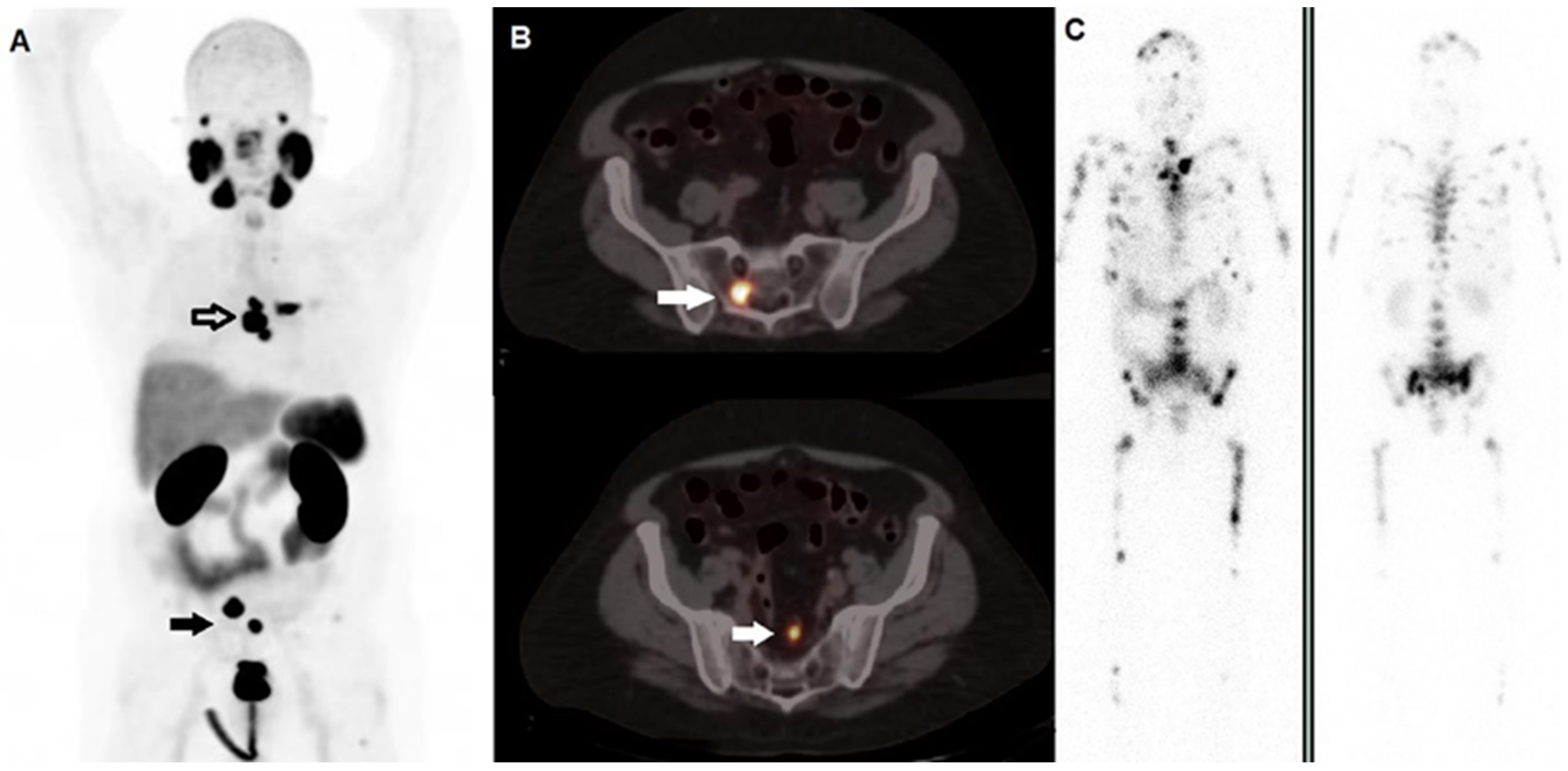
Figure 1. A 59-year-old man affected by prostate cancer, diagnosed in 2016 and submitted to prostatectomy (Gleason score 4 + 5, ISUP 5, pT3b pN1), subsequently treated with adjuvant androgen deprivation therapy. After 2 years, progressively increasing values of PSA were registered, and the patient underwent 2nd generation anti-androgen therapy with abiraterone and, due to progressive skeletal disease, chemotherapy with taxanes. (A) Whole Body 68Ga-PSMA-11 PET/CT demonstrated highly increased tracer incorporation within metastases in the thorax (black bordered arrow) and pelvis (black arrow). (B) Fused PET/CT axial images well depicted PSMA-avid lesions in sacrum (upper row, white arrow) and in a pelvic node (lower row, white arrow). Two months later, he underwent radioligand therapy with 177Lu-PSMA-617; PSMA PET/CT was not repeated due to the shortage of tracer’s availability. (C) Whole planar images (left side; anterior view, right side: posterior view) carried out by scintigraphy on 177Lu’s photopeaks after the 1st cycle of 177Lu-PSMA-617 showed multiple sites of tracer incorporation with a much more extensive skeletal metastatization than that revealed by PET/CT. This discrepancy might be explained by the too long interval of time occurred between the diagnostic phase and the therapeutic procedure in a subject with rapidly progressive disease.
1.6. Radiomedicine
The expansion of nanomedicine has seen the rise of radionanomedicine, which consists of using radionuclides conjugated to nanomaterials for both diagnosis and therapy purposes [22]. An example is nanoparticles of 198 Gold (198Au). The radioactive properties of 198Au such the beta ray of 0.96 MeV and the component gamma ray of 411 KeV make it an ideal candidate for use in diagnosis and therapy. 198Au functionalized with Gum arabic glycoprotein (GA) showed a therapeutic effect in a mouse prostate cancer model after intratumoral injection [23], while 198Au NP-EGCg in a prostate cancer mouse model demonstrated a 72% nanoparticles retention in the tumor after 24 h and 80% reduction in the tumor volume after 28 days [24]. There is an open-word in the therapy and diagnosis of prostate cancer. In this scenario, nuclear medicine and in particular alpha target therapy represent a promising field of research in terms of the therapeutic efficacy, specificity and sensibility of the procedures.
2. Molecular and Metabolic Imaging with PET and SPECT Technology
Since the introduction of personalized medicine, the primary focus of imaging has shifted from detection and diagnosis to tissue characterization, prognosis determination, treatment efficacy prediction, and treatment response measurement. The study of biological processes at the molecular and cellular level is known as molecular imaging. The fast advancement of molecular imaging in recent years has enabled the development of specificity and quantification that are helpful for the early diagnosis and follow-up of the disease. Currently, single photon emission tomography (SPECT) and positron emission tomography (PET) are used for this imaging approach. PET and SPECT radiotracers, which are excellent tools for many medical imaging applications, such as early diagnosis and therapy monitoring in cancer, are becoming more prevalent. The advantage of PET and SPECT is that they provide non-invasive molecular imaging of the entire body, assessing various illness locations [25]. Additionally, serial scanning can be performed, enabling the measurement of functional changes over time during therapeutic interventions.
2.1. Differences between Single Photon Emission Tomography (SPECT) and Positron Emission Tomography (PET)
The main difference between SPECT and PET is the type of radioactive tracer used: while SPECT is based on gamma rays, the decay of the tracers used in PET generates infinitesimal particles known as positrons which decay into two photons emitted in opposite directions. In SPECT, only the direct radiation perpendicular to the detector is recorded; in PET, two detectors hit simultaneously by photons with an oblique direction to the cylinder axis can equally record the radiation. This complex of conditions makes PET faster than SPECT and with higher resolution. The speed of execution is an essential requirement because the radioisotopes used in PET generally have a shorter half-life than those used in SPECT. More and more technological innovations follow one another that allow for progress in the medical field.
2.2. Technologic Progress
The use of WB-SPECT/CT has recently been introduced [26], in which the precision of the CT is added to the function of the SPECT [27][28]. In recent years, digital PET has been introduced through the implementation of silicon photomultipliers (SiMP)-based detectors [29]. This innovative PET detection technology ensures significant improvements over analog technology in terms of better sensitivity and increased spatial and contrast resolution [30][31]. All this technological progress allows for ever more personalized medicine.
3. Targeted Alpha Therapy
Surgery, chemotherapy, and external beam irradiation are frequently utilized as forms of selective therapy in medicine. However, because emitting radionuclides have more precise cell-killing capabilities, there has been some interest in using them in therapy. Targeted radionuclide therapy offers the potential to deliver extremely lethal radiation to cancer cells [31]. This form of treatment can boost the harmful radiobiological effects on cancer cells while decreasing the negative effects of radiation on healthy tissues.
3.1. Physical Characteristics of Alpha Particles and Advantages
The use of alpha particle emitters can help to explain why targeted alpha treatment (TaT) is successful. Such particles have a much higher LET than β particle emitters (50–230 keV/μm vs. 0.1–1.0 keV/μm) with an average energy deposition of 100 keV/μm. The range of α particles in tissues is short, which limits radiation deposition to the target cell and closely neighboring cells [32]. Cellular DNA is the main molecular target of high-LET particles because of the effective production of double-stranded DNA breaks. As a result, TaT can target tumor cells and the tumor microenvironment with high targeted radiation doses while causing the least amount of harm to normal cells. Alpha-emitting particles are preferred over beta particle emitters and external beam radiation for the treatment of malignancies due to all of these advantages. Due to the fact that stable radionuclide sequestration in vivo is an essential part of targeted radiotherapy, the increased interest in TaT has resulted in the development of new chelating agents. Many radionuclides are known which emit one or more α particles during the decay into stable nuclides. Only a small number of them, nevertheless, have been utilized in research projects that examined the effectiveness of targeted radionuclide treatment [33].
3.2. Synthetic Lethality
Not least, the TAT could have a fundamental role for the phenomenon of synthetic lethality [34]. It is defined as “a lethal phenomenon in which the occurrence of a single genetic event is tolerable for cell survival, while the occurrence of multiple genetic events causes cell death”. The variability and complexity of tumor biology, a lack of knowledge of the connections that cause synthetic lethality, drug resistance, and difficulties with clinical screening and translation are the key barriers to synthetic lethality. Recent studies have shown that cancer cells with altered DNA repair mechanisms are ideal candidates for therapies based on synthetic lethality approaches. According to this viewpoint, pharmacological combinations are created with the intention of ultimately curing the condition, but more typically, synergy leads to considerable improvements in treatment outcomes. In a study, Wera and collaborators demonstrated that radiation-induced synthetic lethality could broaden the therapeutic window, thus extending the use of poly(ADP-ribose) polymerase (PARP) inhibitors to patients without BRCAness [35]. In terms of biological tactics, the aim behind synthetic lethality is to take advantage of cancer cells’ reliance on DNA repair in order to maximize their reaction to radiation. They worked within the framework of radiation-induced synthetic lethality; i.e., they used PARPi and RAD51i at concentrations that led to limited cytotoxicity (alone or in combination) but to increased cell death when cells were irradiated with protons or X-rays. The use of TAT could therefore also play a crucial role in implementing a synthetic lethality strategy.
This entry is adapted from the peer-reviewed paper 10.3390/app13031890
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Aurilio, G.; Cimadamore, A.; Mazzucchelli, R.; Lopez-Beltran, A.; Verri, E.; Scarpelli, M.; Massari, F.; Cheng, L.; Santoni, M.; Montironi, R. Androgen Receptor Signaling Pathway in Prostate Cancer: From Genetics to Clinical Applications. Cells 2020, 9, 2653.
- Ricci, M.; Frantellizzi, V.; Bulzonetti, N.; De Vincentis, G. Reversibility of castration resistance status after Radium-223 dichloride treatment: Clinical evidence and Review of the literature. Int. J. Radiat. Biol. 2018, 95, 554–561.
- Yamada, Y.; Beltran, H. The treatment landscape of metastatic prostate cancer. Cancer Lett. 2021, 519, 20–29.
- Crowley, F.; Sterpi, M.; Buckley, C.; Margetich, L.; Handa, S.; Dovey, Z. A Review of the Pathophysiological Mechanisms Underlying Castration-resistant Prostate Cancer. Res. Rep. Urol. 2021, 13, 457–472.
- Martin, S.K.; Kamelgarn, M.; Kyprianou, N. Cytoskeleton targeting value in prostate cancer treatment. Am. J. Clin. Exp. Urol. 2014, 2, 15–26.
- Deschesnes, R.G.; Patenaude, A.; Rousseau, J.L.; Fortin, J.S.; Ricard, C.; Côté, M.F.; Huot, J.; C.-Gaudreault, R.; Petitclerc, E. Microtubule-destabilizing agents induce focal adhesion structure disorganization and anoikis in cancer cells. J. Pharm. Exp. 2007, 320, 853–864.
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746.
- Uo, T.; Sprenger, C.C.; Plymate, S.R. Androgen Receptor Signaling and Metabolic and Cellular Plasticity During Progression to Castration Resistant Prostate Cancer. Front. Oncol. 2020, 10, 580617.
- Jamroze, A.; Chatta, G.; Tang, D.G. Androgen receptor (AR) heterogeneity in prostate cancer and therapy resistance. Cancer Lett. 2021, 518, 1–9.
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422.
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758.
- De Bono, J.S.; De Giorgi, U.; Rodrigues, D.N.; Massard, C.; Bracarda, S.; Font, A.; Arranz Arija, J.A.; Shih, K.C.; Radavoi, G.D.; Xu, N.; et al. Randomized Phase II Study Evaluating Akt Blockade with Ipatasertib, in Combination with Abiraterone, in Patients with Metastatic Prostate Cancer with and without PTEN Loss. Clin. Cancer Res. 2019, 25, 928–936.
- FDA Approves Pluvicto/Locametz for Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2022, 63, 13N.
- Filippi, L.; Chiaravalloti, A.; Schillaci, O.; Cianni, R.; Bagni, O. Theranostic approaches in nuclear medicine: Current status and future prospects. Expert Rev. Med. Devices 2020, 17, 331–343.
- De Vincentis, G.; Gerritsen, W.; Gschwend, J.E.; Hacker, M.; Lewington, V.; O’Sullivan, J.M.; Oya, M.; Pacilio, M.; Parker, C.; Shore, N.; et al. Advances in targeted alpha therapy for prostate cancer. Ann. Oncol. 2019, 30, 1728–1739.
- Frantellizzi, V.; Cosma, L.; Brunotti, G.; Pani, A.; Spanu, A.; Nuvoli, S.; De Cristofaro, F.; Civitelli, L.; De Vincentis, G. Target Alpha Therapy with Thorium-227. Cancer Biother. Radiopharm. 2020, 35, 437–445.
- Tafreshi, N.K.; Doligalski, M.L.; Tichacek, C.J.; Pandya, D.N.; Budzevich, M.M.; El-Haddad, G.; Khushalani, N.I.; Moros, E.G.; McLaughlin, M.L.; Wadas, T.J.; et al. Development of Targeted Alpha Particle Therapy for Solid Tumors. Molecules 2019, 24, 4314.
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223.
- Hammer, S.; Hagemann, U.B.; Zitzmann-Kolbe, S.; Larsen, A.; Ellingsen, C.; Geraudie, S.; Grant, D.; Indrevoll, B.; Smeets, R.; von Ahsen, O.; et al. Preclinical Efficacy of a PSMA-Targeted Thorium-227 Conjugate (PSMA-TTC), a Targeted Alpha Therapy for Prostate Cancer. Clin. Cancer Res. 2020, 26, 1985–1996.
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544.
- Frantellizzi, V.; Verrina, V.; Raso, C.; Pontico, M.; Petronella, F.; Bertana, V.; Ballesio, A.; Marasso, S.L.; Miglietta, S.; Rosa, P.; et al. 99mTc-labeled keratin gold-nanoparticles in a nephron-like microfluidic chip for photo-thermal therapy applications. Mater. Today Adv. 2022, 16, 100286.
- Lee, D.S.; Im, H.-J.; Lee, Y.-S. Radionanomedicine: Widened perspectives of molecular theragnosis. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 795–810.
- Shukla, R.; Chanda, N.; Zambre, A.; Upendran, A.; Katti, K.; Kulkarni, R.R.; Nune, S.K.; Casteel, S.W.; Smith, C.J.; Vimal, J.; et al. Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCg) show efficacy in treating prostate cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12426–12431.
- Hod, N.; Levin, D.; Tiktinsky, K.; Ezroh Katzap, D.; Lantsberg, S. Nuclear Medicine and Molecular Imaging: From Basic Science to the Front in Innovative Imaging and Treatment. Harefuah 2021, 160, 448–454.
- Alqahtani, M.M.; Willowson, K.P.; Constable, C.; Fulton, R.; Kench, P.L. Optimization of (99m) Tc whole-body SPECT/CT image quality: A phantom study. J. Appl. Clin. Med. Phys. 2022, 23, e13528.
- Filippi, L.; Biancone, L.; Petruzziello, C.; Schillaci, O. Tc-99m HMPAO-labeled leukocyte scintigraphy with hybrid SPECT/CT detects perianal fistulas in Crohn disease. Clin. Nucl. Med. 2006, 31, 541–542.
- Frantellizzi, V.; Pontico, M.; Letizia, C.; De Vincentis, G. Bladder wall paraganglioma located using (123)I-mIBG SPECT and CT imaging. Rev. Esp. De Med. Nucl. E Imagen Mol. 2018, 37, 253–254.
- Del Guerra, A.; Belcari, N.; Giuseppina Bisogni, M.; Corsi, F.; Foresta, M.; Guerra, P.; Marcatili, S.; Santos, A.; Sportelli, G. Silicon Photomultipliers (SiPM) as novel photodetectors for PET. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2011, 648, S232–S235.
- Alberts, I.; Hünermund, J.-N.; Sachpekidis, C.; Mingels, C.; Fech, V.; Bohn, K.P.; Rominger, A.; Afshar-Oromieh, A. The influence of digital PET/CT on diagnostic certainty and interrater reliability in Ga-PSMA-11 PET/CT for recurrent prostate cancer. Eur. Radiol. 2021, 31, 8030–8039.
- Filippi, L.; Bagni, O.; Schillaci, O. Digital PET/CT with 18F-FACBC in early castration-resistant prostate cancer: Our preliminary results. Expert Rev. Med. Devices 2022, 19, 591–598.
- Baidoo, K.E.; Yong, K.; Brechbiel, M.W. Molecular pathways: Targeted alpha-particle radiation therapy. Clin. Cancer Res. 2013, 19, 530–537.
- Dahle, J.; Borrebaek, J.; Jonasdottir, T.J.; Hjelmerud, A.K.; Melhus, K.B.; Bruland, O.S.; Press, O.W.; Larsen, R.H. Targeted cancer therapy with a novel low-dose rate alpha-emitting radioimmunoconjugate. Blood 2007, 110, 2049–2056.
- Topatana, W.; Juengpanich, S.; Li, S.; Cao, J.; Hu, J.; Lee, J.; Suliyanto, K.; Ma, D.; Zhang, B.; Chen, M.; et al. Advances in synthetic lethality for cancer therapy: Cellular mechanism and clinical translation. J. Hematol. Oncol. 2020, 13, 118.
- Wéra, A.C.; Lobbens, A.; Stoyanov, M.; Lucas, S.; Michiels, C. Radiation-induced synthetic lethality: Combination of poly(ADP-ribose) polymerase and RAD51 inhibitors to sensitize cells to proton irradiation. Cell Cycle 2019, 18, 1770–1783.
This entry is offline, you can click here to edit this entry!
