1. Spectral Detection Methods
Thinking over the kinds of constraints in each technique, it was sometimes not convincing to prove the existence of OVs with just one technique. In the early research, various electron microscopes and radiation instruments, such as UV-vis and XRD, were used to characterize OVs in perovskite. In recent years, more advanced techniques that included HRTEM and EELs were used to characterize and gain insight into OVs in perovskite catalyst.
1.1. X-ray Photoelectron Spectroscopy (XPS)
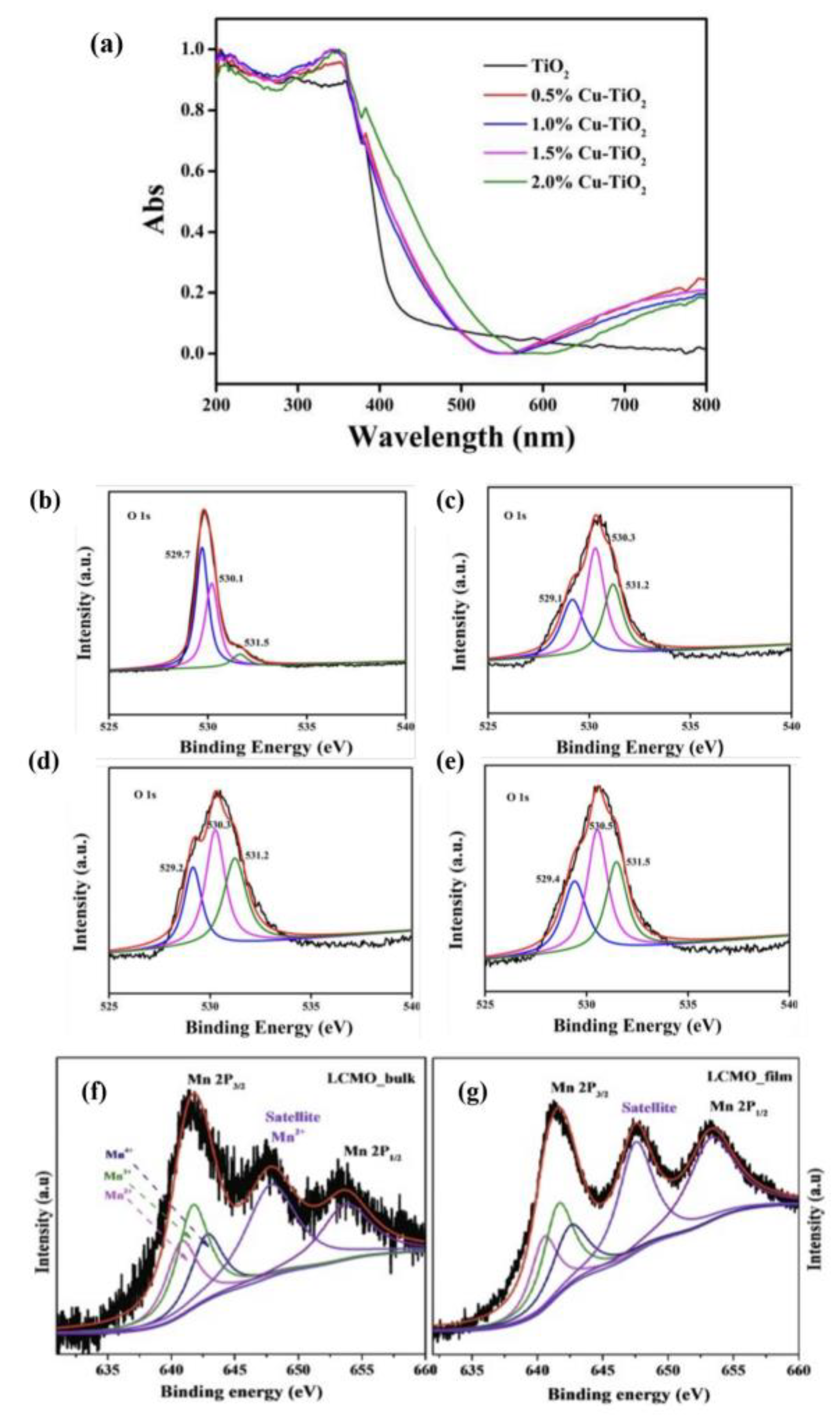
X-ray photoelectron spectroscopy (XPS). It was one of the most important characterization techniques for analyzing OVs, which was characterized by the difference in the atomic number ratio of metal ions to lattice oxygen. XPS and others were employed together to analyze the structures of perovskites and concentrations of OVs. TiO
2 was obtained with distinctive concentrations of OVs by synthesizing a series of TiO
2 samples with different Cu-doping concentrations by Liang et al.
[1]. Although pure TiO
2 could not absorb the visible light, UV-vis diffuse reflectance spectroscopy could easily detect the presence of OVs in TiO
2 samples. As the copper-doping concentration climbed, the intensity of absorption in the visible area increased, which led to the remarkable rising of the concentration of OVs (
Figure 1a). More information about OVs in Cu-doped TiO
2 samples was displayed in the O 1s XPS (
Figure 1b–e). By curve fitting, O 1s area precisely showed three peaks, which corresponded to lattice oxygen, surface hydroxyl oxygen, and adsorbed oxygen. They were located at 529.5, 530.5, and 531.5 eV.
Figure 1. (
a) UV-vis diffuse reflectance spectra of TiO
2 samples with different Cu loadings, XPS spectra of O 1s on the surface of (
b) TiO
2, (
c) 0.5% Cu-doped TiO
2, (
d) 1.5% Cu-TiO
2, and (
e) 2.0% Cu-doped TiO
2 samples, and XPS spectra of Mn 2p core level of (
f) LCMO bulk and (
g) LCMO film
[1][2].
Magray et al.
[2] used a solid–state method and a laser deposition method to prepare La
2CoMnO
6 bulk (LCMO-B) and La
2CoMnO
6 films (LCMO-F), respectively. For the purpose of detecting OVs in the above two perovskites, rapid responding XPS measurements were performed.
Figure 1f,g illustrates the Mn 2p XPS spectrum of LCMO-B and LCMO-F, respectively. Firstly, the background was used for the best fit, and then the normalized spectrum was used to figure out the charge states of Mn ions. Mn
4+, Mn
3+, and Mn
2+ peaks were fitted to the Mn 2p 3/2 peak. Manganese in the sample was present in the Mn
2+ state because of the satellite peak at 647.9 eV. This confirmed the presence of OVs in these samples. However, the peak intensity of Mn
3+ and Mn
2+ in LCMO-F was higher than that in LCMO-B, indicating that LCMO-F had more OVs. XPS detection technology could reflect the presence of OVs from the side, rather than directly detect the concentration.
1.2. Raman Spectroscopy
Raman spectroscopy was widely applied in studying various defects, such as OVs caused by ion doping and so on, because the characterization method was high-efficiency and sensitive to the structure and bond order of perovskites
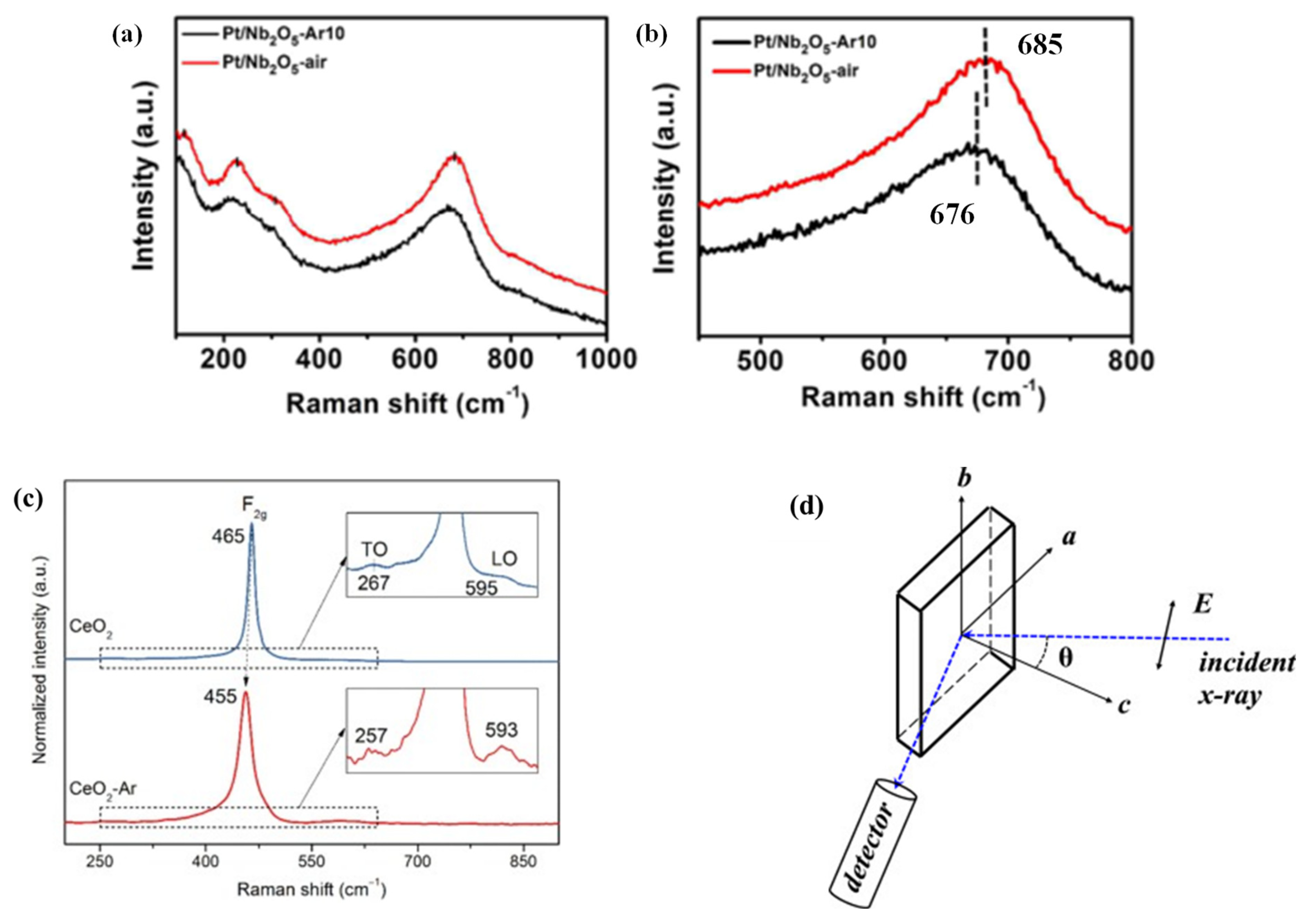
[3]. For example, the chemical coordination structures of Pt/Nb
2O
5-AR
10 (PNO-A) and Pt/Nb
2O
5-air (PNO-a) were studied by Raman spectroscopy. The peaks after several times magnification are shown
Figure 2a,b. It could be clearly seen that in PNO-A, the peak concentrated at 685 cm
−1, while the peak of PNO-a shifted to 676 cm
−1, which could generate more OVs under Ar reaction.
Figure 2. (
a) Raman spectra of the representative Pt–based catalysts and (
b) magnification of the peaks located at 450–800 cm
−1. (
c) Raman spectra of the CeO
2 and CeO
2-Ar catalysts. (
d) Schematic diagram of the polarized EXAFS measurement geometry
[3][4][5][6].
The influence of OVs on NO selective reduction by CeO
2 was also investigated. Zhang et al.
[4] found that the OV of CeO
2 roasted in the Ar atmosphere was higher than that of the roasting in ambient air.
Figure 2c illustrates the Raman spectra of the two catalysts. Usually, the strain and the defect in the lattice caused the Raman band position and shape of the F2g mode at 465 cm
−1. The F2g oscillation frequency of the CeO
2-AR catalyst was changed to 455 cm
−1. The sample was calcined at 600 °C and then cooled to room temperature. Therefore, no residual strain was found in the lattice of CeO
2. Therefore, the inferred Raman band position shift might indicate that there were OVs in CeO
2-AR.
1.3. The X-ray Absorption Microstructural Spectrum (XAFS)
XAFS was developed on the basis of synchrotron radiation. The excited photoelectrons were dispersed by the surrounding atoms as X-rays passed through a sample, which resulted in an energy oscillation. The local electronic and geometric formation of the detected specimen could be obtained by detecting these oscillatory indicators
[5].
The location of OVs of Sr
2CuO
3+δ was developed in a high-temperature superconductor by polarized extended X-ray absorption fine structure (EXAFS) by Wang et al.
[6] The characterization analysis was that OVs were distributed in the material plane and apical position, about 10% and 8%, respectively. By rotating Sr
2CuO
3+δ around the b axis, polarized EXAFS at different incident angles was collected (
Figure 2d), and the DFT was used in the calculation.
Xie et al.
[7] used EXAFS to collect the information of SrTiO
3 (STO) doped with NiP. They concluded that the ratio of OVs in NiP/STO-60 was the highest among all samples; then, the order of oxygen octahedron structure was affected. The STO doped with NiP could gently adjust the relative concentrations of OVs, lattice oxygen, and adsorbed oxygen and significantly improve the catalytic hydrogen evolution activity due to the oxygen balance.
2. Electron Paramagnetic Resonance
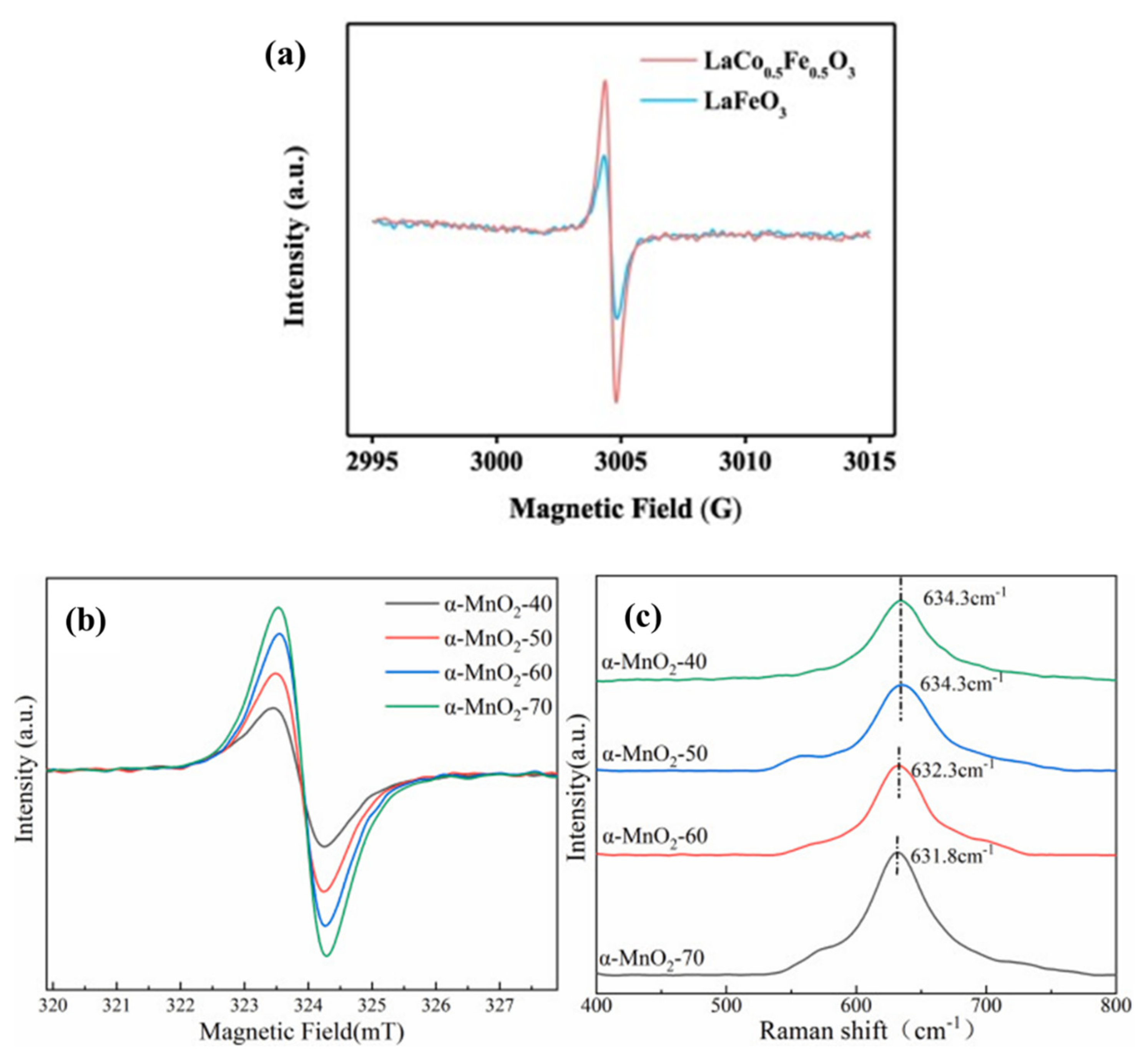
EPR was a kind of magnetic resonance technology that could be used to detect the unpaired electrons contained in atoms or molecules of matter qualitatively and quantitatively. Then, the structural properties of the surrounding environment could be explored. Jing et al.
[8], respectively, synthesized LaFeO
3 (LFO) and 3D micron globular LaCo
0.5Fe
0.5O
3 (LCFO) by the hydrothermal method. Both of these perovskites were used to activate PMS to degrade BPA, and the electron spin resonance (ESR) spectra of the two were compared to detect OVs (
Figure 3a). LCFO exhibited stronger signal intensities due to electron capture sites on OVs. The peak intensity was proportional to the concentration of OVs, which also indicated that there were more OVs in the LCFO material. OVs in LCFO could be increased by ion co-doping in a LFO structure, thereby enhancing the activation ability of PMS
[9].
Figure 3. (
a) LaCo
0.5Fe
0.5O
3 in LaCo
0.5Fe
0.5O
3/PMS system and ESR spectra representing oxygen vacancies in LaFeO
3 and LaCo
0.5Fe
0.5O
3 and EPR (
b) and Raman (
c) profiles of different catalysts
[8][9][10].
The OVs in the various prepared MnO
2-x catalysts were characterized by EPR
[10]. Electron delocalization on OVs might result in more symmetric signal peaks. As can be seen from
Figure 3b,c, each catalyst had a symmetric EPR signal peak, indicating that there were certain OVs in each material. The changing trend of the signal peak intensity of different catalysts was as follows: MnO
2-1.8 (I
peak = 795) > MnO
2-2.0 (I
peak = 758) > MnO
2-1.65 (I
peak = 578) > MnO
2-1.5 (I
peak = 333) > MnO
2-1.8R (I
peak = 226). The content of OVs was directly reflected by the signal peak strength. When x = 1.8 in the prepared MnO
2-x sample, the signal strength of the catalyst was the strongest, suggesting that the MnO
2-1.8 catalyst had the highest OVs’ concentration. Conversely, when x = 1.8R, the signal strength of the catalyst was the lowest, which indicated that it possessed the least amount of OVs
[11].
Yang et al.
[12] utilized EPR to probe into the hydrothermal α-Fe
2O
3 film and explored the kinetic and thermodynamic properties of its photoanodes in the presence or absence of OVs, understanding the effect of OVs on the water oxidation of the film. The consequence was that the film had an obvious EPR signal. Along with the time of hydrothermal treatment, the signal intensity of the material strengthened, indicating the increase in OVs.
3. Kelvin Probe Force Microscope and Electron Microscopy
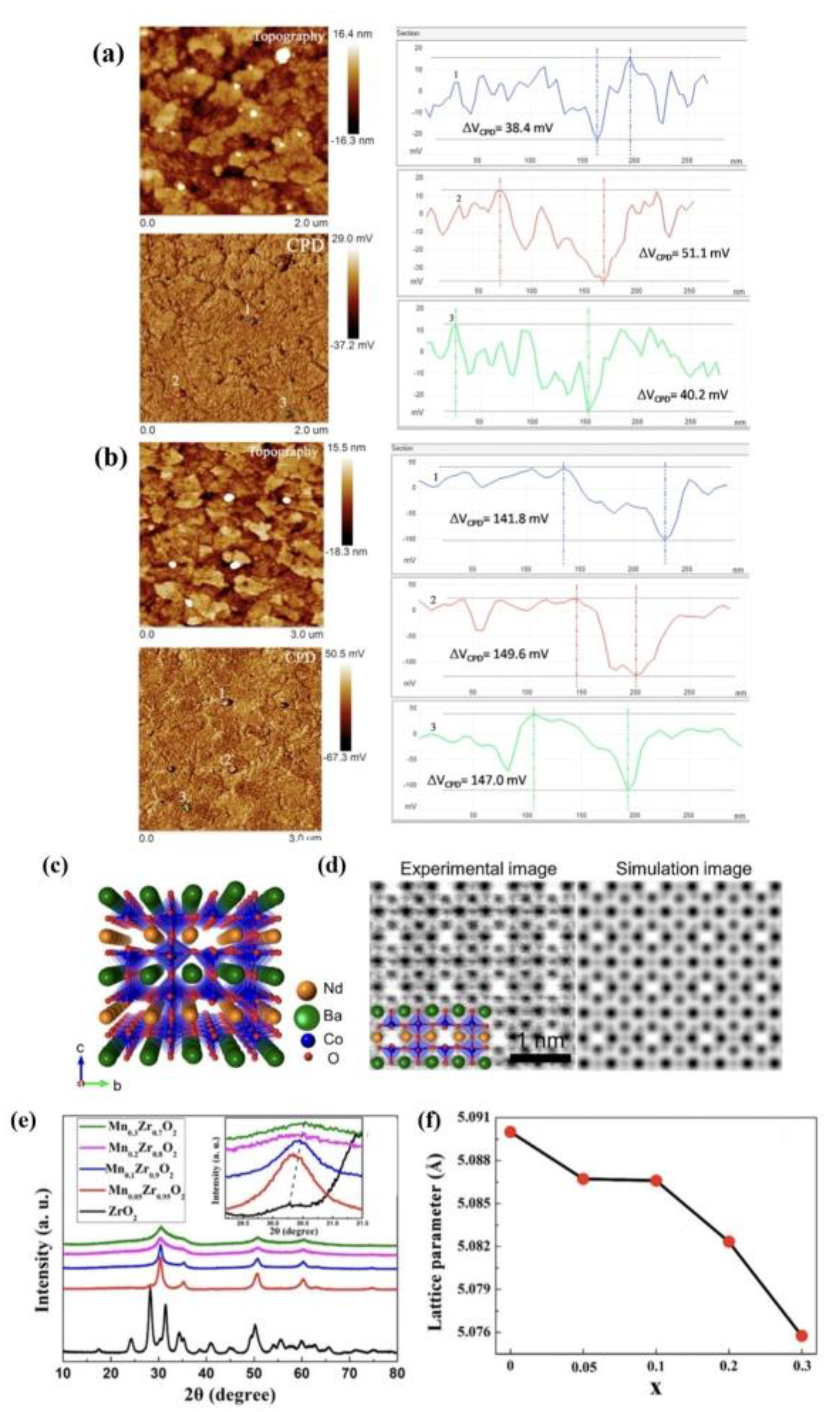
The Kelvin probe force microscope (KPFM) was one of the most widely used instruments, which was susceptive to detect the concentrations of local OVs. The electronic characterization of semiconductor surfaces also could be performed by it. The preparation of BiFeO
3 (BFO) and hydrogenated BFO (HB-180-2-8) on the ITO surface was measured simultaneously with KPFM, respectively, and the morphology and CPD images are illustrated in
Figure 4a,b. The variation of CPD cross-section variation values (ΔVCPD) was ~40 mV and ~140 mV, respectively, which were the original BFO and the hydrogenated BFO (HB-180-2-8); both them were clearly marked in CPD images with the marks of one, two, and three. The surface potential of unhydrogenated BFO nanocrystals was less than negative than that of hydrogenated BFO nanocrystals. The large number of OVs on the particle surface was due to the surface potential with a more negative shift of hydrogenated BFO, and these OVs could be used as active sites for electron capture
[13]. The BFO particle electronic structure surface was actually altered by the use of a hydrogenation method in the image of KPFM. In an effort to better study the distribution and kinetics of OVs in NdBaCo
2O
5.5, the oxygen ions and their occupied positions were analyzed by means of neutron powder diffraction (NPD) and annular dark-field scanning transmission electron microscopy (ADF-STEM). A schematic diagram of the crystal structure of NdBaCo
2O
5.5 (NBCO
5.5) in TEM image is shown in
Figure 4c. The image could be used to distinguish OVs and positions of oxygen atoms by the way of improving spatial resolution.
Figure 4d displays the ordered arrangement of OVs’ channels in the ND-O layer due to alternating the CoO
5 square cone and CoO
6 octahedron along the B-axis. ADF-STEM image simulation was carried out on the measured grid parameters and the optimized DFT model in order to demonstrate the experimental results. The image showed that OVs’ channels were empty, and the atomic columns of Nd, Ba and Co were murky, which was consistent with the experimental image primely
[14].
Figure 4. Topography and contact potential difference (CPD) images of as-prepared (
a) BFO and (
b) hydrogenated BFO (HB−180−2−8) nanoparticles on ITO surface measured by KPFM. The cross−se−tional line profiles of CPD are achieved along the lines indicated by numbers 1, 2, and 3 in CPD images and direct observation of oxygen vacancy channels in NdBaCo
2O
5.5 (NBCO
5.5) double perovskite. (
c) Schematic view of the structure of NBCO
5.5. (
d) ADF STEM and simulated images of NBCO
5.5 taken in the
[10] zone axis. (
e) HAADF image of NBCO
5.5 with the EDS elemental map of Nd, Ba, and Co. (
f) XRD patterns of MnxZr
1−xO
2 catalysts
[13][14][15].
It was confirmed that the addition of Mn2 ions into a ZrO2 lattice could lead to a large amount of OVs, which was probably one of the causes of the reduction in lattice parameters.
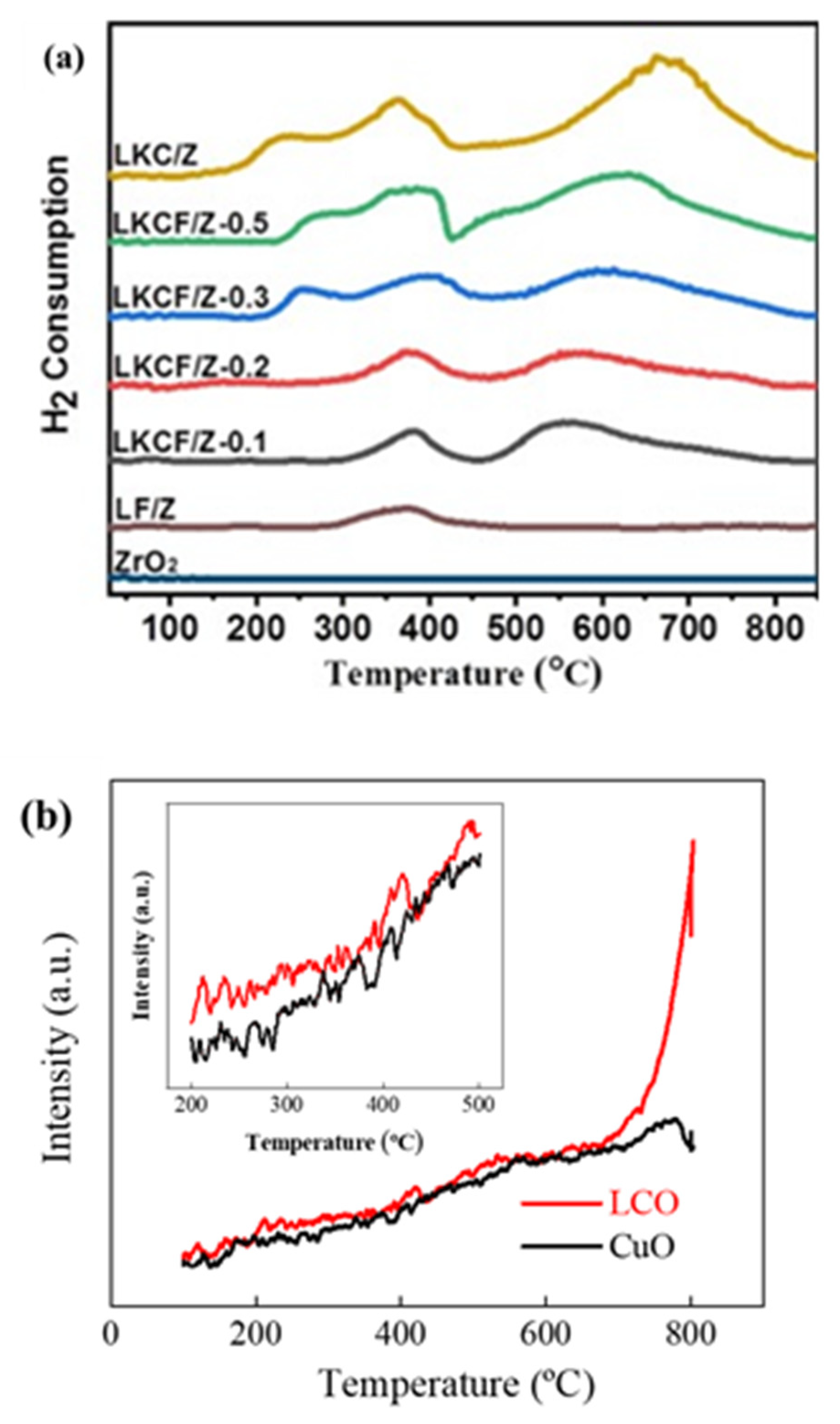
4. H2-TPR, O2-TPD
Temperature programmed desorption (TPD) was a technique used to measure the surface properties of an active center. Temperature programmed reduction (TPR) could measure the reducibility of a substance. La
0.9K
0.
1CoO
3/ZrO
2 (LKC/Z), LaFeO
3/ZrO
2 (LF/Z), and pure LaFeO
3 (Pure-LF) were prepared by Guo et al.
[16] The TPR profiles of all samples are shown in
Figure 5a. The reduction in Fe
4+ to Fe
3+ occurred in two places: one was a small H
2 consumption peak of LF/Z material at around 370 °C, and the other was a relatively obvious H
2 consumption peak, which appeared in the sample of x = 0.2. Another significant H
2 consumption peak appeared in the x = 0.1 sample due to the reduction in Co
3+ to Co
2+ at low temperature. The reduction in the above metal ions led to the formation of OVs on the surface of perovskite. The O
2-TPD spectra (
Figure 5b) of CuO and La
2CuO
4−δ (LCO) showed LCO had more surface chemisorbed oxygen because several of the main low-peak strengths of CuO were less than LCO at 800 °C
[17].
Figure 5. (
a) The TPR profiles of LKCF/Z-x (x = 0.1–0.3 and 0.5), LKC/Z, LF/Z and ZrO
2 after calcination
[16] and (
b) O
2-TPD profiles of LCO and CuO
[17].
5. Gravimetric Method
The gravimetric method was the way to determine the component content of the measured substance by weighing the mass of the substance. For the purpose of quantitatively studying the OVs’ concentration, the OVs-rich α-Fe2O3 was obtained by the gravimetric method. Three specimens were prepared on the basis that the molar ratios of α-Fe2O3 and tartaric acid were, respectively, 1/5, 1/7, and 1/10, expressed as S1, S2, and S3. The OVs-rich sample, in which mv was 1000 mg, was transformed into an OVs-free sample (mo) after oxygen annealing at 400 °C for 2 h. where Δmx was the evaporation of α-Fe2O3 at 400 °C, and the weight of absorbed water was calibrated by repeated blank tests.
In the absence of OVs, the mean weight loss of α-Fe
2O
3 (Δ
mx) was −3.4. For OVs-rich α-Fe
2O
3 (Δ
m), S2 and S3 were, respectively, +1.4 mg and −2.0 mg. As a result, S2 and S3 had an OVs mass (Δ
m − Δ
mx) of +4.8 mg and +1.4 mg. Then, in accordance with Equation (2), S3 and S2 were calculated to be 1.6% and 0.4%, respectively. The results for S1 and S4 were not clear, mainly due to the fact that they were too low to weigh (<0.1%), which exceeded the capacity of the device
[18]. Other methods for the quantitative detection of OVs according to different concentrations were also studied
[19].
This entry is adapted from the peer-reviewed paper 10.3390/catal13010148