Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Hydrogen production involves different resources and energy loads, depending on the production method used. Therefore, the industry has tried to set a classification code for this energy carrier. This is done by using colors that reflect the hydrogen production method, the resources consumed to produce the required energy, and the number of emissions generated during the process.
- hydrogen production technologies
- hydrogen colors
- water electrolysis
- green hydrogen
- grey hydrogen
- blue hydrogen
1. Introduction
Hydrogen has the advantage of being clean, non-polluting, storable, flexible, and renewable. It could be considered the “ultimate energy” of the 21st century. It is used in several fields, which include construction, industry, electricity, and transportation. Hydrogen occurs naturally on Earth in compound form with other elements in gases, liquids, and solids. It can be combined with oxygen, which results in water, or with carbon, to form different compounds, such as the hydrocarbons present in natural gas, coal, or petroleum. Consequently, hydrogen must be separated from the other elements to be consumed alone. Of course, it takes more energy to produce and purify hydrogen than what it delivers when converted to useful energy.
Different production methods can be found, depending on the original hydrogen source (e.g., water, natural gas, coal, petroleum). Some production methods are still under research. The most common production methods are steam methane reforming (SMR), which accounts for most commercially produced hydrogen, and electrolysis, which currently stands for less than 4% of the total hydrogen production. These methods will be explained in detail later on. As is known, hydrogen is a colorless gas, but there are around nine colors for identifying hydrogen. These colors refer to the process used to produce the hydrogen, the assessment of the production method, the resources consumed to obtain the required energy, and the amount of polluting emissions generated. The colors that identify these factors are green, grey, brown or black, blue, aqua, turquoise, purple, pink, red, yellow, and white.
2. Types of Hydrogen
As mentioned, hydrogen can be produced using different primary energy sources. Thus, these technologies are classified into different colors depending on the production process, the kind of used energy, the hydrogen costs, and the related emissions. The different colors are green, blue, aqua, and white, called low-carbon hydrogen, and then grey, brown or black, yellow, turquoise, purple or pink, and red.
2.1. Green Hydrogen
Green hydrogen, which is also often called “clean hydrogen”, “renewable hydrogen”, or “low-carbon hydrogen”, is, by definition, the hydrogen produced with water electrolysis using electricity from renewable energy sources. By using renewable energy, green hydrogen production does not generate carbon dioxide (CO2) emissions at any point. This kind of hydrogen is particularly interesting in the energy transition towards a more sustainable energy and transport system.
Nowadays, green hydrogen only represents a tiny percentage of the total hydrogen production because of the high costs involved in its process. However, it has an excellent projection towards the future, being the cleanest type of hydrogen, which will help to satisfy net-zero carbon plans. The following years will determine whether this technology evolves into a feasible option or not.
2.1.1. Production Methods
Electrolysis of Water
Water electrolysis is currently a mature technology applied in several industrial applications. The main advantage of this method is that the used electricity has the potential to be generated with renewable energy that can come from low-carbon or even carbon-free methods [1]. Thus, this technology is seen as the most promising green hydrogen production method, assuming the electrical energy used to power the water electrolyzer comes exclusively from renewable energy sources.
The process of hydrogen production from water electrolysis depends on two half-reactions: the cathodic hydrogen evolution reaction (HER) and the anodic oxygen evolution reaction (OER). Currently, this technology includes four main approaches: alkaline water electrolysis (AWE), proton-exchange membrane (PEM) electrolysis, the solid oxide electrolysis cell (SOEC), and anion-exchange membrane (AEM) electrolysis [1].
The AWE technology belongs to the earliest industrialization time due to its reliability, low cost, and easy operation. Its main drawback is that it occupies a large area. The electrolyzer mainly comprises the electrolyte, diaphragm (generally asbestos), cathode, and anode. In normal conditions, it works at low temperatures, and the pressure between the anode and the cathode must be balanced to avoid an explosion caused by the interpenetration of hydrogen or oxygen. AWE has a relatively long cold start time (ca. 50 min), meaning it starts slowly and requires a long response time [2].
PEM technology is easy to integrate and has high conversion efficiency but is very expensive. The catalyst price is one of the main reasons why this technology is not yet applied on a large scale. Still, the small size of the electrolysis cell makes it easier to couple it with wind energy and photovoltaics, which makes it a potential option for the future. The primary device is an electrolytic cell with a polymeric cation-exchange membrane as the core part. There is a bipolar plate that is responsible for connecting multiple membrane–electrode assemblies (MEAs), where the cathode and anode catalysts are deposited over the membrane [3]. Water is fed to the anode side, where its oxidation generates O2 and H+, with the latter crossing the PEM membrane to the cathode to form H2.
SOECs use high working temperature conditions and are subjected to many restrictions, requiring a high standard of the used materials [4]. For this technology, the cathode is usually a nickel-based porous cermet. At the same time, the anode is based on a perovskite oxide containing rare-earth elements, and the ceramic electrolyte is an oxygen ion (O2−) conductor.
AEM electrolysis is still in the research stage, with many aspects under development. An anion-exchange membrane capable of good ion conduction at low temperatures is used as the separator [5]. This system is similar to the PEM electrolyzer, with the water being fed to the cathode side instead, and has a fast start-up time.
All these hydrogen production technologies via water electrolysis are continuously under research and innovation seeking to reduce costs and improve efficiency. Depending on the technology, several components are studied and tested, such as the catalysts, the used materials, or the ion-exchange membranes. The water electrolysis process will undoubtedly have a cheaper cost in the future, as this is one of the main goals of accomplishing massive green hydrogen production.
Photocatalysis
Fujishima and Honda discovered in 1972 that photocatalytic water splitting on a TiO2 electrode could produce hydrogen. Photocatalysis is the process where a catalyst absorbs photons to generate high-energy electrons and holes, followed by a redox reaction, as represented in Figure 1 [6]. This is performed by using a semiconductor photocatalyst to generate an electron–hole pair under the irradiation of light. H2 can be obtained as the target product in the reduction reaction [1]. With this method, solar energy can be converted or stored as chemical energy, one of the most promising advantages of green hydrogen production. Thus, photocatalytic water splitting is seen as the cleanest way to produce hydrogen, as it only uses sunlight to split water directly into H2 and O2.
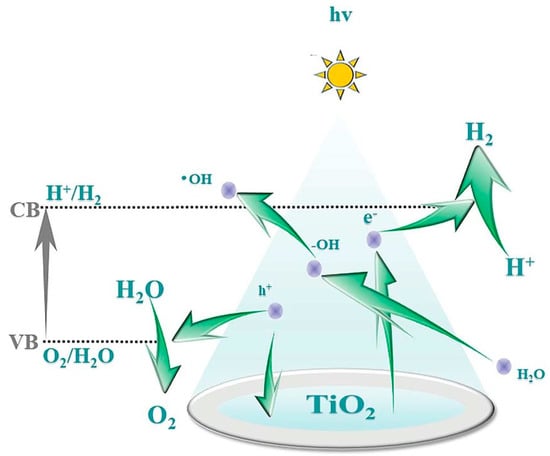
Figure 1. Mechanism of photocatalytic water splitting for hydrogen production [6].
Besides water, other compounds such as alcohols and biomass have been studied as photocatalytic raw materials for H2 production. One of the keys in the application of photocatalysis is the recombination of photogenerated charge and poor light absorption. Several options are being tested, seeking to improve photocatalytic performance. Considering the low radiation efficiency of the light source, poor reactivity is the main limitation for large-scale commercial applications.
Additionally, the photocatalytic mechanism for hydrogen production is uncertain, with most studies focusing on the energy band and the catalyst. The reasonable design of reactors and devices is also crucial for enhancing catalytic activity, but such research is still limited. As it is a production method that converts photons directly into H2, it is expected that this technology will evolve into a more efficient one in the coming years, playing a pivotal role in green hydrogen production.
2.1.2. Renewable Energy Sources
Wind Energy
Wind energy is one of the most promising sources for producing green hydrogen. The wind energy is first converted into electricity using wind generators, and the hydrogen is later generated with water electrolysis. Depending on whether the wind generators are connected to the grid or not, they are divided into three types [7]:
-
Grid-connected system, where the wind turbines are connected to the grid, thus obtaining the electricity from the grid (100% renewable energy) and producing the hydrogen by electrolyzing water. This method is commonly used for wind consumption and energy storage of large-scale wind sites.
-
Off-grid system, where the electricity is generated by single or multiple fans directly connected to the electrolysis equipment, without connecting to the grid. It is often used for distributed hydrogen production systems.
-
Grid-connected without transmission, where the turbines are connected to the grid, but the energy is not transmitted to other sites. It only meets local hydrogen production demand.
Solar Energy
Solar energy is the other primary renewable source participating in green hydrogen production. There are two ways to connect the solar energy source with the application [8]:
-
A direct coupling system achieves an optimal structure matching between the photovoltaic array and the electrolyzer, using a DC–DC controller and a storage battery. In this case, maximum power point tracking (MPPT) is not included.
-
Indirect connection involves the use of photovoltaic and control modules, batteries, and hydrogen storage systems and is the most commonly used method for photovoltaic-water electrolysis hydrogen production systems. As it requires electronic equipment, such as the MPPT and DC–DC controllers, some power transmission losses may occur, dropping the efficiency and increasing the cost.
Other Renewable Energy Sources
The renewable energy sources that currently take the most significant role in green hydrogen production are wind and solar energy. However, other renewable sources produced without any greenhouse gas (GHG) emissions can also be considered, such as hydropower. There are plans to start working with hydro energy as a source for green hydrogen production, which can be seen in a project aiming to prepare hydrogen production maps for regions in Turkey based on the hydro energy potential for use in electrolyzers [9]. In the near future, some other technologies may appear to obtain the greenest energy possible from every kind of renewable energy source.
2.2. Purple (Violet), Pink and Red Hydrogen
According to the literature, pink hydrogen is produced with water electrolysis using electricity from a nuclear power plant. It was also considered that purple hydrogen is obtained by using nuclear power and heat through combined electrolysis and thermochemical water splitting [10]. Red hydrogen is generated through the high-temperature catalytic splitting of water using nuclear thermal power as the energy source. Some may establish that they can be considered the same.
The use of nuclear electricity for hydrogen production is not significantly promoted in the European Union’s hydrogen strategies; however, it may be a useful alternative for other regions, such as Russia and China. France is also pushing for this new technology, and attaching a hydrogen production facility can help reduce the curtailment of nuclear plants [11]. Figure 2 shows hydrogen production with different sources of energy [12].
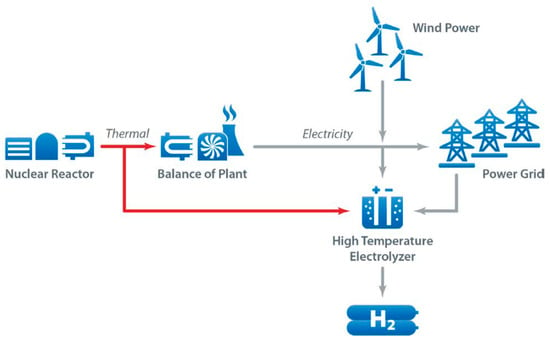
Figure 2. Energy sources for pink, green, and yellow hydrogen production [12].
2.3. Yellow Hydrogen
Yellow hydrogen is produced with electrolysis using electricity from the energy grid. Carbon emissions vary significantly in time, depending on the grid’s energy sources [13]. The grid results from the injection of electricity from every power source available. The sources and technologies have changed over time, and some are used more than others, depending on the country.
Using Spain as an example, the electricity generated and fed to the grid during 2021 came from the following power mix. With a share of 23.3%, wind energy was the most commonly used source, followed closely by nuclear power, with nearly 21% of the electricity generation [14]. Later, there was the combined cycle with 17.1% and hydropower generation with 12.4% of the total share, followed by cogeneration at 10% and solar photovoltaic at 8%.
It is important to mention the chlor-alkali industry as an essential part of the chemical industry. It produces chlorine and sodium hydroxide through the electrolysis of brine, a saturated sodium chloride solution obtained from natural salt deposits [16]. In this process, hydrogen is classified as a byproduct that may be combusted for process heat, sold as a commodity to the external market, or wasted by simply venting it into the atmosphere. Thus, the byproduct hydrogen from the chlor-alkali industry can help meet the market’s increasing demand [17]. As the process involves electrolysis and the energy source is typically the grid, it can also be considered yellow hydrogen, even though some sources consider it white hydrogen. Some authors recognize yellow hydrogen as being produced through electrolysis via solar power, although this designation is now becoming obsolete.
2.4. Grey Hydrogen
Grey hydrogen denotes hydrogen produced from steam methane reforming, partial oxidation, or autothermal reforming. Currently, most of the produced hydrogen corresponds to grey hydrogen. It is important to highlight that 40% of grey hydrogen is a byproduct of other chemical processes. Grey hydrogen is generally used in the petrochemical industry and for ammonia production [18].
Around 6% of the worldwide extracted natural gas and 2% of coal are used to produce grey hydrogen. The main disadvantage of grey hydrogen is related to the high CO2 emissions during hydrogen production (ca. 830 Mt CO2 per year) [20].
2.4.1. Production Methods
Steam Methane Reforming
Steam methane reforming (SMR) is a mature technology that, in combination with the water–gas shift reaction, allows the production of grey hydrogen. It is currently the most common and cost-effective method to produce hydrogen, accounting for 80% of the global demand [21]. Steam reforming was first implemented in the industry in the 1930s in the United States, where methane was highly available. However, it was not until the 1960s that this technique broke into the syngas and methanol industry, with naphtha mainly being used as feedstock across Europe [22]. Natural gas has proven to be the most suitable raw material for steam reforming due to its high availability, ease of transportation, and higher composition homogeneity compared to other fossil fuels. Most of this hydrogen (around 95%) is used as feedstock for ammonia, methanol, and the synthesis of liquid fuels (by Fischer–Tropsch processing) [21].
The steam reforming process involves a reaction between hydrocarbons and steam to produce a mixture of hydrogen and carbon monoxide, known as syngas [23].This process requires high temperatures and pressures, ranging from 700 °C to 1000 °C, and 5–20 bar, depending on the desired yields, the reactors, and the catalysts employed. With this being an endothermic process with a high-temperature demand, the supply of thermal energy becomes a remarkable aspect. Usually, on a large scale, this supply is provided through fossil fuels. However, some experimental facilities worldwide carry out this reaction by providing heat via concentrated solar power. Some of the most advanced pilot plants can be found in Almeria (Spain), Zurich, Rome, and Colorado. However, high surface areas are required to reach these temperatures and carbon depositions due to the added difficulties in controlling the reactor [24].
Partial Oxidation
Partial oxidation is a process where grey hydrogen is obtained from hydrocarbons, typically natural gas. This technology is commercially available and can be considered mature. In this process, the combustion is carried out with a limited amount of oxygen. As a result of the sub-stoichiometric oxygen supply, only partial oxidation of the carbon takes place, so CO is produced instead of CO2. The other reaction products are hydrogen and nitrogen if the air is used instead of pure oxygen. Due to the high temperatures, using a catalyst is not mandatory. However, the catalyst significantly increases the reaction yield [25].
The partial oxidation process is faster than steam reforming and involves a smaller reactor vessel. A disadvantage is that less hydrogen is produced per input fuel than with the steam reforming process. Finally, the gas stream is cleaned of CO2 and other impurities [26].
2.5. Turquoise Hydrogen
Turquoise hydrogen also uses methane as the feedstock but is produced via methane pyrolysis. Contrary to SMR, the byproduct is solid carbon appearing as filamentous carbon or carbon nanotubes. This type of byproduct can be further used and is easier to store, thus having a lower carbon footprint [27]. Furthermore, it may be sold for other applications.
The pyrolysis of methane can be carried out through three approaches: thermal decomposition, plasma decomposition (known as the Kvaerner process), and catalytic decomposition. This technique has already been known for decades and is technically performed in several processes. Still, it has recently been considered an interesting option for hydrogen production [28]. So far, pyrolysis has never been commercialized as a hydrogen production method. The thermal process is being further developed with the target of producing hydrogen in large quantities.
2.6. Brown and Black Hydrogen
Considering production from coal, the brown and black hydrogen colors refer to the type of lignite (brown) and bituminous (black) coal. It is regarded as the least environmentally friendly hydrogen production method, creating as much CO2 as burning the source fuel would have in the first place. Around 20 kg of CO2 is released for every kg of brown/black hydrogen produced [29]. This is a highly used hydrogen production method, as coal is the fossil energy source with the largest worldwide reserves. Notably, China produces large amounts of hydrogen through coal gasification due to high natural gas prices and large coal reserves [30].
Although some authors claim that hydrogen from biomass gasification should be seen as green, assuming the whole lifecycle of the biomass is carbon-neutral, the undeniable high CO2 emissions of the process lead most authors to consider it brown hydrogen.
2.6.1. Production Methods
Coal Gasification
Coal gasification is one of the most important hydrogen production methods and can involve different techniques. Gasification is known as the process of converting any carbon-based raw material into syngas using air, steam, or oxygen. Gasification techniques can effectively convert many raw materials and wastes (e.g., coal, car tires, sewage sludge, sawdust, wood, and plastic waste) into valuable outputs. The end product gas of the gasification process can include some (or all) of the following compounds: CO, H2, CH4, ash, tar, H2S, NH3, HCl, and HCN. Purification of the product gas is then required to remove contaminants, particles, and other substances, thus decreasing its calorific value. This may involve applying several gas clean-up processes to adequately separate the useful gases, such as CO, H2, and CH4.
In the gasification process, four types of coal are usually utilized (Figure 3). These are lignite (low rank), sub-bituminous coal (low rank), bituminous coals (medium rank), and anthracites (high rank) [31].

Figure 3. Different types of coal [31].
It should be noted that these materials are generally gasified at temperatures higher than 900 °C by applying fixed bed gasification, moving bed gasification, fluidized bed gasification, entrained flow gasification, or plasma gasification. Particularly, the latter is recognized as a relatively recent technology that uses plasma torches for producing clean and renewable fuels. Among gasification processes, the entrained and plasma gasification of coal are usually carried out at higher temperatures of ca. 1200 and 1700 °C, respectively, whereas the other processes require operating temperatures lower than 1200 °C [33].
Coal gasification still seems to be an important process for relatively cleaner and cost-effective production of energy and chemical products. Compared to the traditional coal combustion process, one can point out the following advantages of coal gasification:
-
Higher efficiency in the conversion of coal’s high moisture and ash content into valuable products.
-
Production of high-calorific-value syngas.
-
This hydrogen-rich syngas leads to higher-efficiency electricity generation, significantly decreasing carbon emissions.
Co-Gasification of Coal
Researchers have studied the effect of mixing different biomass ratios on the performance of coal gasification. It was demonstrated that the coal gasification rate improved with the amount of biomass in the mixture. Furthermore, hydrogen production increased with the increase in temperature. Studies were conducted to design a combined cycle power plant involving the co-gasification of coal with biomass coupled with a carbon capture and storage (CCS) unit. The hydrogen and electricity generation performance in this integrated process was evaluated using four gasification approaches: 100% coal and 80–20% combinations of coal–sawdust, coal–sewage sludge, coal–meat, and coal–bone meal. The coal–meat and coal–bone meal mixtures exhibited the best results for hydrogen production [34].
The gasification of mixtures of coal with small amounts of meat and bone powder (MBM) in a fluidized bed reactor as an alternative for waste management was also evaluated. The work assessed the influence of bed temperature (800–900 °C), equivalence ratio (0.25–0.35), and MBM ratio in the feed (0.1 wt.%) on the quality of the produced syngas. When using air as a gasifying agent, it was seen that MBM had a minimal effect on the amount of H2, CO, and CO2 synthesis gases. The work demonstrated that the hydrogen ratio in the produced syngas increased with the temperature and decreased with the equivalence ratio [34].
Biomass
Using biomass as raw material can be another method used to obtain hydrogen. Biomass gasification, involving conversion, decarbonization, and separation, is a promising approach for obtaining pure hydrogen [35]. At the same time, hydrogen-rich compounds such as methane, methanol, or ethanol can be obtained by other processes, including enzymatic hydrolysis and fermentation. High-purity hydrogen can be reached by reforming these hydrogen compounds through catalytic reactions.
There are two pathways to produce hydrogen from biomass: biological or thermochemical hydrogen production. The biological pathway is performed using hydrogen-producing microorganisms and can involve anaerobic fermentation or photosynthetic routes. Still, the possibility of large-scale production is limited due to its poor yield and stability. The thermochemical method may involve the gasification or pyrolysis of the hydrocarbon components of biomass into syngas, followed by the water–gas shift reaction to increase the hydrogen yield [36].
The production of hydrogen from biomass has the basic technological development conditions for industrialization. Even though this process has CO2 emissions, it can be considered a net-zero carbon emissions process, as the carbon emitted is also incorporated as part of the gasified biomass life cycle. For that reason, some authors claim this type of hydrogen should be also considered green. However, as the definition of green hydrogen refers specifically to that produced by water electrolysis using renewable energy sources, some experts defend that a new color should be created to define the hydrogen produced from biomass gasification. Recent suggestions point out to be named "beige hydrogen", as the historical origins of this light neutral color referred to the color of natural wool that had been neither bleached nor dyed.
2.7. Blue Hydrogen
A very appealing option for low-carbon hydrogen is blue hydrogen. It is based on producing hydrogen from fossil fuels, but with a carbon capture, utilization, and storage (CCUS) system. Utilization is not mandatory to qualify as blue hydrogen. Being produced from fossil fuels, blue hydrogen currently has lower costs than green hydrogen.
As no CO2 is emitted, the blue hydrogen production process is categorized as carbon-neutral. Blue hydrogen is considered an alternative solution during the energy transition, as it still offers the possibility of consuming fossil fuels, but with a reduction in the carbon footprint. Doing that provides a sustainable vision for some fossil fuel-producing countries (e.g., Canada, Iran, Norway, Qatar, Russia, United States).
Different methods can be used to produce blue hydrogen, some of which are the conventional ones to produce grey, brown, or black hydrogen, although carbon is captured and stored in the former case. There are also several ways of capturing carbon, depending on the stage of the production method. After capturing it, CO2 can be either utilized for other purposes or transported and stored.
2.7.1. CO2 Capture
There are several available technologies for carbon capture during hydrogen production at different stages of development and commercialization. The principal CO2 capture technologies are adsorption, absorption, cryogenic separation, membrane separation, calcium looping, chemical looping, and direct separation calcination technology. Carbon capture technologies can be divided into pre-combustion, post-combustion, and oxyfuel combustion [37].
Pre-Combustion
Pre-combustion carbon capture is the process of CO2 removal from fossil fuels before combustion is completed [38]. Pre-combustion technology is the most adopted since it is a terminal technology that can be easily incorporated into any existing system.
Post-Combustion
Post-combustion capture denotes CO2 removal from fossil fuels after combustion is completed. Among the available technologies, the most suited for pre-combustion capture are physical absorption, pressure-swing adsorption (PSA), and membrane separation [39]. Post-combustion CO2 capture can be schematically divided into three blocks: biological, physical, and chemical methods. The biological method refers to the photosynthesis of plants, algae (terrestrial but also marine or freshwater microalgae), and photosynthetic bacteria without energy consumption.
Oxyfuel Combustion
Oxyfuel combustion processes use nearly pure oxygen instead of air for the combustion of fuel [40]. The combustion produces an exhaust gas mainly made up of vapor H2O and CO2 that can be easily processed through dehydration to obtain a high-purity CO2 stream [41]. Generally, oxyfuel combustion provokes the recirculation of flue gas to obtain a lower flame temperature, promoting it as a highly efficient combustion technology in terms of energy saving [42]. The salient features can be summarized as follows:
-
Pure oxygen replaces air during fuel combustion to obtain high CO2 concentrations.
-
Some of the flue gas must be recirculated to control the temperature of the furnace flame and preserve pertinent heat transfer characteristics.
-
Beneficial for CO2 capture and subsequent compression.
One technology developed that has gained interest is the ion-transport membrane technology, which could considerably contribute to improving the performance efficiency of oxyfuel combustion and the integration of carbon capture in integrated gasification combined cycle systems [39].
As of today, SMR satisfies most of the hydrogen demand thanks to its low cost and high efficiency. Indeed, 9 kg of CO2 is generated for every kg of H2 produced. Figure 4 schematically represents the operations of a modern SMR hydrogen plant fed by natural gas [43]. Of the total CO2 production, approximately 60% is produced in the water–gas shift reactor and PSA tail gas. In comparison, the remaining 40% comes from the combustion of additional fuel gas (non-renewable) required by the steam reformer [43]. CO2 can be captured from any of the three streams (Figure 4), with a removal efficiency of about 90% from PSA tail gas and steam reforming flue gas, and over 99% from raw H2.
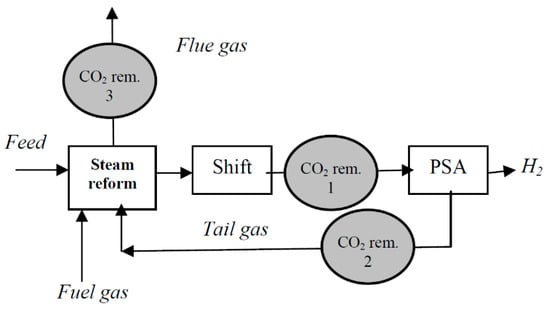
Figure 4. Scheme of a modern steam methane reforming H2 plant with CO2 removal [43].
2.8. Aqua Hydrogen
Canada is a major producer of hydrogen from natural gas through SMR, thus producing vast amounts of GHG emissions. The University of Calgary and the company “Proton Technologies” (based in Western Canada) have developed a carbon emission-free technology that allows for extracting hydrogen from oil sands (natural bitumen) and conventional oil fields [44]. This hydrogen can be a zero-carbon approach to enhancing energy recovery from Canada’s oil and gas resources.
The aqua hydrogen technology involves placing oxygen into a sealed fuel deposit between grains of rock, using unswept petroleum as the fuel and the ground as the reactor vessel [45]. The process starts by separating atmospheric air into nitrogen and oxygen using air separation units. The oxygen is then sent to an underground reservoir, where the temperature is increased, promoting water–gas shift, hot gasification, and aqua thermolysis reactions within the fuel reservoir, thus generating syngas.
Lastly, the H2 is extracted using membranes in the production wells. These membranes are composed of a palladium alloy, where carbon oxides stay underground and H2 reacts with palladium [45]. The process has high efficiency because all energy conversion takes place underground. This technology may be a clean way to generate hydrogen using economically unrecoverable oil reserves, bringing only hydrogen to the surface.
2.9. White Hydrogen
White hydrogen refers to naturally occurring hydrogen. It is found in nature as a free gas in layers of the continental crust, deep in the oceanic crust, or in volcanic gases, geysers, and hydrothermal systems. It seems to be present in a wide range of rock formations and geological regions.
The processes involved in natural hydrogen formation are not well understood. Some hypotheses are hydrogen degassing from the Earth’s core, water reacting with ultrabasic rocks (i.e., serpentinization) or with reducing agents in the mantle, natural radiolysis (i.e., water dissociation by uranium or plutonium), and decomposition of organic matter. Some of this hydrogen has been found in New Caledonia. It is believed to have been formed by serpentinization, with the water reacting with local rocks (ophiolites) and releasing hydrogen.
As is known, steam reforming produces grey hydrogen with high efficiency and low cost, but also with a catastrophic carbon footprint. As for electrolysis, it generates green hydrogen from renewable energy sources but loses almost 70% of the energy input throughout the process. Naturally formed white hydrogen appears as a promising carbon-free and abundant source that requires minimal infrastructure for its exploitation [46]. Due to a low level of research on the topic, it is still difficult to assess worldwide white hydrogen resources. The EartH2 initiative has been assisting the scientific community and industry in gathering forces and increasing the knowledge on white hydrogen. A hydrogen economy based on white and green may be the answer for the transition to a carbon-free society, enabling a brighter tomorrow for planet Earth and humankind. As mentioned before, other sources may consider hydrogen produced as a byproduct of chemical processes to also be referred to as white hydrogen [47].
This entry is adapted from the peer-reviewed paper 10.3390/gases3010002
This entry is offline, you can click here to edit this entry!
