Neurons are the basic building blocks of the human body’s neurological system. Atrophy is defined by the disintegration of the connections between cells that enable them to communicate. Peripheral neuropathy and demyelinating disorders, as well as cerebrovascular illnesses and central nervous system (CNS) inflammatory diseases, have all been linked to brain damage, including Parkinson’s disease (PD). It turns out that these diseases have a direct impact on brain atrophy. However, it may take some time after the onset of one of these diseases for this atrophy to be clearly diagnosed. With the emergence of the Coronavirus disease 2019 (COVID-19) pandemic, there were several clinical observations of COVID-19 patients.
1. Introduction
Cerebrovascular illness affects the brain’s blood flow and blood vessels. Blood flow issues may be caused by blood vessel constriction, clot development, artery blockage, or rupture. SARS-CoV-2 is an opportunistic brain infection that causes substantial respiratory distress, as well as neurological signs. COVID-19 is linked to symptoms including neurological complications and brain death.
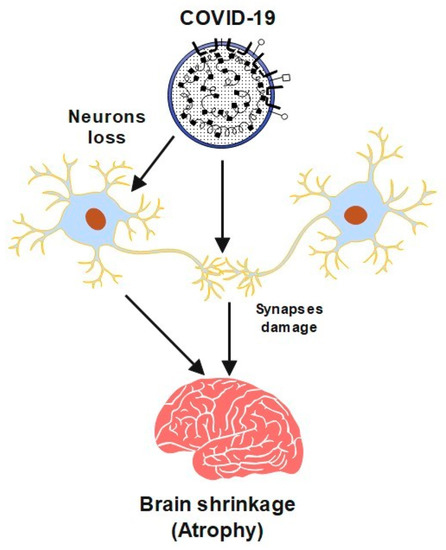
However, studies have shown that this virus may cause neurological problems, which can sometimes precede usual symptoms like a fever and a cough. It may cause serious problems, including cerebrovascular disease (CVA), convulsions, or paralysis. The virus has caused serious neurological symptoms in older people and severely ill patients. Furthermore, COVID-19 can cause damage to neurons and their synapses. In this case, the brain could shrink, which leads to a brain disease called atrophy. A semantic diagram is presented in
Figure 1 to show a summary of this process. It also has a significant influence on global mental health [
1].
Figure 1. A schematic diagram of COVID-19 effects brain shrinkage.
SARS-CoV-2 may trigger intrinsic and innate immunological responses in the host, including enhanced cytokine release, tissue damage, and high neurosusceptibility to COVID-19, particularly in hypoxic settings induced by lung injury. In immunocompromised people, the virus may enter the brain through the vasculature and peripheral nerves [
2].
SARS-CoV-2 was detected in nasal swabs and a computerized tomography (CT) scan revealed widespread enlargement of the brain stem. A woman in her 59th year visited the emergency room after experiencing recurrent, fleeting episodes of vacant staring and speech arrest. These symptoms were accompanied by flexion of both shoulders and a brief generalized tonic-clonic seizure (GTCS), which was followed by postictal reduced consciousness. Due to her diminished state of awareness, she needed intubation and artificial ventilation.
It was found that the patient’s health had worsened with symmetrical hemorrhagic lesions in the brain stem, amygdalae, and thalamic nuclei. The findings matched hemorrhagic ANE with early brain stem involvement. The patient died after eight days of steroid treatment [
3]. Postmortem brain magnetic resonance imaging (MRI) shows hemorrhagic and Posterior reversible encephalopathy syndrome (PRES)-related brain lesions in COVID-19 non-survivors, perhaps caused by virus-induced endothelium abnormalities and brainstem absence.
COVID-19 MRI abnormalities do not support brain-related respiratory distress [
4]. SARS-CoV-2 may target deeper brain regions such as the thalamus and brainstem via trans-synaptic transmission, as seen in other viral illnesses. The virus may then infect the respiratory center of the brain, causing respiratory failure in COVID-19 patients. COVID-19 patients should be screened for neurological symptoms and the collapse of the respiratory center in the brainstem [
5].
The pandemic’s neurological effects are becoming clearer, and COVID-19 does not seem to pass the blood-brain barrier [
6]. The ubiquitous danger to our desire for human connection may combine with “brain styles,” which we previously characterized as “biotypes” informed by a neural taxonomy, to explain the mental health repercussions of COVID-19. This essay aims to promote research on COVID-19’s mental health effects from an individualized, brain-based approach that recognizes the virus’s grave danger to our core human drives [
7].
Recent research shows that a cytokine storm promotes brain inflammation, and hence neurological symptoms during the COVID-19 epidemic. Targeting brain inflammation may help cure SARS-CoV-2’s neurologic consequences. Vascular Endothelial Growth Factor (VEGF), which is extensively distributed in the brain, may have a role in brain inflammation by promoting the recruitment of inflammatory cells and regulating angiopoietin II levels [
8]. The vast variety of neurologic imaging results underscores the necessity for future investigations to improve therapy for these individuals [
9]. The involvement of medulla oblongata brainstem structures in food intake and vomiting control has been studied, as well as the virus’s probable neurotropic and hematogenous routes to the brainstem [
10].
Neurological exams are required for all SARS-CoV-2 patients, symptomatic or not, for COVID-19, and during hospitalization. Even after recovery from COVID-19, people should be considered at risk for neurological problems. Long-term neurologic monitoring following COVID-19 recovery will show whether this sickness is linked to treatable neurodegenerative illnesses.
COVID-19 patients and their families should get mental health assistance when the condition has stabilized [
11]. Many COVID-19 patients remain unconscious following a serious illness. We don’t know how structural brain abnormalities affect brain function or prognosis. This population has yet to be studied in terms of prognostic neuroimaging. Despite chronic non-responsiveness and anatomical brain abnormalities, a patient with severe COVID-19 showed intact functional network connections and, weeks later, regained command-following ability [
12].
There is a link between anxiety and cognitive impairment in SARS-CoV-2-infected people with moderate or no respiratory symptoms and changed cerebral cortical thickness. Of all COVID-19 deaths, 19% had brain injuries. SARS-CoV-2 foci were found in astrocytes in all the afflicted brain regions. Neurotransmitter synthesis and energy consumption are altered in neural stem cell-derived astrocytes infected with a secretory phenotype that lowers neuronal survival [
13].
Further study is required to understand the precise mechanisms and pathways of infectivity underpinning CNS pathology [
14]. COVID-19 affects transcription in all cortical, parenchymal, and choroid plexus cell types. As a result of the SARS-CoV-2 infection of the cerebral vasculature and meninges, enhanced inflammatory signaling in the brain occurs. Parallel to this, peripheral immune cells penetrate the brain, microglia activate programs that mediate the phagocytosis of living neurons, and astrocytes dysregulate genes important in neurotransmitter balance. All these processes are part of the neuroinflammatory response [
15]. COVID-19 symptoms may affect other organs, including the brain, and data on SARS-CoV-2 neuropathological characteristics is scarce [
16].
The effect of the COVID-19 quarantines on Traumatic brain injury (TBI) patients in Tyrol was investigated to ensure that neurosurgical treatment is available even during pandemic lockdown [
17]. The COVID-19 infection can cause severe pneumonia, as well as systemic thrombotic complications, including cerebrovascular disease [
18]. Infections of the CNS cause aseptic meningitis or encephalitis. The respiratory system is the major target of COVID-19. However, it is also a neuropathogen. The hallmark clinical feature ranges from mild confusion to profound coma. Most encephalitis patients are severely ill [
19]. SARS-CoV-2 enters the brain through olfactory nerves via angiotensin-converting enzyme 2 (ACE2) and cytokine storms, a process called “cell reprograming” [
20]. As shown in
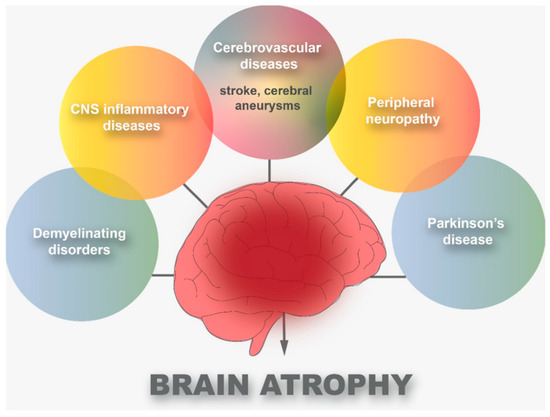
Figure 2, there are six main diseases that can cause brain atrophy: cerebrovascular diseases, CNS inflammatory diseases, peripheral neuropathy, demyelinating disorders, and PD.
Figure 2. Common diseases that cause brain atrophy.
2. Cerebrovascular Diseases and COVID-19
Inhibitions of COVID-19 receptor ACE2 expression in a high-risk cohort for coronary heart disease (CHD) and stroke will be discussed next. A functioning ACE2 receptor has recently been identified in COVID-19, which may cause serious cerebrovascular disorders, including strokes, in people with risk factors for severe cerebrovascular diseases (CVD), such as diabetes and smoking. The effects of cigarette smoke extract (CSE) and hyperglycemia on ACE2 expression in arteries were studied in a rat MCAO model. After ischemic damage, the cortical penumbra expressed more ACE2. CSE increased ACE2 expression in the human brain vasculature. In diabetic primary cultured human blood vessels, ACE2 expression was increased [
21]. Strokes have been reported in young people under fifty years old, with no cardiovascular risk factors associated with COVID-19. As of now, just a few instances have been reported, so it’s possible that the illness promotes its growth. Elderly people with stroke risk factors such as hypertension, diabetes, and increased fibrin D-dimers had higher cerebrovascular occurrences. COVID-19 contains several CVD cases reports and series. The mechanism that causes COVID-19 cerebral ischemia is unknown [
22].
While the presence of both respiratory and cerebral injuries would suggest a greater severity, COVID-19 stroke patients might present with minor or non-respiratory symptoms. The severity of anterior circulation big vessel blockage strokes in COVID-19 patients was compared. Anterior circulation, big artery blockages and early brain imaging within 3 h of commencement were compared to a control group hospitalized over the same calendar month in 2019. Patients with COVID-19 had more severe big vessel occlusion strokes. The pandemic’s neurovascular effects should be feared given the enormous number of afflicted patients [
23].
The pathogenetic effects of SARS-CoV-2 in the novel COVID-19 infection on the brain implies both direct and indirect harm. The goal of the research is to learn more about ischemic strokes in COVID-19 patients. Patients with COVID-19 need close monitoring of their coagulation system and aggressive thrombosis prevention. Providing ischemic stroke treatment in the context of COVID-19 adds to the organizational challenges, affecting intra-hospital logistics and care quality [
24]. Stroke patients with COVID-19 had a 9-fold poorer prognosis than those without it [
25]. SARS-CoV-2 induces COVID-19, a global condition. Swelling, cytokine storms, and an increase in heart injury biomarkers occur in the moderate and severe phases of infection. There may also be a link between COVID-19 and neurological problems. After being admitted to the Reanimation Unit, two patients with severe COVID-19 infection died. Moderate to severe COVID-19 patients should get high-dose antithrombotic prophylaxis [
26].
COVID-19 seems to cause neurological complications, including bleeding and infarction. Individuals who are at high risk of coagulopathy should be investigated, but the risk of bleeding must be considered. Patients with COVID-19 should also have stringent blood pressure management. Acute cerebral infarction in COVID-19 individuals should be treated with thrombolytics [
27].
According to reports, patients with COVID-19 have suffered from benign intracranial hypertension, seizures, ischemic and hemorrhagic cerebrovascular disorders, acute necrotizing encephalopathy, meningitis, and delirium. SARS-CoV-2 involvement in the central nervous system may worsen neurodegenerative problems in the future. The SARS-CoV-2 virus may harm the CNS. In turn, this leads to more severe symptoms and a higher chance of negative consequences [
28]. SARS-CoV-2 has been shown to infect the CNS. The existing research on its neurological symptoms and pathological processes has not been systematic.
Patients may present with encephalopathy, encephalitis, seizures, cerebrovascular events, acute polyneuropathy, headache, hypogeusia, hyposmia, and other non-specific symptoms. However, they may indicate direct SARS-CoV-2-related neuronal injury [
29]. COVID-19 has been linked to ischemic strokes. There is an increasing indication that COVID-19 effects extend beyond the lungs. Cerebrovascular disease is one of the most common neurologic symptoms [
30].
The following aspects of cerebrovascular disease in COVID-19 patients will be discussed next: imaging, histology, and clinical features. There is a 1.4% incidence of cerebral vascular accident (CVA) in COVID-19 patients with substantial morbidity and death. Endotheliopathy with a haemorrhagic propensity has been linked to thrombotic microangiopathy [
31]. SARS-CoV-2 infection may cause significant and multi-site vascular involvement. Results were classified as “relevant” or “other/incidental,” indicating the necessity for prompt patient care and treatment. The study groups were compared using the Student T-test, Mann-Whitney U-test, or Fisher exact test. Vascular involvement is not overlooked in COVID-19 and may have a substantial impact on disease behavior and prognosis [
32].
The top protein of SARS-CoV-2 interacts with the ACE2 receptor. Twelve nucleotides are inserted into the S-polyphasic protein’s furin cleavage side at S1/S2 to slow down six receptor-binding domain (RBD) amino acids. Children with severe COVID-19 infections may benefit from angiotensin II receptor blockers [
33].
Brainstem perivascular lymphocytic infiltration and microthrombi are common neurological consequences in COVID-19 patients, although little neuropathological research exists. A recent case report identified neocortical infarcts with tiny haemorrhagic and non-haemorrhagic white matter lesions, suggesting an evolving pattern of distinctive changes, which were also detected radiologically [
34].
COVID-19 targets the lungs, causing an abrupt respiratory collapse. COVID-19 neurological symptoms include strokes, encephalitis, and neuropathy. The aspects of multiple sclerosis patient treatment during the COVID-19 epidemic are described [
35]. Although SARS-CoV-2 usually affects the lungs, neurological symptoms are becoming more common. In three case studies of brain autopsies, no consistent pathobiological mechanism of CNS involvement in severe COVID-19 has been documented. Acute strokes, herniation, or olfactory bulb injuries were not seen [
36].
Neuronal degeneration in COVID-19 individuals includes non-specific alterations in nerve cells. COVID-19 victims’ brain histopathology shows CNS impairment. Hypoxia from respiratory failure and individual risk factors including cerebrovascular atherosclerosis and hypertension induce ischemic strokes in COVID-19 patients [
37]. SARS-CoV-2, which produced COVID-19, was recently found in postmortem neural and capillary endothelial cells of the frontal lobe of a patient. Seizures and early Guilli-an-Barre-like symptoms in SARS-CoV-2 patients have prompted concerns regarding the possible effects of SARS-CoV-2 neuroinvasion. The pathogenesis of SARS-CoV-2 infection is unknown [
38]. Two COVID-19 patients had structural brainstem injuries. Two COVID-19-dead patients and two COVID-19-negative patients were studied for neuropathological characteristics. Histopathological, CA/mm
2 and neuronal injury were measured. The medulla oblongata of COVID-19 patients had greater neuronal damage. SARS-CoV-2 in the brainstem and medullary damage in respiratory centers clearly imply a neurogenic component to COVID-19-related respiratory failure pathogenesis. This suggests viral trafficking between the brainstem and lungs [
39].
This entry is adapted from the peer-reviewed paper 10.3390/brainsci13010131