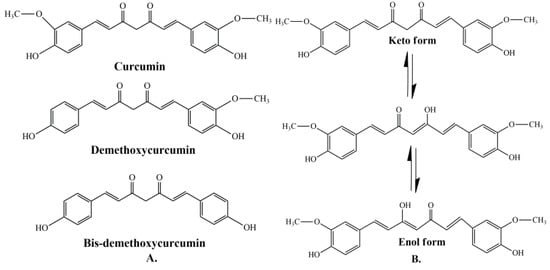
Curcuminoids are the major component of turmeric, which are responsible for its orange color.
Taken together, the biological and chemical features of curcumin exposed in the current context highlight the need to approach from a new thermodynamic perspective as well as bioavailability with respect to the pharmacological or biotechnological uses of curcumin.
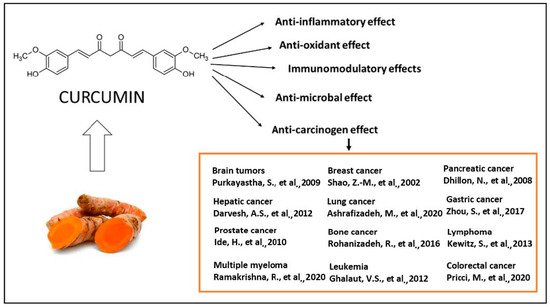
2. Biological Actions of Curcumin
2.1. General Bioactivities and Limitations
The majority of systemically administered synthetic drugs has undesirable side effects or toxicity and can spread to nontarget body parts. For this reason, it is desirable to use safe compounds that also present a good bioavailability. CCM is a hydrophobic polyphenol [
21], with numerous pharmacological properties (
Figure 2), such as antiproliferative, anticancer [
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33], antidiabetic, antioxidant [
34], and anti-inflammatory activities [
35,
36,
37] and antimicrobial properties [
38]. In general, the well-documented antiviral [
38,
39,
40], anti-inflammatory, and immunomodulatory effects [
41] of CCM, along with the evidence on anti-fibrotic [
42] and lung protective properties [
43] of this phytochemical in lung tissue, make it a likely molecule for the prevention and treatment of the infection with the SARS-CoV19 virus [
44]. The virus binds to the target cell receptor, the angiotensin converting enzyme 2 receptor (ACE2R). Research has proved that CCM inhibits this virus ACE2R by inhibiting the spike protein and the function of the ACE2 receptor [
45].
Figure 2. The therapeutical effects of CCM are reflected by a large spectrum of action, as reported by the literature. The antitumor action of CCM was extensively explored in clinical and experimental conditions [
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33] as well as the antioxidant [
34], anti-inflammatory [
35,
36,
37], antibacterial [
38,
39,
40] and immunomodulatory effects [
41].
CCM is a unique molecule due to its wide range of useful biological functions. Clinical studies have shown that a daily dose of 8 g (considered high) of CCM has no adverse effects on the human organism [
46].
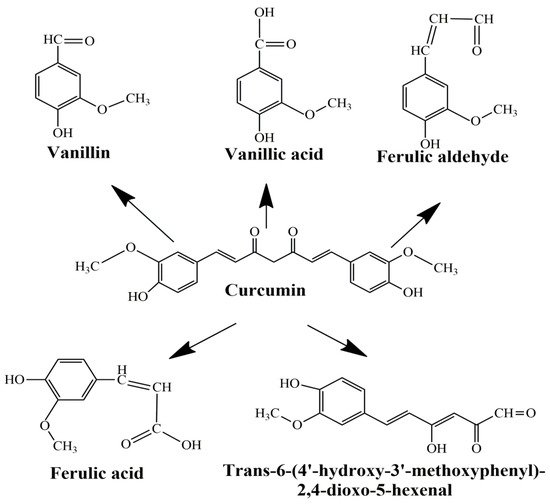
The main limitations of CCM are low water solubility, low absorption, rapid systemic elimination from the human body, degradation at alkaline pH, and limited oral bioavailability [
47,
48,
49]. Under alkaline conditions, CCM degrades to
trans-6-(40-hydroxy-30-methoxyphenyl)-2, 4-dioxo-5-hexanal, ferulic acid, feruloyl methane, and vanillin (
Figure 3) very quickly (~30 min) [
50,
51], while under acidic conditions, less than 20% of CCM was decomposed at 1 h. Only a small portion of CCM ingested orally is adsorbed in the intestine because a major part of CCM is excreted [
49]. In addition, the absorbed CCM went through a quick metabolism in the liver and plasma [
52]. Several studies show that decomposition products such as ferulic acid and vanillin also have antimicrobial, antiarrhythmic, antithrombotic, and antioxidant properties and can inhibit cancer development [
53,
54]. However, the biological activities of ferulic acid and vanillin are weaker compared to CCM [
55].
Figure 3. Product decomposition of curcumin in alkaline condition [
56,
57].
2.2. Antibacterial Properties
Although progress has been made over the years in the development of methods to treat infections caused by bacteria, the emergence of multi-resistant strains requires the study of new treatment methods. The aqueous extract obtained from the rhizome of
C. longa demonstrated antimicrobial effects. The minimum inhibitory concentrations (MIC) obtained had values between 4 and 16 g/L, and the minimum bactericidal concentrations (MBC) varied between 16 and 32 g/L against
S. epidermis, Staph. aureus, Klebsiella pneumoniae and
E. coli [
38,
58]. Although the methanolic extract showed good inhibitory effects against
S. aureus and
B. subtillis the best results were obtained using hexanes and ethanol turmeric extract and curcuminoids against 24 bacterial species, namely
Vibrio harveyi, V. alginolyticus, V. vulnificus, V. parahaemolyticus, V. cholerae, Bacillus subtilis, B. cereus, Aeromonas hydrophila, Streptococcus agalactiae, Staphilococcus aureus, S. intermedius, S. epidermidis, etc. [
38,
59]. A potential aim for antibacterials could be the stability and assembly of FtsZ protofilaments, an important factor for bacterial cytokinesis. By inducing, CCM abolished
B. subtilis cytokinesis and Z-ring formation. A possible antibacterial mechanism of action produced by CCM is the inhibition of FtsZ assembly on the Z-ring and the suppression of bacterial cells [
60].
In burn units, infections with
Pseudomonas aeruginosa are the most common, and the multi-resistance to antibiotics acquired by this microorganism causes a major problem, complicating the treatment of patients. Shariati et al. (2019) used the wet-milling method to synthesize CCM nanoparticles with sizes between 2 and 40 nm. This large surface area enhanced aqueous solubility and produced better inhibitory effects on MDR
P. aeruginosa than CCM extracts. These nanoparticles produced cell wall destruction, cell lysis, and death. It was demonstrated that nano-CCM produced a decrease in the number of genes responsible for the pathogenicity of the microorganism and the formation of biofilms. These effects were stronger than those caused by CCM due to the increased stability and solubility of nanoparticles [
61].
Furthermore, the increase in the number of antibiotic-resistant strains led to research on the synergistic effect of antibiotics in combination with plant extracts. Combining CCM with antibiotics such as ampicillin, oxacillin, ciprofloxacin, and norfloxacin showed synergistic effects against the methicillin-resistant
S. aureus strain, although the CCM-ciprofloxacin combination showed antagonistic effects against
S. typhi and
S. typhimuriumin [
62,
63].
2.3. Antifungal Effects
CCM also showed inhibitory effects on fungi. The methanolic extracts of
C. longa showed inhibitory activity toward
Cryptococcus neoformans (MIC-128 μg/mL) and
Candida albicans (MIC-256 μg/mL), the hexane extract of the plant at 1 g/L was proved to have antifungal proprieties in the case of
Rhizoctonia solani, Phytophthora infestans, and
Erysiphe graminis, and the ethyl acetate extract, at the same concentration, had an inhibitory effect towards
R. solani, P. infestans, P. recondita, and
Botrytis cinerea [
38,
64].
An in vivo work on guinea pigs infected with
T. rubrum confirmed that the cutaneous application of turmeric oil in a 1:80 dilution resulted in improved wound healing for a period of 2–5 days, and after 6–7 days the lesions disappeared [
58]. Additionally, turmeric oil proved to be efficient against pathogens such as
Sporothrix schenckii,
Exophiala jeanselmei,
Fonsecaea pedrosoi and
Scedosporium apiospermum that produce inhibitory effects [
38]. The foremost reason to examine the synergistic effect of CCM with the current biocidal chemical employed to eradicate parasitic fungi might be the potent antifungal properties of
C. longa alongside its reduced side effects. The synergistic activity of CCM with several drugs was studied: voriconazole, itraconazole, ketoconazole, miconazole, nystatin, amphotericin B, and fluconazole (for the last two drugs, this synergistic activity could be understood by ROS accumulation [
38]). These combinations exhibited a 10- to 35-time decrease in the MIC values of the fungicides toward 21 clinical isolates of
C. albicans [
65].
A plausible mechanism of cell death of
Candida species induced by CCM treatment could be acidification of the intracellular environment by inhibiting H
+ extrusion and targeting the global uptake suppressor thymidine 1, which resulted in the inhibition of hyphal development [
65,
66].
2.4. Antiviral Actions
Since most diseases caused by viruses do not have adequate treatments, or existing treatments are poorly tolerated or very expensive, researchers’ attention has turned to other treatment methods. Due to phytochemical compounds with multiple biological activities, plants were a subject of interest in these investigations [
38]. Among the most important effects produced against viruses is the effect of CCM against HIV (human immunodeficiency virus). Due to effects on the function of some viral proteins, counting viral integrase, protease, and transactivator of transcription protein (Tat), CCM can inhibit HIV replication. CCM can inhibit viral integrase by directly interacting with the catalytic center of the protein and, if the Tat protein is considered, CCM prompts protein degradation at doses between 20 and 120 µM [
40].
Curcumin-stabilized silver nanoparticles (CCM-AgNPs) have likewise been tested for their effectiveness toward HIV. When comparing the treatment of ACH-2 cells with CCM-AgNP with CCM alone, it was demonstrated that the nanoparticles were more effective in decreasing HIV-LTR expression even though CCM-AgNP did not entirely abolish the expression of HIV-1 p24 because the levels of the studied protein were constantly elevated during the infection. The expression of TNF-α (tumor necrosis factor alpha), interleukin-6, interleukin-1 β and nuclear factor kappa B decreased following CCM-AgNP treatment [
67].
CCM has shown activity against the PR8, H1N1, and H6N1 influenza viruses. The results of the in vitro tests showed a more than 90% decrease in virus production in cell culture using low concentrations of CCM. Curcumin and its products such as gallium-curcumin and copper-curcumin have also produced antiviral effects towards other RNA viruses such as: Zika virus, by directly inhibiting cell attachment or inactivating the virus; Dengue virus, through indirect inhibition by influence on cellular systems rather than in a direct way on viral functions; and HCV, by decreasing replication by overturning the Akt-SREBP-1 path [
58]. Against the hepatitis B virus, the
C. longa extract blocked its replication by rising the rate of p53 protein by improving protein stability and activating the transcription of the p53 gene [
38].
Human papillomaviruses (HPV) are also responsible for the appearance of cervical cancer, the viral oncoproteins E6 and E7 being responsible for this. CCM had a hindering activity towards the expressed genes of the E6 and E7 proteins of HPV-16 and HPV-18 as two of the main oncogenic HPV [
68]. CCM alongside its analogs is able to inhibit the replication of numerous viruses through various mechanisms. However, because it is rapidly metabolized, CCM has low bioavailability, thus reducing curcumin efficiency as an antiviral compound [
40].
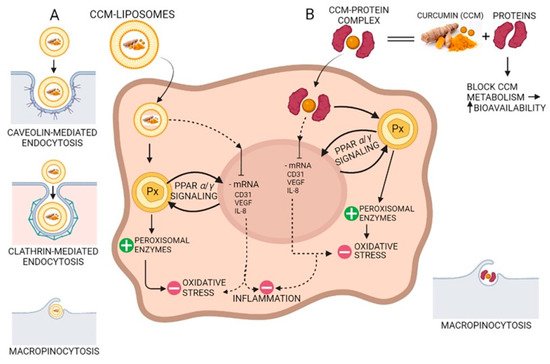
2.5. Antioxidant and Anti-Inflammatory Action of Curcumin
Due to the hydroxyl and methoxy groups, CCM can have antioxidant and anti-inflammatory effects. Acting on different molecular targets such as interleukins and cytokines, CCM inhibits the proliferation of inflammatory cells, metastases, and angiogenesis via CD31 (cluster of differentiation 31), VEGF (vein endothelial growth factor), and IL-8 (interleukin 8) signaling as shown in
Figure 4. CCM has proven to be efficient in the management of several inflammatory illnesses such as diabetes, obesity, and various neurological and cardiovascular diseases [
69]. Many inflammatory transcription factors, cytokines, and enzymes play a significant part in the onset and evolution of type II diabetes and the main cause of the development is inflammation. CCM can increase antihyperglycemic and insulin sensitivity by reducing glucose produced in the liver, decreasing the inflammatory response arising from hyperglycemia, increasing the expression of GLUT2, GLUT3, and GLUT4 genes, cellular glucose uptake, and activating AMPK [
70]. CCM increases adiponectin production, and decreases macrophage infiltration, leptin, and leptin receptor levels in the case of inflammation-related obesity. The increase in adiponectin production leads to a decrease in NF-kB activity, having a positive effect against obesity [
69]. CCM treatment activates the Nrf2-dependent antioxidant response, inhibits TNF-α in blood vessel smooth muscle cells, and increases p21 expression. A study conducted on individuals suffering from coronary heart disease proved that CCM can lower serum levels of triglycerides, LDL, and VLDL cholesterol, although no significant effects on inflammatory markers were observed [
71]. New aspects about the antioxidant effect of curcumin suggest that CCM stimulates the peroxisomal enzymes such as catalase, as a prominent antioxidant factor reducing the oxidative stress via the PPRA α/γ (peroxisome proliferator-activated receptor alfa/gamma) pathway (
Figure 4).
Figure 4. The antioxidant and anti-inflammatory action of curcumin (CCM). (A) CCM-liposomes are internalized in the cell by three routes: (i) caveolin-mediated or (ii) clathrin-mediated endocytosis, and also (iii) macropinocytosis. CCM targets the PPARα/γ (peroxisome proliferator-activated receptor alpha/gamma) signaling and potentiates peroxisome activity by increasing peroxisomal enzymes (e.g., catalase, superoxide-dismutase). Increasing the activity of peroxisomal-derived antioxidant enzymes leads to a reduction in reactive oxidative species (ROS) and a decrease in oxidative stress. Additionally, CCM inhibits mRNA of CD31 (cluster of differentiation 31), IL-8 (interleukin 8), and VEGF (vascular endothelial growth factor) with anti-inflammatory effects in the cell. (B) Complexes of CCM-proteins act on the peroxisome by increasing peroxisomal enzymes, thus decreasing oxidative stress by their scavenging action on the ROS. Furthermore, like CCM internalized by caveolin/clathrin-mediated endocytosis, the CCM-protein complex inhibits mRNA of CD31, IL-8, and VEGF, thereby demonstrating anti-inflammatory activity due to CCM increased bioavailability.
Curcumin also has a role in the treatment of some neurodegenerative disorders caused by oxidative or inflammatory effects. In the case of Alzheimer’s disease, CCM acts as an antioxidant, improving cognitive functions and delaying neuronal damage. Due to CCM’s inhibitory effect on inflammatory cytokines and the associated JAK-STAT, AP-1 and NF-kB signaling pathways, it can be used in the treatment of multiple sclerosis, a chronic inflammatory autoimmune disease that results in the degradation of the myelin sheath of neurons. CCM also suppresses Th17 cell differentiation, which is a significant factor in the development of multiple sclerosis [
69].
2.6. Antitumoral Effects of Curcumin
Curcumin has antitumor effects affecting two primary processes: tumor growth and angiogenesis. CCM has effects against angiogenesis through different mechanisms: acting on transcription factors NF-kB and AP-1; limiting the expression of IL-8 in pancreatic cancer and head and neck cancer cell lines; inhibiting COX-2 (cyclo-oxygenase-2) and 5-LOX (5-lipo-oxygenase); inhibiting angiogenesis mediated by NO and iNOS (inducible nitric oxide synthase). CCM induces cell death in several cancer cell lines, including melanoma, leukemia, lung, colon, ovarian, liver, and breast carcinomas. The induction of apoptosis is linked to the action of CCM on the proteasome. In small doses, CCM activates the proteasome leading to cell survival, while in high doses, it inhibits the proteasome, leading to apoptosis. In addition, the dose of CCM can affect the type of cell death. High doses reduce the fabrication of reactive oxygen species and cause cell death through necrosis, while small doses result in oxidative stress and apoptosis [
72].
An in vivo study performed in mice receiving a brain bolus of B16F10 melanoma cells showed that CCM effectively inhibited P-NF-kB, BclXL, cyclin D1, P-Akt and VEGF, elucidating its effectiveness in blocking proliferation, survival, and invasion of B16F10 cells in the nervous system [
22].
A study carried out in Wistar rats showed that CCM prevents the formation of hyperplastic liver nodules [
73]. Microcapsules containing curcumin exhibited high, dose-dependent cytotoxicity on HCT-116 colorectal carcinoma cell culture [
74]. Composites containing curcumin showed increased cytotoxicity in human breast carcinoma (MCF-7) and human lung adenocarcinoma (A549) [
75]. Topical application of CCM significantly inhibited the formation of papilloma in female CD-1 mice [
76].
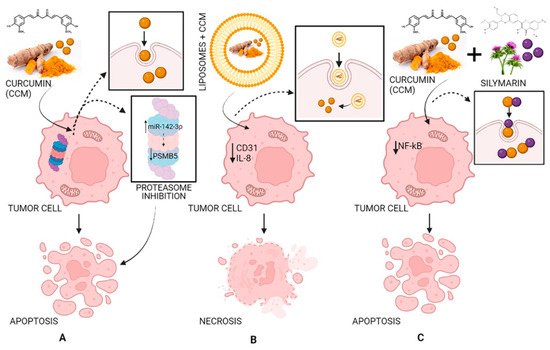
In a xenograft model study, pancreatic cancer cells were injected subcutaneously into female nude mice. It was demonstrated that liposomes loaded with CCM can have antitumor effects by decreasing the expression of CD31 as well as that of VEGF and IL-8, demonstrating that CCM blocked the evolution of pancreatic carcinoma in these types of models and inhibited tumor angiogenesis (
Figure 5) [
72,
77,
78].
Figure 5. The antitumor activity of CCM (A), of liposomes loaded with CCM (B), and of CCM complexes with silymarin (C). Strategy and pathway (A): CCM inhibits the proteasome by increasing miR-142–3p expression leading to negatively regulating PSMB5 (proteasome 20S subunit beta 5) leading to cell apoptosis; strategy and pathway (B): Liposomes loaded with CCM decrease the expression of CD31 (cluster of differentiation 31) and IL-8 (interleukin 8) leading to cell necrosis; strategy and pathway (C): CCM complexes with silymarin inhibit NF-κB (nuclear factor-κB) leading to cell apoptosis.