Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Juvenile psoriatic arthritis (JPsA) accounts for 1–7% of all cases of juvenile idiopathic arthritis (JIA) and its definition has been a matter of controversy among pediatric rheumatologists for many years. The traditional attribution of JPsA to the spondyloarthropathy group was challenged in the early 1990s, whereas demonstrations of its heterogenous nature have led to questions about its identification as a distinct category in JIA classification.
- juvenile idiopathic arthritis
- juvenile psoriatic arthritis
- psoriasis
- childhood arthritis
1. Introduction
Juvenile idiopathic arthritis (JIA) is an umbrella term that encompasses all forms of arthritis of unknown origin that last for >6 weeks and have their onset before 16 years of age. It is the most common chronic inflammatory rheumatic condition in childhood and is a leading cause of acquired disability in the pediatric period [1][2][3]. Juvenile psoriatic arthritis (JPsA) is one of the seven JIA categories outlined by the International League of Associations for Rheumatology (ILAR) classification for JIA [4], and accounts for 1–7% of all cases of JIA (Table 1) [5].
Table 1. Worldwide frequency of the ILAR categories of JIA.
| ILAR JIA Categories | Frequency 1 |
|---|---|
| Systemic arthritis | 4.2–33% |
| Oligoarthritis | 10.8–56.7% |
| Rheumatoid-factor-positive polyarthritis | 1.3–11.2% |
| Rheumatoid-factor-negative polyarthritis | 12.7–31.5% |
| Enthesitis-related arthritis | 5.4–29.8% |
| Psoriatic arthritis | 1.3–7.1% |
| Undifferentiated arthritis | 3.7–10.4% |
1 The reported frequencies refer to the percentage of all juvenile idiopathic arthritis patients. Data are extracted from Ref. [5]. Abbreviations: ILAR, International League of Associations for Rheumatology; JIA, juvenile idiopathic arthritis.
The concept of JPsA has been a matter of debate for many years among pediatric rheumatologists [6][7][8][9][10]. The initial area of controversy regarded the attribution of JPsA to the spectrum of spondyloarthropathies and its relationship to the forms of adult psoriatic arthritis that belong to this disease family. More recently, the characterization of JPsA as a single homogeneous disease entity has been challenged as many studies have shown considerable heterogeneity within its clinical phenotype. These controversies are reflected in the evolution of the classification of JPsA over the years. In a recent Delphi survey conducted by the Pediatric Rheumatology International Trials Organization (PRINTO) in the context of an effort aimed to revise the current classification criteria for JIA, no consensus was reached on the definition of JPsA [11].
2. History of JPsA Classification
In 1962, Ansell and Bywaters published the first observation of psoriasis in seven children with chronic arthritis [12]. Some years later, Angevine and colleagues described the case of a young boy with psoriasis who developed a persistent non-migratory polyarthritis, and highlighted the similarity between this patient’s disease and adult psoriatic arthritis [13]. JPsA was initially grouped within the seronegative spondyloarthropathies, like its adult counterpart [14]. As a result, it was not included in the American College of Rheumatology (ACR) classification for juvenile rheumatoid arthritis, which excluded the spondyloarthropathies [15], whereas it was placed within the spondyloarthropathies category in the European League for Rheumatology juvenile chronic arthritis classification [16].
In 1976, the first case series of JPsA was reported by Lambert and colleagues, who retrospectively evaluated 43 children [17]. An important observation from that study was that arthritis preceded psoriasis in approximately half the patients. The researchers proposed the first definition of psoriatic arthritis in childhood, by stating that JPsA could be diagnosed in any child with arthritis who develop psoriasis at any time up to 15 years after the onset of joint disease. This proposal was supported by a subsequent case series of 60 patients with JPsA by Shore and Ansell [18], who observed that a family history of psoriasis, the presence of nail pits, and a cumulative asymmetrical arthropathy could be predictive of JPsA before psoriasis onset. However, it was noticed that the application of the definition by Lambert et al. only seldom allowed the diagnosis of JPsA early in the disease course [18][19].
To address the concern that JPsA could be underdiagnosed in case of delayed occurrence of the typical psoriatic rash, Southwood and co-workers devised a new definition of JPsA, based on the so called “Vancouver criteria” (Table 2), which was aimed to enable the diagnosis of JPsA in the absence of overt psoriasis [19]. These criteria allowed the definition of JPsA in the presence of arthritis and a psoriasis-like rash or other clinical features, including dactylitis, nail pits, or a family history of psoriasis. The new categorization led to the identification of a population of patients of a younger age compared to those reported in previous series [20].
| Vancouver Criteria for JPsA 1 | ILAR Classification Criteria for JPsA 1 | |
|---|---|---|
| Inclusion criteria | Definite JPsA: Arthritis plus typical psoriasis or Arthritis plus at least three of the following minor criteria:
Arthritis plus two of the minor criteria listed above |
Arthritis plus psoriasis or Arthritis plus at least two of:
|
| Exclusion criteria | None |
|
1 For both criteria, arthritis must begin before the 16th birthday, persist for at least 6 weeks, and be of unknown origin. Abbreviations: JPsA, juvenile psoriatic arthritis; ILAR, International League of Associations for Rheumatology; HLA-B27, human leukocyte antigen-B27; IgM, immunoglobulin M; JIA, juvenile idiopathic arthritis.
Southwood et al. also noticed that JPsA was more similar to classic JIA than to spondylarthritis and, thus, questioned the inclusion of JPsA within the spectrum of these conditions [19]. This view was reinforced by Ross Petty who, in a review published in 1994 [6], criticized the inclusion of JPsA within the spondyloarthritis group based on the following considerations: (i) the higher frequency of female sex and antinuclear antibody (ANA) positivity in JPsA compared to spondyloarthropathies; (ii) the lower prevalence of positive HLA-B27, axial skeleton arthritis, and enthesitis, which are hallmarks of spondyloarthritis, in JPsA; (iii) the different characteristics of uveitis, which is an important complication of both conditions, but is typically acute and self-limited in spondyloarthritis and chronic in JPsA. However, the pattern of joint disease, especially the presence of dactylitis and the asymmetry of arthritis affecting both large and small joints, led Petty to also set JPsA apart from oligoarticular JIA [6].
Subsequently, the ILAR Pediatric Task Force outlined the JPsA category in the JIA classification, which was promulgated in 1994 [21], and revised in 1997 [22] and then in 2001 [4] (Table 2). The diagnosis of JPsA by ILAR criteria requires the simultaneous presence of arthritis and a typical psoriatic rash (Figure 1) or, if the rash is absent, the presence of arthritis and any two of the following: family history of psoriasis in a first-degree relative; dactylitis (swelling of one or more fingers that extends beyond the joint margins); and nail pitting (pits in at least two nails) (Figure 2). With the aim of delineating homogeneous and mutually exclusive categories of JIA, the ILAR criteria did not allow the diagnosis of JPsA in patients with positive rheumatoid factor (RF) test, family history of an HLA-B27-associated disorder in a first-degree relative, onset of arthritis in a HLA-B27 positive male aged more than 6 years, or systemic arthritis [4].
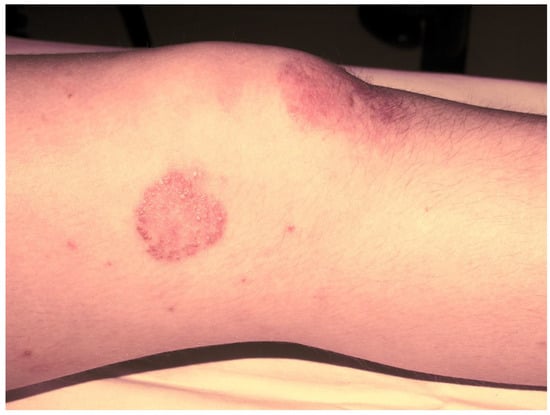
Figure 1. Psoriatic skin lesion in a child with JPsA.
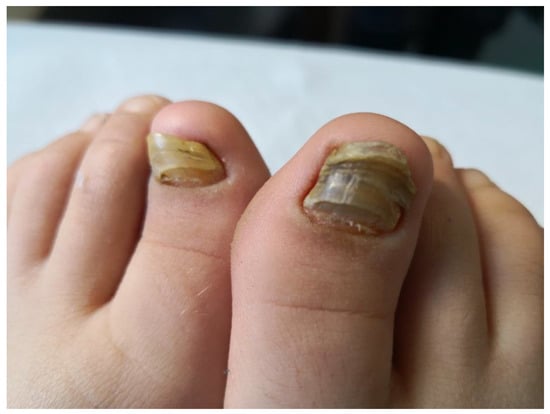
Figure 2. Nail changes in a child with JPsA.
Recently, the research network PRINTO challenged the validity of the ILAR classification system, especially its assumption of defining homogeneous disease categories, and proposed new preliminary criteria for JIA aimed to identify more homogeneous entities [11]. These criteria were developed through international expert consensus and will be formally validated by means of a prospective collection of clinical and laboratory data, which is currently ongoing. Notably, no consensus was reached on a provisional definition of JPsA, and the criteria for this condition were intended to be established after the analysis of prospective data.
3. The Clinical Spectrum of JPsA
The clinical features of JPsA appear to be quite heterogeneous [23]. The age at onset is bimodally distributed, with a first peak occurring during the preschool years and a second during mid-adolescence [19][24][25]. In about 80% of children with JPsA, joint disease begins as an oligoarthritis, and the onset is not uncommonly marked by a monoarthritis [23]. Even though the most affected joint is the knee, followed by the ankle, most patients develop arthritis of both small and large joints, with an asymmetrical distribution [19][25][26][27]. Without an effective treatment, progression from oligoarticular to polyarticular involvement is observed in 60–80% of patients [18][19][20]. Axial disease is seen in up to 25% of patients [18][25], mainly with asymmetric sacroiliitis. Dactylitis is recorded in 20–40% of children with JPsA; it usually affects one or a few digits, and can be symptomatic or asymptomatic [23]. Dactylitis consists of the variable combination of inflammatory synovial and extra-synovial digital lesions, such as flexor tenosynovitis, enthesitis, and peritendinous tissue inflammation [28][29]. In contrast with the adult population with psoriatic arthritis, bone erosions are not commonly observed in the hand joints of children with JPsA [28].
Overt psoriasis is seen in 40–60% of patients with JPsA [23], and occurs after the onset of arthritis in about half of the cases [27]. Nail changes, including pits, onycholysis, horizontal ridging and discoloration, are detected in 50 to 80% of patients (Figure 2) [23]. Chronic uveitis occurs in 10–15% of children with JPsA, and is indistinguishable from that seen in oligoarticular and polyarticular arthritis; acute anterior uveitis is rare [19][20][26][30]. Laboratory findings are generally unremarkable, with the ANA test being positive in about 50% of cases [19][20][24].
Based on the review of the existing literature, Martini noticed in an editorial published in 2003 [7] that early-onset JPsA is more common in young girls presenting with an asymmetric oligoarthritis that can extend over time; in these patients, chronic anterior uveitis occurs with a frequency similar to that seen in early-onset oligoarticular JIA (roughly 20%) and is associated with ANA positivity [31]. Conversely, the older group of children with JPsA has a male predominance and present some features typical of enthesitis-related arthritis (ERA) (the pediatric counterpart of adult undifferentiated spondyloarthritis), such as axial involvement and enthesitis. These observations were later corroborated by Stoll and colleagues in a retrospective study that described two distinct populations of patients within a cohort of 139 patients meeting the Vancouver criteria for JPsA [24]. Children with early disease onset were more likely to be female, to have dactylitis and polyarticular onset, and to be ANA positive, but less likely to have frank psoriasis, enthesitis, or axial disease, which were mainly detected in patients with late-onset JPsA. Similar findings were observed when the data were reanalyzed using the stricter ILAR criteria [32]. The recent analysis of 361 children of the JPsA cohort recruited within the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry also supports the dissection of patients with JPsA into two clinical subgroups, each characterized by a different age at onset and the respective above-mentioned clinical features [25].
A number of studies have shown a wide, though still unexplained, variability of the frequency of the JIA categories, including JPsA, across geographic areas and ethnic groups [33]. The epidemiology of the JIA categories was recently investigated in a more systematic way in the EPOCA (EPidemiology, treatment, and Outcome of Childhood Arthritis) study [5], a multinational, cross-sectional, observational cohort investigations that collected the demographic, clinical, and therapeutic data of 9081 patients with JIA living in 49 countries. The frequency of JPsA among the total cases of JIA ranged from 1.3% in Southeast Asia to 7.1% in North America. The frequency of uveitis in JPsA was also variable, with figures similar to those of oligoarthritis, RF-negative polyarthritis, and undifferentiated arthritis in southern and western Europe and lower than those of these categories in other geographical areas. Altogether, these findings suggest that some clinical features of children currently classified as JPsA may differ throughout the world (Table 3).
Table 3. Demographic and clinical features of patients with JPsA enrolled in the EPOCA study [5].
| Northern Europe |
Western Europe |
Southern Europe |
Eastern Europe |
North America |
Latin America |
Africa and Middle East | Southeast Asia |
|
|---|---|---|---|---|---|---|---|---|
| Epidemiologic data | ||||||||
| Girls | 25 (71.4) | 27 (67.5) | 63 (71.6) | 36 (66.7) | 24 (64.9) | 8 (61.5) | 17 (45.9) | 2 (40) |
| Boys | 10 (28.6) | 13 (32.5) | 25 (28.4) | 18 (33.3) | 13 (35.1) | 5 (38.5) | 20 (54.1) | 3 (60) |
| Median (IQR) age of onset (years) | 10.5 (6.2–12.9) | 9.6 (3.9–11.4) | 4.4 (2–10.4) | 10.7 (6.4–13.6) | 7.8 (5.6–11.9) | 9.2 (5.9–11.7) | 8.4 (4.5–11.8) | 5.5 (2.3–12.) |
| Frequency of extra-articular features | ||||||||
| Psoriasis | 27 (77.1) | 24 (60.0) | 52 (59.1) | 37 (68.5) | 29 (78.4) | 8 (61.5) | 24 (64.9) | 5 (100.0) |
| Dactylitis | 7 (20.0) | 9 (22.5) | 34 (38.6) | 19 (35.2) | 4 (10.8) | 4 (30.8) | 14 (37.8) | 3 (60.0) |
| Nail pitting or onycholysis | 3 (8.6) | 8 (20.0) | 18 (20.5) | 13 (24.1) | 7 (18.9) | 5 (38.5) | 12 (32.4) | 0 (0.0) |
| Psoriasis in a first-degree relative | 13 (37.1) | 17 (42.5) | 39 (44.3) | 20 (37.0) | 8 (21.6) | 5 (38.5) | 14 (37.8) | 0 (0.0) |
| Clinical and laboratory features at visit | ||||||||
| Median (IQR) number of active joints | 0 (0–1) | 0 (0–1) | 0 (0–1) | 2 (0–5) | 0 (0–1) | 1 (0–4.5) | 0 (0–2.5) | 0 (0–4.5) |
| Enthesitis | 6 (17.1) | 5 (12.8) | 3 (3.4) | 5 (9.6) | 2 (5.7) | 1 (7.7) | 8 (21.6) | 0 (0.0) |
| Sacroiliitis | 4 (11.4) | 0 (0.0) | 1 (1.1) | 8 (14.8) | 0 (0.0) | 0 (0.0) | 4 (10.8) | 0 (0.0) |
| Median (IQR) ESR (mm/h) 2 | 7 (5–13) | 10 (4.5–14) | 10.5 (6–18) | 9 (5–19) | 7 (4–14) | 17 (8.75–20) | 12.5 (7.5–20.5) | 20 (8–34) |
Data are extracted from the EPOCA study [5] and include unpublished data. Data are n (%) unless otherwise indicated. 1 Erythrocyte sedimentation rate was available for 219 patients. 3 The frequency of uveitis was calculated in patients with at least 2 years of disease duration (N = 231) [5]. IQR, interquartile range; ESR, erythrocyte sedimentation rate.
4. Genetics of JPsA
Data from genetic analyses conducted specifically in JPsA patients are available. Some single-nucleotide polymorphisms (SNPs) have been found to be associated with JPsA. Using the same approach of testing genetic associations previously applied in adult psoriatic arthritis in 1244 JIA cases, of whom 93 had JPsA, and 5200 controls, Hinks et al. identified a negative association between JPsA and a SNP linked to the minor protective allele of interleukin 23R, the IL-23 receptor gene, which was not observed in the overall JIA cohort or in different JIA subtypes [34]. Furthermore, two SNPs, rs224204 in the MEFV gene and rs3806265 in the NLRP3 gene, were found to be associated with JPsA by Day et al., who tested genes that cause monogenic disorders with some phenotypic overlap with JIA [35]. However, the majority of genetic data on JPsA refers to the study of HLA associations and their results are not conclusive. The first of such investigations, published in 1993 by Ansell et al., describes a statistically significant association with HLA-B27 in 70 patients with JpsA compared to 310 controls [31]. Several years later, Flatø and coworkers showed that the HLA-DRB1*11/12 status differentiates JPsA patients from those with either oligoarthritis or polyarthritis [36]. In a more recent study by Hinks and colleagues describing the HLA associations across each JIA category in 5043 JIA cases, 112 of whom had JPsA, diverse associations with HLA alleles and JPsA were found: HLA-DQA1*0401 (p = 0.0001), HLA-DRB1*08 (p = 0.0003), HLA-DQB1*0402 (p = 0.0008), HLA-C*0602 (p = 0.008), and HLA-B*27 (p = 0.003). However, none of those associations reached the genome-wide level of significance (p < 5 × 10−8) [37]. According to researchers’ interpretation, the observed mixed HLA associations in JPsA could be explained as the result of some misclassification (that is, the JPsA sample might have contained subjects with oligoarthritis, RF-negative polyarthritis, or ERA categories). It could, however, be argued that these findings reflect the heterogeneity of the JPsA category, as defined by ILAR criteria.
5. Treatment of JPsA
Only a few data are available on the specific management of JPsA and the overall treatment approaches for this illness do not differ from those used in other JIA subtypes. Notably, the recently updated ACR guidelines for the treatment of non-systemic JIA were based on broader clinical phenotypes, rather than on ILAR categories, and were guided by the number of involved joints, the presence of sacroiliitis or enthesitis, and the involvement of the temporomandibular joint [38][39].
The following therapeutic choices recommended by these guidelines can be applied to JPsA clinical phenotypes. Intra-articular glucocorticoids and nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended as first-line therapy in oligoarthritis, and as adjunct therapy in polyarthritis. Conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), with methotrexate as the preferred agent, should be started in the case of inadequate response to the first-line treatment in oligoarthritis and as an initial therapy in polyarthritis. Treatment with a biological DMARD (bDMARD) is recommended in case of incomplete response to (or intolerance of) csDMARDs, in patients with active sacroiliitis and enthesitis despite NSAIDs or, as part of initial therapy, in the presence of high disease activity and risk factors in polyarthritis [38][39].
Data on the use of the tumor necrosis factor inhibitor (TNFi) etanercept (ETN) in JPsA have been provided by the CLIPPER study, which demonstrated the effectiveness and safety of ETN in a JIA cohort including patients with extended oligoarticular JIA, ERA, and JPsA [40][41][42]. Among other TNFi, adalimumab and infliximab are the preferred agents when active uveitis is present, irrespective of the ILAR category, in the case of incomplete response to topical therapy and csDMARDs or in the presence of sight-threatening complications [43]. Although TNFi are the most frequently used bDMARDs in children, non-TNFi agents of proven efficacy in the treatment of JIA, such as abatacept or tocilizumab, may be used or preferred in certain scenarios, based on patient features and preferences [38][39].
In the last few years, the introduction of small molecules, such as Janus kinase (JAK) inhibitors, hold promise for the treatment of chronic arthritis. A pivotal trial has recently shown the effectiveness of tofacitinib, an oral JAK inhibitor, in a cohort of patients with polyarticular course JIA, including 20 subjects with JPsA [44]. This molecule has been recently approved by European Medicine Agency (EMA) for the treatment of polyarticular JIA and JPsA. The blockade of the IL-17 and IL-12/23 pathways has shown robust therapeutic effect in adult psoriasis and PsA. A monoclonal antibody against IL17A, secukinumab, is currently approved by the EMA for refractory PsA and ankylosing spondylitis in adults, and has yielded significantly longer time to disease flare than placebo and a good safety profile in children with ERA and JPsA in a randomized phase 3 trial [45]. A multicenter, open-label trial (ClinicalTrials.gov Identifier: NCT04527380) is currently ongoing to assess the efficacy and safety in children with ERA and JPsA of another anti-IL17A, ixekizumab, which has already been approved for the treatment of refractory PsA and axial spondyloarthritis, and adult and juvenile plaque psoriasis. Recently, the US Food and Drug Administration approved an anti-IL-12/23 monoclonal antibody, ustekinumab, for the treatment of patients with JPsA 6 years or older, based on the extrapolation [46] of data from studies about the use of ustekinumab in pediatric patients with psoriasis, including a small sample of JPsA [47][48] and in adults with PsA [49].
6. JPsA vs. Adult-Onset PsA
While the prevalence of arthritis in children with psoriasis is approximately 2% [50], up to 30% of adult patients with psoriasis develop joint inflammation. In PsA, unlike JPsA, the onset of psoriasis generally precedes that of arthritis, which usually occurs within 10 years after the diagnosis of psoriasis [51][52]. Peripheral arthritis is the most common presentation both in adults and children, whereas axial disease is more frequent in adults. Dactylitis is a distinctive feature of PsA, both in pediatric and adult populations, while solely the late-onset subgroup of JPsA displays enthesitis. Adult PsA often pursues a severe course, with a high frequency of bone and cartilage destruction in the joints and pathologic new bone formation [53]. A sizeable proportion of patients (about 25%) with JPsA develop radiological articular damage [19][25], although bone erosions are not commonly observed in the hand joints of children with JPsA [28].
The ClASsification criteria for Psoriatic ARthritis (CASPAR), which were developed to standardize enrollment in PsA clinical trials, provide guidance for PsA diagnosis in adulthood [54]. These criteria incorporate cutaneous and radiographic features and musculoskeletal manifestations, including both peripheral and axial arthritis [54]. In contrast, according to ILAR criteria, many children with axial arthritis are classified as having ERA or undifferentiated JIA [4]. The application of CASPAR criteria to the CARRA JIA cohort led to the identification of additional patients who would be classified as PsA by adult rheumatologists; those patients were likely to have less psoriasis but more enthesitis and sacroiliitis/inflammatory back pain, which are manifestations of spondyloarthritis [25]. Notably, in a study aimed to determine how JIA patients older than 18 fulfil the classification criteria for adult rheumatic diseases, all patients with JPsA fulfilled the CASPAR criteria for PsA [55].
RF is typically negative in both conditions and its presence excludes the diagnosis of JPsA by ILAR criteria and its absence is one of the CASPAR criteria for PsA [4]. RF positivity is reported in about 5% of cases in both PsA and JPsA. In line with what is observed in JPsA, the prevalence of ANA in PsA has been reported to be about 50% [56].
This entry is adapted from the peer-reviewed paper 10.3390/jcm12010367
References
- Martini, A.; Lovell, D.J.; Albani, S.; Brunner, H.I.; Hyrich, K.L.; Thompson, S.D.; Ruperto, N. Juvenile idiopathic arthritis. Nat. Rev. Dis. Primers 2022, 8, 5.
- Prakken, B.; Albani, S.; Martini, A. Juvenile idiopathic arthritis. Lancet 2011, 377, 2138–2149.
- Ravelli, A.; Martini, A. Juvenile idiopathic arthritis. Lancet 2007, 369, 767–778.
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.M.; et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392.
- Consolaro, A.; Giancane, G.; Alongi, A.; van Dijkhuizen, E.H.P.; Aggarwal, A.; Al-Mayouf, S.M.; Bovis, F.; De Inocencio, J.; Demirkaya, E.; Flato, B.; et al. Phenotypic variability and disparities in treatment and outcomes of childhood arthritis throughout the world: An observational cohort study. Lancet Child Adolesc. Health 2019, 3, 255–263.
- Petty, R.E. Juvenile psoriatic arthritis, or juvenile arthritis with psoriasis? Clin. Exp. Rheumatol. 1994, 12 (Suppl. 10), S55–S58.
- Martini, A. Are the number of joints involved or the presence of psoriasis still useful tools to identify homogeneous disease entities in juvenile idiopathic arthritis? J. Rheumatol. 2003, 30, 1900–1903.
- Nigrovic, P.A. Juvenile psoriatic arthritis: Bathwater or baby? J. Rheumatol. 2009, 36, 1861–1863.
- Ravelli, A.; Consolaro, A.; Schiappapietra, B.; Martini, A. The conundrum of juvenile psoriatic arthritis. Clin. Exp. Rheumatol. 2015, 33, S40–S43.
- Stoll, M.L.; Mellins, E.D. Psoriatic arthritis in childhood: A commentary on the controversy. Clin. Immunol. 2020, 214, 108396.
- Martini, A.; Ravelli, A.; Avcin, T.; Beresford, M.W.; Burgos-Vargas, R.; Cuttica, R.; Ilowite, N.T.; Khubchandani, R.; Laxer, R.M.; Lovell, D.J.; et al. Toward new classification criteria for juvenile idiopathic arthritis: First steps, Pediatric Rheumatology International Trials Organization International Consensus. J. Rheumatol. 2019, 46, 190–197.
- Ansell, B.M.; Bywaters, E.G. Diagnosis of “probable” Still’s disease and its outcome. Ann. Rheum. Dis. 1962, 21, 253–262.
- Angevine, C.D.; Pless, I.B.; Baum, J.; Jacox, R.F. Psoriatic arthritis in a child. Arthritis Rheum. 1973, 16, 278–283.
- Moll, J.M.; Wright, V. Psoriatic arthritis. Semin. Arthritis Rheum. 1973, 3, 55–78.
- Brewer, E.J.; Bass, J.; Baum, J.; Cassidy, J.T.; Fink, C.; Jacobs, J.; Hanson, V.; Levinson, J.E.; Schaller, J.; Stillman, J.S. Current proposed revision of JRA Criteria. JRA Criteria Subcommittee of the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Section of the Arthritis Foundation. Arthritis Rheum. 1977, 20, 195–199.
- Wood, P. Special Meeting on: Nomenclature and classification of arthritis in children. In The Care of Rheumatic Children; Munthe, E., Ed.; EULAR Publisher: Basel, Switzerland, 1978; pp. 47–50.
- Lambert, J.R.; Ansell, B.M.; Stephenson, E.; Wright, V. 2—Psoriatic arthritis in childhood. Clin. Rheum. Dis. 1976, 2, 339–352.
- Shore, A.; Ansell, B.M. Juvenile psoriatic arthritis—An analysis of 60 cases. J. Pediatr. 1982, 100, 529–535.
- Southwood, T.R.; Petty, R.E.; Malleson, P.N.; Delgado, E.A.; Hunt, D.W.; Wood, B.; Schroeder, M.L. Psoriatic arthritis in children. Arthritis Rheum. 1989, 32, 1007–1013.
- Roberton, D.M.; Cabral, D.A.; Malleson, P.N.; Petty, R.E. Juvenile psoriatic arthritis: Followup and evaluation of diagnostic criteria. J. Rheumatol. 1996, 23, 166–170.
- Fink, C.W. Proposal for the development of classification criteria for idiopathic arthritides of childhood. J. Rheumatol. 1995, 22, 1566–1569.
- Petty, R.E.; Southwood, T.R.; Baum, J.; Bhettay, E.; Glass, D.N.; Manners, P.; Maldonado-Cocco, J.; Suarez-Almazor, M.; Orozco-Alcala, J.; Prieur, A.M. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J. Rheumatol. 1998, 25, 1991–1994.
- Nigrovic, P.A.; Sundel, R.P. Juvenile psoriatic arthritis. In Textbook of Pediatric Rheumatology, 7th ed.; Petty, R.E., Laxer, R., Lindsley, C., Wedderburn, L., Eds.; Elsevier: Philadelphia, PA, USA, 2015.
- Stoll, M.L.; Zurakowski, D.; Nigrovic, L.E.; Nichols, D.P.; Sundel, R.P.; Nigrovic, P.A. Patients with juvenile psoriatic arthritis comprise two distinct populations. Arthritis Rheum. 2006, 54, 3564–3572.
- Zisman, D.; Gladman, D.D.; Stoll, M.L.; Strand, V.; Lavi, I.; Hsu, J.J.; Mellins, E.D.; CARRA Legacy Registry Investigators. The juvenile psoriatic arthritis cohort in the CARRA registry: Clinical characteristics, classification, and outcomes. J. Rheumatol. 2017, 44, 342–351.
- Huemer, C.; Malleson, P.N.; Cabral, D.A.; Huemer, M.; Falger, J.; Zidek, T.; Petty, R.E. Patterns of joint involvement at onset differentiate oligoarticular juvenile psoriatic arthritis from pauciarticular juvenile rheumatoid arthritis. J. Rheumatol. 2002, 29, 1531–1535.
- Stoll, M.L.; Nigrovic, P.A. Subpopulations within juvenile psoriatic arthritis: A review of the literature. Clin. Dev. Immunol. 2006, 13, 377–380.
- Lee, E.Y.; Sundel, R.P.; Kim, S.; Zurakowski, D.; Kleinman, P.K. MRI findings of juvenile psoriatic arthritis. Skelet. Radiol. 2008, 37, 987–996.
- Tuttle, K.S.; Vargas, S.O.; Callahan, M.J.; Bae, D.S.; Nigrovic, P.A. Enthesitis as a component of dactylitis in psoriatic juvenile idiopathic arthritis: Histology of an established clinical entity. Pediatr. Rheumatol. Online J. 2015, 13, 7.
- Baquet-Walscheid, K.; Rothaus, K.; Niewerth, M.; Klotsche, J.; Minden, K.; Heiligenhaus, A. Occurrence and risk factors of uveitis in juvenile psoriatic arthritis: Data from a population-based nationwide study in Germany. J. Rheumatol. 2022, 49, 719–724.
- Ansell, B.; Beeson, M.; Hall, P.; Bedford, P.; Woo, P. HLA and juvenile psoriatic arthritis. Rheumatology 1993, 32, 836–837.
- Toll, M.L.S.; Lio, P.; Sundel, R.P.; Nigrovic, P.A. Comparison of Vancouver and International League of Associations for rheumatology classification criteria for juvenile psoriatic arthritis. Arthritis Rheum. 2008, 59, 51–58.
- Consolaro, A.; Ravelli, A. Unraveling the phenotypic variability of juvenile idiopathic arthritis across races or geographic areas—Key to understanding etiology and genetic factors? J. Rheumatol. 2016, 43, 683–685.
- Hinks, A.; Martin, P.; Flynn, E.; Eyre, S.; Packham, J.; Barton, A.; Worthington, J.; Thomson, W.; Childhood Arthritis Prospective Study-CAPS; BSPAR Study Group; et al. Subtype specific genetic associations for juvenile idiopathic arthritis: ERAP1 with the enthesitis related arthritis subtype and IL23R with juvenile psoriatic arthritis. Arthritis Res. Ther. 2011, 13, R12.
- Day, T.G.; Ramanan, A.V.; Hinks, A.; Lamb, R.; Packham, J.; Wise, C.; Punaro, M.; Donn, R.P. Autoinflammatory genes and susceptibility to psoriatic juvenile idiopathic arthritis. Arthritis Rheum. 2008, 58, 2142–2146.
- Flatø, B.; Lien, G.; Smerdel-Ramoya, A.; Vinje, O. Juvenile psoriatic arthritis: Longterm outcome and differentiation from other subtypes of juvenile idiopathic arthritis. J. Rheumatol. 2009, 36, 642–650.
- Hinks, A.; Bowes, J.; Cobb, J.; Ainsworth, H.C.; Marion, M.C.; Comeau, M.E.; Sudman, M.; Han, B.; Becker, M.L.; Bohnsack, J.F.; et al. Fine-mapping the MHC locus in juvenile idiopathic arthritis (JIA) reveals genetic heterogeneity corresponding to distinct adult inflammatory arthritic diseases. Ann. Rheum. Dis. 2017, 76, 765–772.
- Onel, K.B.; Horton, D.B.; Lovell, D.J.; Shenoi, S.; Cuello, C.A.; Angeles-Han, S.T.; Becker, M.L.; Cron, R.Q.; Feldman, B.M.; Ferguson, P.J.; et al. 2021 American College of Rheumatology Guideline for the treatment of juvenile idiopathic arthritis: Therapeutic approaches for oligoarthritis, temporomandibular joint arthritis, and systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 2022, 74, 553–569.
- Ringold, S.; Angeles-Han, S.T.; Beukelman, T.; Lovell, D.; Cuello, C.A.; Becker, M.L.; Colbert, R.A.; Feldman, B.M.; Ferguson, P.J.; Gewanter, H.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the treatment of juvenile idiopathic arthritis: Therapeutic approaches for non-systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Rheumatol. 2019, 71, 846–863.
- Foeldvari, I.; Constantin, T.; Vojinović, J.; Horneff, G.; Chasnyk, V.; Dehoorne, J.; Panaviene, V.; Sušić, G.; Stanevicha, V.; Kobusinska, K.; et al. Etanercept treatment for extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis, or psoriatic arthritis: 6-year efficacy and safety data from an open-label trial. Arthritis Res. Ther. 2019, 21, 125.
- Constantin, T.; Foeldvari, I.; Vojinovic, J.; Horneff, G.; Burgos-Vargas, R.; Nikishina, I.; Akikusa, J.D.; Avcin, T.; Chaitow, J.; Koskova, E.; et al. Two-year efficacy and safety of etanercept in pediatric patients with extended oligoarthritis, enthesitis-related arthritis, or psoriatic arthritis. J. Rheumatol. 2016, 43, 816–824.
- Horneff, G.; Burgos-Vargas, R.; Constantin, T.; Foeldvari, I.; Vojinovic, J.; Chasnyk, V.G.; Dehoorne, J.; Panaviene, V.; Susic, G.; Stanevica, V.; et al. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: Part 1 (week 12) of the CLIPPER study. Ann. Rheum. Dis. 2014, 73, 1114–1122.
- Angeles-Han, S.T.; Ringold, S.; Beukelman, T.; Lovell, D.; Cuello, C.A.; Becker, M.L.; Colbert, R.A.; Feldman, B.M.; Holland, G.N.; Ferguson, P.J.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis-associated uveitis. Arthritis Rheumatol. 2019, 71, 864–877.
- Ruperto, N.; Brunner, H.I.; Synoverska, O.; Ting, T.V.; Mendoza, C.A.; Spindler, A.; Vyzhga, Y.; Marzan, K.; Grebenkina, L.; Tirosh, I.; et al. Tofacitinib in juvenile idiopathic arthritis: A double-blind, placebo-controlled, withdrawal phase 3 randomised trial. Lancet 2021, 398, 1984–1996.
- Brunner, H.I.; Foeldvari, I.; Alexeeva, E.; Ayaz, N.A.; Calvo Penades, I.; Kasapcopur, O.; Chasnyk, V.G.; Hufnagel, M.; Żuber, Z.; Schulert, G.; et al. Secukinumab in enthesitis-related arthritis and juvenile psoriatic arthritis: A randomised, double-blind, placebo-controlled, treatment withdrawal, phase 3 trial. Ann. Rheum. Dis. 2022.
- Leu, J.H.; Shiff, N.J.; Clark, M.; Bensley, K.; Lomax, K.G.; Berezny, K.; Nelson, R.M.; Zhou, H.; Xu, Z. Intravenous golimumab in patients with polyarticular juvenile idiopathic arthritis and juvenile psoriatic arthritis and subcutaneous ustekinumab in patients with juvenile psoriatic arthritis: Extrapolation of data from studies in adults and adjacent pediatric populations. Paediatr. Drugs 2022, 24, 699–714.
- Philipp, S.; Menter, A.; Nikkels, A.F.; Barber, K.; Landells, I.; Eichenfield, L.F.; Song, M.; Randazzo, B.; Li, S.; Hsu, M.C.; et al. Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients (≥6 to <12 years of age): Efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br. J. Dermatol. 2020, 183, 664–672.
- Landells, I.; Marano, C.; Hsu, M.C.; Li, S.; Zhu, Y.; Eichenfield, L.F.; Hoeger, P.H.; Menter, A.; Paller, A.S.; Taieb, A.; et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: Results of the randomized phase 3 CADMUS study. J. Am. Acad. Dermatol. 2015, 73, 594–603.
- McInnes, I.B.; Kavanaugh, A.; Gottlieb, A.B.; Puig, L.; Rahman, P.; Ritchlin, C.; Brodmerkel, C.; Li, S.; Wang, Y.; Mendelsohn, A.M.; et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013, 382, 780–789.
- Brandon, T.G.; Manos, C.K.; Xiao, R.; Ogdie, A.; Weiss, P.F. Pediatric psoriatic arthritis: A population-based cohort study of risk factors for onset and subsequent risk of inflammatory comorbidities. J. Psoriasis Psoriatic Arthritis 2018, 3, 131–136.
- FitzGerald, O.; Ogdie, A.; Chandran, V.; Coates, L.C.; Kavanaugh, A.; Tillett, W.; Leung, Y.Y.; deWit, M.; Scher, J.U.; Mease, P.J. Psoriatic arthritis. Nat. Rev. Dis. Primers 2021, 7, 59.
- Tillett, W.; Charlton, R.; Nightingale, A.; Snowball, J.; Green, A.; Smith, C.; Shaddick, G.; McHugh, N. Interval between onset of psoriasis and psoriatic arthritis comparing the UK Clinical Practice Research Datalink with a hospital-based cohort. Rheumatology 2017, 56, 2109–2113.
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017, 376, 2095–2096.
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H.; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673.
- Oliveira-Ramos, F.; Eusébio, M.; Martins, F.M.; Mourão, A.F.; Furtado, C.; Campanilho-Marques, R.; Cordeiro, I.; Ferreira, J.; Cerqueira, M.; Figueira, R.; et al. Juvenile idiopathic arthritis in adulthood: Fulfilment of classification criteria for adult rheumatic diseases, long-term outcomes and predictors of inactive disease, functional status and damage. RMD Open 2016, 2, e000304.
- Silvy, F.; Bertin, D.; Bardin, N.; Auger, I.; Guzian, M.C.; Mattei, J.P.; Guis, S.; Roudier, J.; Balandraud, N. Antinuclear antibodies in patients with psoriatic arthritis treated or not with biologics. PLoS One 2015, 10, e0134218.
This entry is offline, you can click here to edit this entry!
