Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The term “rheology” stands for the study of a material’s flow behavior under applied deformation forces or stress. The RPR method is considered a “pollution-less method” to prepare any metal oxides with high crystallinity, phase purity, and fewer agglomerations depending on the proper raw materials and the right temperature conditions are being chosen.
- rheological phase reaction
- metal oxides
- battery
1. Introduction
Metal oxides are an extremely important class of materials from both scientific and technological viewpoints. Hence the different approaches to making metal oxides both in bulk and nano size have become a vital area of research. Several methods, such as sol-gel, hydrothermal, reflux method, ball milling, etc., have been utilized for the preparation of metal oxides [1][2][3][4]. But each method has serious limitations, for example, not being suitable for mass production, expensive, time consuming, composed of toxic materials, and others. Apart from these limitations, the major concern about all the above methods of preparation is the environmental pollution they cause. Environment pollution happens when the contaminated solvents utilized in the metal oxide preparation experiments are exposed to their surroundings. Whereas metal oxides are utilized for the removal of heavy metal ions [5], dye degradation [6], energy storage devices [7], solar cells [8], and other applications, the method of preparing metal oxides by themselves should not harm the environment.
On the other hand, research activities on metal oxide preparations cannot be stopped as they are the major steps in achieving high efficiency in a particular process such as dye degradation or the production of high-performance devices. The material preparation plays a major role in determining the performance and as well as the cost of the final devices. The preparation method influences the properties of metal oxides such as phase purity, particle size, surface area, and others, where the metal oxides utilized in lithium ion batteries (LIBs) is just one of the examples. LIB has applications for all portable electronic devices such as mobile phones, laptops, etc. But still, improvements in energy density and power density are always needed owing to customer expectations. There is a growing demand for high-performing LIB for electric vehicles. To increase the performance and efficiency of LIBs utilized in electronically powered devices and electric vehicles [9][10], the cathode materials such as LiCoO2, LiMnO2, LiCoPO4, LiMn2O4, LiNiO2, LiFePO4, Li2FeSiO4, and Li3V2(PO4)3 have been explored by preparing them through various preparation routes [11][12][13][14][15][16][17][18]. Among the several methods such as solid-state reaction [19], sol-gel [20], hydrothermal [21], rheological phase reaction (RPR) [22], ultrasonic treatment [23], freeze-drying [24], microwave-assisted sol-gel [25], and spray pyrolysis [26], the RPR method was found to be very promising as an eco-friendly mass production method and meets the industrial demands for preparation of metal oxides for high performance LIBs.
Lithium trivanadate LiV3O8 (LVO) is the highly explored material prepared by the RPR method. Since most of the above cathode materials for LIBs have practical capacities lower than 200 mAh/g [10], LiV3O8, proposed by Wadsley in 1957, rapidly developed among them due to its high theoretical capacity of 352.5 mAh/gas a cathode for the lithium ion battery [27][28]. At the same time, LVO exhibits serious limitations such as fast capacity decay, multiple plateau regions during the charge/discharge process, growth of dendrite, irreversible phase transition, and dissolution of LVO grains during cycling [10]. Although the above limitations were successfully overcome by hetero-ions doping, conductive layer coating, and morphology tuning [22][29][30][31][32][33][34][35][36], still the preparation method adopted plays a crucial role. The phase purity and tuning the morphology of the particles by optimizing the calcination temperatures and time have a major impact [37][38][39][40][41].
Although there are plentiful review reports on different types of cathode materials for LIBs [9][39][40][41], only seldom are there reports on the review of vanadium oxides for LIBs [42], and particularly the RPR’s method of reviewing metal oxides is not a focus in any of the reports.
2. What Is Meant by the Rheological Phase Reaction (RPR) Method?
The term “rheology” stands for the study of a material’s flow behavior under applied deformation forces or stress. Every material has a property known as viscoelasticity, which indicates both the viscous and elastic portions. If the material is more viscous, it is a liquid; if it is more elastic, it is solid. A proper mixture of metal oxides with appropriate water or organic solvent provides a viscoelastic nature, a property similar to honey. This is called the solid-liquid rheological mixture. In this nature, there is close contact with the reactants and any external heat applied will be uniformly distributed among the reactants with no overheating.
The process of preparing compounds from the solid-liquid rheological mixture through chemical reactions is called the Rheological Phase Reaction (RPR) method. Initially, the stoichiometric molar ratios of the solid reactants (raw materials) are mixed thoroughly using mortar and pestle. Later, the solid–liquid rheological mixture is obtained by adding an appropriate amount of water or other organic solvents (one or two drops) to the well mixed solid reactants, in which the solid particles and liquid substance are uniformly distributed. The resultant mixture will be smooth and viscoelastic as the honey (Figure 1a–c). Finally, the obtained rheological body is transferred to the high pressure reactor and kept at a constant temperature and pressure for an appropriate time. The rheological body collected from the high pressure reactor is then dried in a petri dish for the desired time until the dry powders are obtained. The smooth powders are then calcinated at the desired temperature and time to obtain the required compounds. The schematic illustration of rheological phase reaction method is shown in Figure 1d.
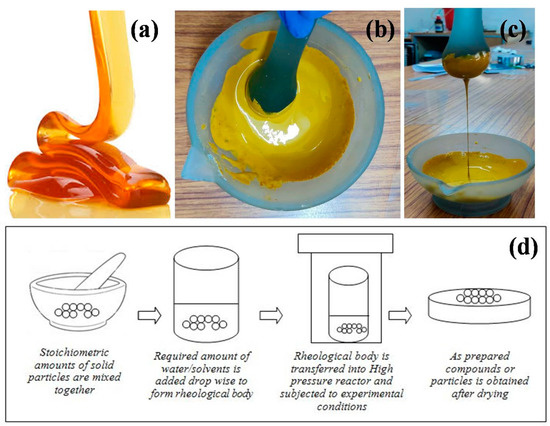
Figure 1. (a) Viscoelastic honey (b,c) a rheological mixture from the laboratory; (d) Schematic illustration of rheological phase reaction method.
Although both the water and organic solvents could be used as the rheological liquid, when water is used as the solvent, there is a great chance that the product may be agglomerated; as the use of an organic solvent is supposed to decrease the particle size. Still, the researchers prefer water as the solvent due to its eco-friendly nature and can overcome the agglomerations through inventive steps. Hence, the RPR method could be classified as the aqueous RPR and non-aqueous RPR method based on the solvent chosen. Usually, methods such as coprecipitation, sol-gel, hydrothermal, etc., when using high amounts of water or organic solvents are regarded as the best methods for making nanomaterials for mass production. However, all these preparation routes utilize a high volume of water or organic solvents. As a result, the waste water or solvents containing transition metal ions, and other groups such as sulphides, chlorine, fluorine, etc. are harmful to the land and aquatic bodies. In this perspective, the RPR method is considered a “pollution-less method” to prepare any metal oxides with high crystallinity, phase purity, and fewer agglomerations depending on the proper raw materials and the right temperature conditions arebeing chosen.
This entry is adapted from the peer-reviewed paper 10.3390/en16020841
References
- Dhas, S.; Maldar, P.; Patil, M.; Waikar, M.; Sonkawade, R.; Moholkar, A.V. Sol-gel synthesized nickel oxide nanostructures on nickel foam and nickel mesh for a targeted energy storage application. J. Energy Storage 2022, 47, 103658.
- Yewale, M.; Jadhavar, A.; Kadam, R.; Velhal, N.; Nakate, U.; Teli, A.; Shin, J.; Nguyen, L.; Shin, D.; Kaushik, N. Hydrothermal synthesis of manganese oxide (Mn3O4) with granule-like morphology for supercapacitor application. Ceram. Int. 2022, 48, 29429–29437.
- Manafi, S.; Tazikeh, S.; Joughehdoust, S. Synthesis and characterization of indium tin oxide nanoparticles via reflux method. Mater. Sci. Pol. 2017, 35, 799–805.
- Palem, R.; Shimoga, G.; Rabani, I.; Bathula, C.; Seo, Y.-S.; Kim, H.-S.; Kim, S.-Y.; Lee, S.-H. Ball-milling route to design hierarchical nanohybrid cobalt oxide structures with cellulose nanocrystals interface for supercapacitors. Int. J. Energy Res. 2022, 46, 8398–8412.
- Rajput, A.; Sharma, P.; Yadav, V.; Gupta, H.; Kulshrestha, V. Synthesis and characterization of different metal oxide and GO composites for removal of toxic metal ions. Sep. Sci. Technol. 2019, 54, 426–433.
- Sumantha, H.; Rajagopal, S.; Shashank, M.; Nagaraju, G.; Pattar, V.; Shanmugaraj, P.; Ayyasamy, S.; Suresha, B. Green synthesis and characterization of Mn3O4 nanoparticles for photocatalytic and supercapacitors. Ionics 2022.
- Veena, B.; Pavithra, S.; Seetha, M. Cr doped CeO2 nanoparticles as supercapacitor electrodes Cr doped CeO2 nanoparticles as supercapacitor electrodes. AIP Adv. 2022, 12, 125310.
- Chen, X.; Zhang, Z.; Fang, J.; Zhang, Y.; Zhao, C.; Wu, Y.; Li, W. TiO2 nanoparticles via simple surface modification as cathode interlayer for efficient organic solar cells. Org. Electron. 2022, 101, 106422.
- Eng, J.; Bensalah, N.; Dawood, H. Review on Synthesis, Characterizations, and Electrochemical Properties of Cathode Materials for Lithium Ion Batteries. J. Mater. Sci. Eng. 2016, 5.
- Xie, L.; Ge, P.; Zhu, L.; Cao, X. Stabilization of LiV3O8 Rod-like Structure by Protective Mg3(PO4)2Layer for Advanced Lithium Storage Cathodes. Energy Technol. 2018, 6, 2479–2487.
- Ying, J.; Jiang, C.; Wan, C. Preparation and characterization of high-density spherical LiCoO2 cathode material for lithium ion batteries. J. Power Sources 2004, 129, 264–269.
- Xu, H.; Sun, J.; Gao, L. Hydrothermal synthesis of LiMnO2 microcubes for lithium ion battery application. Ionics 2013, 19, 63–69.
- Huang, X.; Ma, J.; Wu, P.; Hu, Y.; Dai, J.; Zhu, Z.; Chen, H.; Wang, H. Hydrothermal synthesis of LiCoPO4 cathode materials for rechargeable lithium ion batteries. Mater. Lett. 2005, 59, 578–582.
- Kim, D.; Muralidharan, P.; Lee, H.; Ruffo, R.; Chan, C.; Peng, H.; Huggins, R.; Cui, Y.; Kim, D.; Muralidharan, P.; et al. Spinel LiMn2O4 Nanorods as Lithium Ion Battery Cathodes. Nano Lett. 2008, 8, 3948–3952.
- Wang, G.; Zhong, S.; Bradhurst, D.; Dou, S.; Liu, H. Synthesis and characterization of LiNiO2 compounds as cathodes for rechargeable lithium batteries. J. Power Sources 1998, 76, 141–146.
- Higuchi, M.; Katayama, K.; Azuma, Y. Synthesis of LiFePO4 cathode material by microwave processing. J. Power Sources 2003, 121, 258–261.
- Abouimrane, A.; Armand, M. Electrochemical performance of Li2FeSiO4 as a new Li-battery cathode material. Electrochem. Commun. 2005, 7, 156–160.
- Gaubicher, J.; Wurm, C.; Goward, G. Rhombohedral Form of Li3V2(PO4)3 as a Cathode in Li-Ion Batteries. Chem. Mater. 2000, 2, 3240–3242.
- Wu, W.; Ding, J.; Peng, H.; Li, G. Synthesis and electrochemical properties of single-crystalline LiV3O8 nanobelts for rechargeable lithium batteries. Mater. Lett. 2011, 65, 2155–2157.
- Liu, X.; Wang, J.; Zhang, J.; Yang, S. Sol–gel template synthesis of LiV3O8 nanowires. J. Mater. Sci. 2007, 42, 867–871.
- Yan, H.; Wang, H.; Qiang, Z. Novel chemical method for synthesis of LiV3O8 nanorods as cathode materials for lithium ion batteries. Electrochim. Acta 2004, 49, 349–353.
- Feng, C.; Chew, S.; Guo, Z.; Wang, J.; Liu, H. An investigation of polypyrrole—LiV3O8 composite cathode materials for lithium-ion batteries. J. Power Sources 2007, 174, 1095–1099.
- Kumagai, N. Ultrasonically Treated LiV3O8 as a Cathode Material for Secondary Lithium Batteries. J. Electrochem. Soc. 1997, 144, 830.
- Huang, S.; Lu, Y.; Wang, T.; Gu, C.; Wang, X.; Tu, J. Polyacrylamide-assisted freeze drying synthesis of hierarchical plate-arrayed LiV3O8 for high-rate lithium-ion batteries. J. Power Sources 2013, 235, 256–264.
- Wu, F.; Wang, L.; Wu, C.; Bai, Y.; Wang, F. Study on Li1+xV3O8 synthesized by microwave sol–gel route. Mater. Chem. Phys. 2009, 115, 707–711.
- Ju, S.; Kang, Y. Electrochimica Acta Morphological and electrochemical properties of LiV3O8 cathode powders prepared by spray pyrolysis. Electrochim. Acta 2010, 55, 6088–6092.
- Fang, D.; Cui, M.; Bao, R.; Yi, J.; Luo, Z. In-situ coating polypyrrole on charged BiVO4 nanowire arrays to improve lithium-ion storage properties. Solid State Ionics 2020, 346, 115222.
- Scientific, C. Crystal chemistry of non-stoichiometric pentavalent vandadium oxides: Crystal structure of Li1+xV3O8. Acta Crystallogr. 1957, 10, 261–267.
- Pistoia, G.; Wang, G.; Zane, D. Mixed Na/K vanadates for rechargeable Li batteries. Solid State Ionics 1995, 76, 285–290.
- Feng, Y.; Li, Y.; Hou, F. Preparation and electrochemical properties of Cr doped LiV3O8 cathode for lithium ion batteries. Mater. Lett. 2009, 63, 1338–1340.
- Song, H.; Liu, Y.; Zhang, C.; Liu, C.; Cao, G. Mo-doped LiV3O8 nanorod-assembled nanosheets as a high performance cathode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 3547–3558.
- Jiao, L.; Li, H.; Yuan, H.; Wang, Y. Preparation of copper-doped LiV3O8 composite by a simple addition of the doping metal as cathode materials for lithium-ion batteries. Mater. Lett. 2008, 62, 3937–3939.
- Zhao, M.; Jiao, L.; Yuan, H.; Feng, Y.; Zhang, M. Study on the silicon doped lithium trivanadate as cathode material for rechargeable lithium batteries. Solid State Ionics 2007, 178, 387–391.
- Feng, Y.; Li, Y.; Hou, F. Boron doped lithium trivanadate as a cathode material for an enhanced rechargeable lithium ion batteries. J. Power Sources 2009, 187, 224–228.
- Fang, H.; Mo-ran, S.; Yu-sheng, W.; Chun-hua, Z. The synthesis and electrochemical performance of LiV3O8 cathode with Lanthanum-doped. Adv. Mater. Res. 2012, 1, 860–863.
- Ng, S. Low-temperature synthesis of polypyrrole-coated LiV3O8 composite with enhanced electrochemical properties. J. Electrochem. Soc. 2007, 154, 3–9.
- Liu, Q.; Liu, H.; Zhou, X.; Cong, C.; Zhang, K. A soft chemistry synthesis and electrochemical properties of LiV3O8 as cathode material for lithium secondary batteries. Solid State Ionics 2005, 176, 1549–1554.
- Qiao, Y.; Wang, X.; Zhou, J.; Zhang, J.; Gu, C.; Tu, J. Synthesis and electrochemical performance of rod-like LiV3O8 cathode materials for rechargeable lithium batteries. J. Power Sources 2012, 198, 287–293.
- Daniel, C.; Mohanty, D.; Li, J.; Wood, D.; Daniel, C.; Mohanty, D.; Li, J.; Wood, D. Cathode materials review. AIP Conf. Proc. 2015, 1597, 26.
- Xu, B.; Qian, D.; Wang, Z.; Meng, Y. Recent progress in cathode materials research for advanced lithium ion batteries. Mater. Sci. Eng. R Rep. 2012, 73, 51–65.
- Kraytsberg, A.; Ein-eli, Y. Higher, Stronger, Better…A Review of 5 Volt Cathode Materials for Advanced Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 922–939.
- Mai, L.; Xu, X.; Xu, L.; Han, C.; Luo, Y. Vanadium oxide nanowires for Li-ion batteries. J. Mater. Res. 2011, 26, 2175–2185.
This entry is offline, you can click here to edit this entry!
