Soil salinity is one of the major abiotic constraints in agricultural ecosystems worldwide. High salinity levels have negative impacts on plant growth and yield, and affect soil physicochemical properties. Salinity also has adverse effects on the distribution and abundance of soil microorganisms. Halotolerant plant growth-promoting rhizobacteria (HT-PGPR) secrete secondary metabolites, including osmoprotectants, exopolysaccharides, and volatile organic compounds. The importance of these compounds in promoting plant growth and reducing adverse effects under salinity stress has been widely recognised. HT-PGPR are emerging as effective biological strategies for mitigating the harmful effects of high salinity; improving plant growth, development, and yield; and remediating degraded saline soils.
- exopolysaccharides
- osmoprotectants
- growth hormones
- soil microbes
- volatile organic compounds
1. Introduction
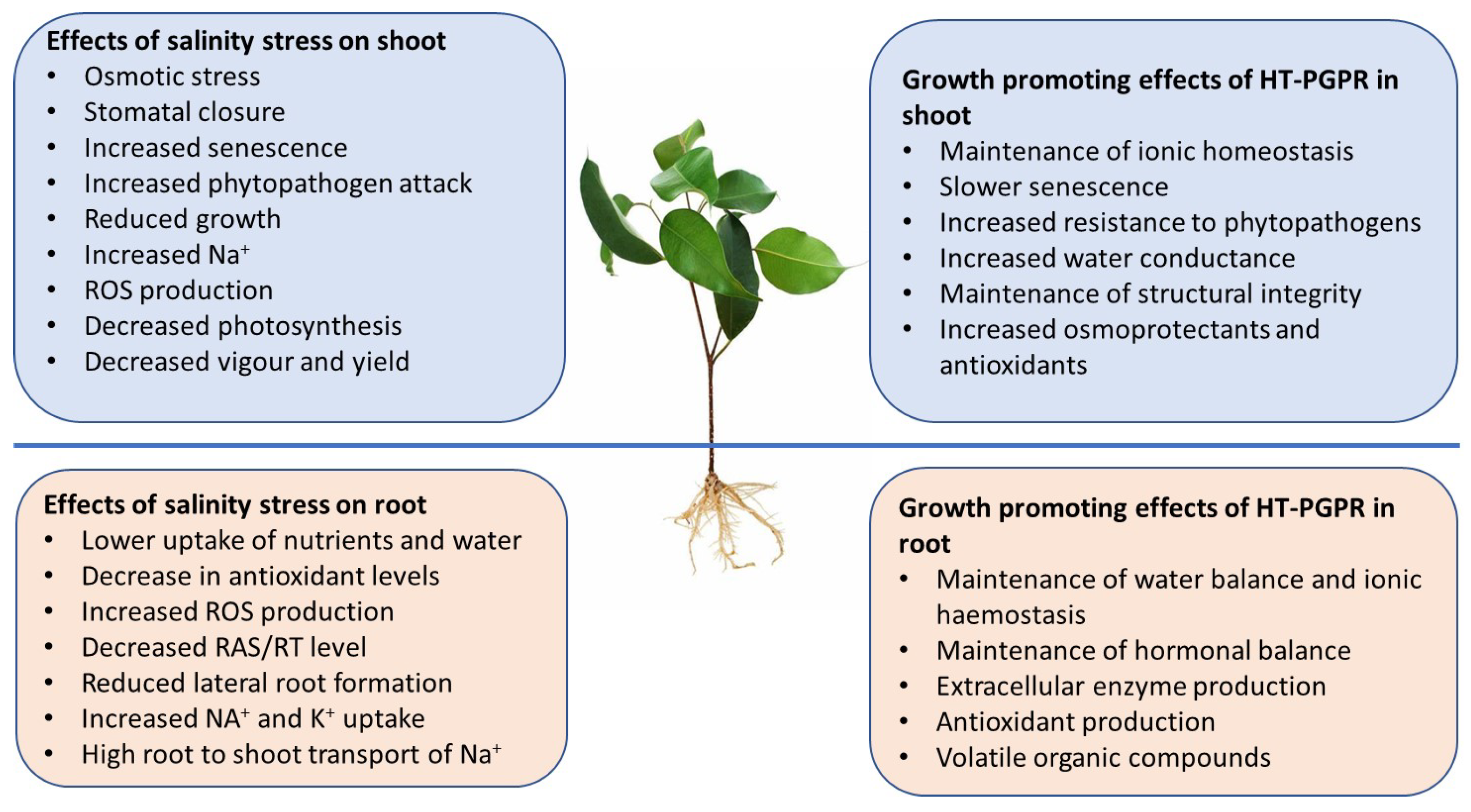
2. HT-PGPR: Diversity and Their Effect on Crop Production
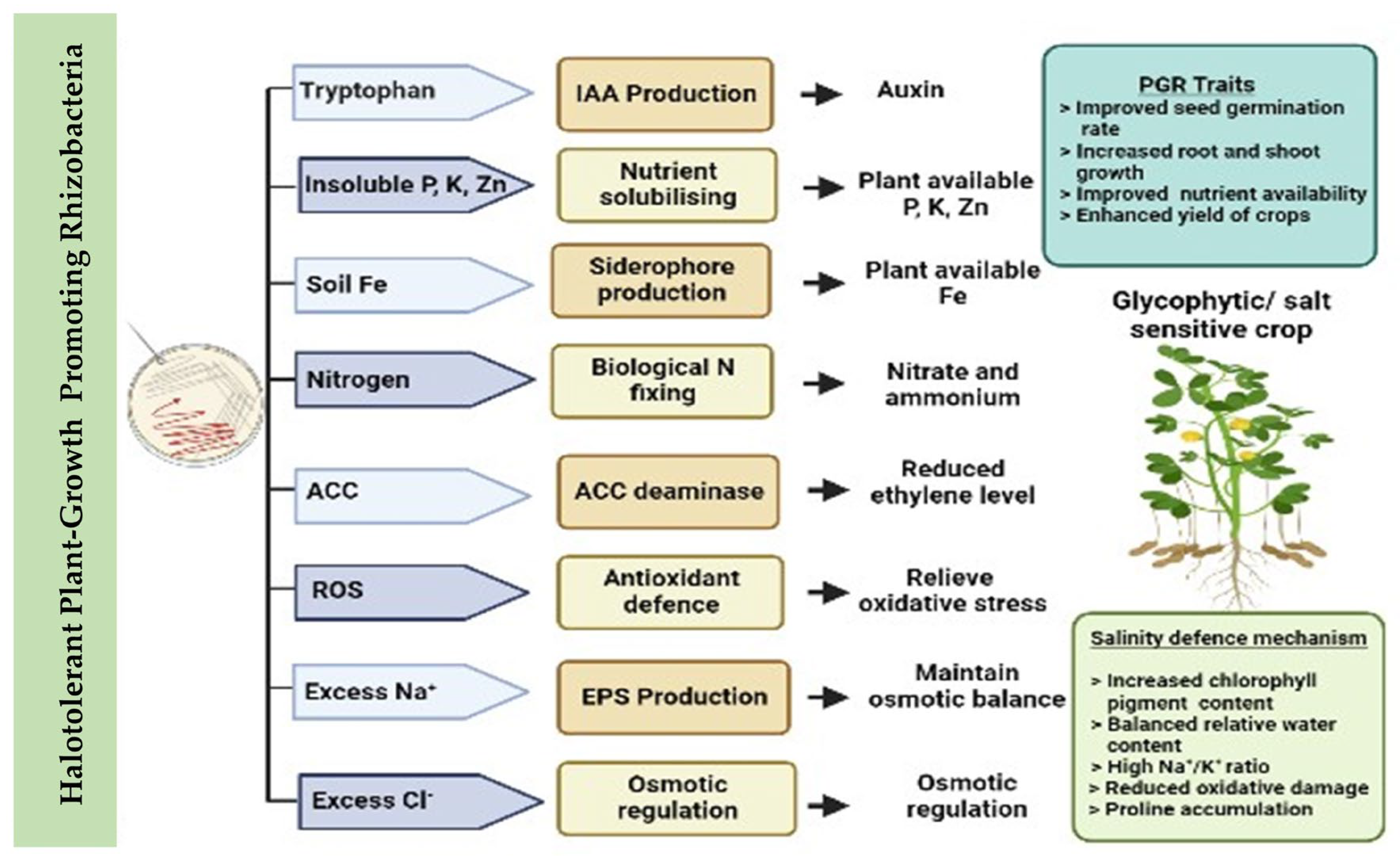
3. HT-PGPR and Their Effects in Mitigating Salt Stress in Crops
| Plant Species |
HT-PGPR Species |
Gene/s Involved |
Mechanism to Mitigate Stress | Effect Observed | References |
|---|---|---|---|---|---|
| Arabidopsis thaliana L. | Bacillus oryzicola YC7007 | RD22, KIN1, RD29B, RD20, RD22, and ERD1 | Stem and the root of the seedlings released stress-related genes | Enhanced plant tolerance to salt stress | [35] |
| Pseudomonas putida PS01 |
APX2 and GLYI7 | APX2 and GLYI7 genes were downregulated | ABA signalling, jasmonic acid production route, ROS scavenging, detoxification | [36] | |
| Pseudomonas knackmussii MLR6 | NHX1, HKT1, SOS2, SOS3, SAG13, and PR1 | Enhanced stomatal conductance, transpiration rate, chlorophyll, and carotenoid levels | Reduced electrolyte leakage and priming ROS accumulation increasing cell membrane stability | [37] | |
| Bacillus amyloliquefaciens SQR9 | NHX1 and NHX7 | Involved in reducing GSH biosynthesis | Reduced ion toxicity by sequestering Na+ into vacuoles and releasing Na+ from the cell | [38] | |
| Burkholderia phytofirmans PsJN | Upregulation of RD29A and GLYI7, and downregulation of LOX2 | Enhancement of proline and transcription of genes related to abscisic acid signalling and downregulated gene Lipoxygenase 2 | Abscisic acid signalling, ROS reduction, detoxifying, jasmonate synthesis, and ion transport | [39] | |
| Paenibacillus yonginensis DCY84T | AtRSA1, AtVQ9 and AtWRKY8 | Upregulated salt-stress genes | Promoted more resistance to salinity, drought, and aluminium stresses | [40][41] | |
| Enterobacter sp. EJ01 | DREB2b, RD29A, RD29B, RAB18, P5CS1, P5CS2, MPK3, and MPK6 | Upregulated salt-stress genes | Promoted more resistance to salinity and enhanced plant growth | [42] | |
| Bacillus subtilis GB03 | HKT1 | Down- and upregulates HKT1 in roots and shoots, respectively | Decreased total plant Na+ accumulation | [23] | |
| Bacopa monneri L. | Dietzia natronolimnaea STR1 | SOS1, SOS4, TaST, TaNHX1, TaHAK, and TaHKT1 | Reduction in ABA-signalling, upregulated TaABARE and TaOPR1 | Abscisic acid signalling, ROS scavenging, antioxidant enzyme activity, enhanced ion transporter expression, high K+/Na+ ratio | [24] |
| Bacillus pumilus STR2, Exiguobacterium oxidotolerens STR36 |
- | Mixture of plant growth-promoting traits under primary and secondary saline condition | Produced higher yield, high proline/lipid content peroxidation | [2] | |
| Cicer arietinum L. | Planococcus rifietoensis (RT4) and Halomonas variabilis (HT1) | - | Biofilm and exopolysaccharides production | Improved crop growth, soil aggregation, and soil fertility | [43] |
| Glycine max L. | Arthrobacter woluwensis AK1 | - | Reduced endogenous ABA and controlled antioxidant activity | Mitigated salinity stress and increased plant growth | [44] |
| Microbacterium oxydans, Arthrobacter woluwensis, Arthrobacter aurescens, Bacillus aryabhattai, and Bacillus megaterium |
- | Increased production of IAA, GA, siderophores, and phosphate solubilisation | Increased antioxidant enzymes and K absorption; reduced Na+ in plant tissue; phytohormone | [45] | |
| Pseudomonas simiae AU | P5CS, PPO and HKT1 | Downregulated HKT1, LOX, PPO, and P5CS genes | Increased chlorophyll, phosphate solubilisation, IAA, and siderophores; decreased root surface in saline | [46] | |
| Pseudomonas sp. strain AK-1 | HTK1 | Improve K+/Na+ ratio and Exopolysaccharide production binds free Na+ from soil | Increased shoot/root length and decreased Na+/K+ ratio | [33] | |
| Pseudomonas simiae AU | VSP2 | Increase vegetative storage protein (VSP), gamma-glutamyl hydrolase (GGH), and RuBisCo proteins | Reduced Na, increased K and P in soybean seedling roots, high proline and chlorophyll content | [47] | |
| Helianthus annuus L. | Pseudomonas libanensis TR1 | - | ACC-deaminase and exopolysaccharide production | Ni and Na+ accumulation potential increased along with plant growth. | [48] |
| Pseudomonas spp. | - | Upregulating of ACC deaminase | Improved P and K contents, and K+/Na+ ratio in shoot | [49] | |
| Hordeum vulgare L. | Bacillus mojavensis, B. pumilus and Pseudomonas fluorescens | S1 and S3 | ACC deaminase, IAA, and proline production | Reduced plant Na concentration, stimulated root growth, improved water and nutrient absorption | [50] |
| B. aryabhattai MS3 | BZ8, SOS1, GIG, and NHX1 | Increased salt stress resistance and accumulation | Adaptation of plant under saline condition | [51] | |
| Bacillus amyloliquefaciens SN13 | DHN | Upregulated salt stress-responsive genes and protein-related genes | Lipid peroxidation and electrolyte leakage reduced; increased rice biomass, water content, proline, and total soluble sugar | [52] | |
| Bacillus megaterium ST2-1 | - | IAA production | Stimulated the growth of rice roots and dry biomass | [53] | |
| Pseudomonas pseudoalcaligenes ST1, Bacillus pumilus ST2 |
EU440977 and FJ840535 | Accumulation of proline decrease with inoculation, antioxidative activity | Enhanced plant growth by ROS scavenging and higher accumulation of osmoprotectant | [54] | |
| Puccinellia tenuiflora L. | Bacillus subtilis (GB03) |
- | Upregulated PtHKT1;5 and PtSOS1 genes, downregulated PtHKT2;1 |
Na homeostasis modulation, exclusive K+ absorption | [55] |
| Solanum lycopersicum L. | Leclercia adecarboxylata MO1 | - | ACC deaminase and IAA production | Increased soluble sugars: organic glucose, sucrose, fructose, malic, amino acid, and proline | [56] |
| Sphingobacterium sp. BHU-AV3 | - | Reduction in ROS concentration in plant | Enhanced antioxidant activities and energy metabolism | [57] | |
| Enterobacter sp. EJ01 | DREB2b, RD29A, RD29B, and RAB18 | Downregulated P5CS1 and P5CS2, and upregulated MPK3 and MPK6 | Biosynthesis, defence pathway modulation, salt response |
[42] | |
| Pseudomonas putida UW4 | Toc GTPase | Toc GTPase genes were upregulated and reduction in ACC deaminase | Increased shoot length and chlorophyll concentration | [58] | |
| Trifolium repens L. | Bacillus subtilis (GB03) | - | Reduced shoot and root Na+, improving K+/Na+ ratio | Decreased Na+, increased chlorophyll, leaf osmotic potential, cell membrane integrity | [59] |
| Triticum aestivum L. | Pseudomonas aeruginosa GI-1, and Burkholderia gladioli GI-6 | - | P solubilisation, catalase activity, IAA production, N assimilation, and siderophores production | Encouraged growth and yield and improve soil fertility | [60][61] |
| Arthrobacter nitroguajacolicus | - | Upregulated 152 genes whereas 5 genes were downregulated | Amplified ACC, IAA, siderophore, and phosphate solubility. ROS detoxification, Na+ homeostasis, abiotic stress | [62] | |
| Serratia marcescens CDP-13 | - | Increased salt tolerance in plant | ACC deaminase, phosphate solubilisation, siderophore, indole acetic acid, N fixation, and ammonia synthesis | [63] | |
| Pseodomonas sp and Enterobacter cloacae (R-10) | B-22 and S-49 | K and Zn solubilisation for identifying antifungal activity | Enhanced K+ uptake, dry matter of wheat | [64] | |
| Hallobacillus sp. SL3 Bacillus halodenitrificans PU62 |
acdS | IAA production and siderophore production, phosphate solubilising, and siderophore production | Increased root elongation and dry weight | [65] | |
| Zea mays L. | Serratia liquefaciens KM4 | Upregulation of stress-related genes (APX, CAT, SOD, RBCS, RBCL, H+-PPase, HKT1, and NHX1) | Regulating redox potential and stress-related gene expression | Higher leaf gas exchange, osmoregulation, antioxidative defence mechanisms, and nutrient uptake boosted maize growth and biomass production | [66] |
| Azospirillum lipoferum, Azospillum sp., Azotobacter chroococcum, Azotobacter sp., and Bacillus sp. | - | Exopolysaccharide inoculation in the soil | Increased root and shoot dry weights, chlorophyll and carotenoids, restricted Na and Cl uptake, and increased shoot N, P, and K | [67] | |
| Abelmoschus esculentus L. |
Enterobacter sp. UPMR18 |
X55749 | ROS pathway upgradation and enhancement in antioxidant enzyme activities | Higher germination, growth, and chlorophyll improved salt tolerance | [68] |
This entry is adapted from the peer-reviewed paper 10.3390/agriculture13010168
References
- Myo, E.M.; Ge, B.; Ma, J.; Cui, H.; Liu, B.; Shi, L.; Jiang, M.; Zhang, K. Indole-3-Acetic Acid Production by Streptomyces Fradiae NKZ-259 and Its Formulation to Enhance Plant Growth. BMC Microbiol. 2019, 19, 155.
- Bharti, N.; Barnawal, D.; Awasthi, A.; Yadav, A.; Kalra, A. Plant Growth Promoting Rhizobacteria Alleviate Salinity Induced Negative Effects on Growth, Oil Content and Physiological Status in Mentha Arvensis. Acta Physiol. Plant 2014, 36, 45–60.
- Morris, J.; González, J.E. The Novel Genes EmmABC Are Associated with Exopolysaccharide Production, Motility, Stress Adaptation, and Symbiosis in Sinorhizobium Meliloti. J. Bacteriol. 2009, 191, 5890–5900.
- Ladeiro, B. Saline Agriculture in the 21st Century: Using Salt Contaminated Resources to Cope Food Requirements. J. Bot. 2012, 2012, 310705.
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Zaman, M., Shahid, S.A., Heng, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 43–53. ISBN 978-3-319-96190-3.
- Hernandez Fernandez, M.T.; Mataix-Solera, J.; Lichner, L.; Štekaurová, V.; Zaujec, A.; Garcia Izquierdo, C. Assessing the Microbiological, Biochemical, Soil-Physical and Hydrological Effects of Amelioration of Degraded Soils in Semiarid Spain. Biologia 2007, 62, 542–546.
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR Inoculation on Growth and Antioxidant Status of Wheat under Saline Conditions. Plant Biol. 2012, 14, 605–611.
- Orhan, F. Alleviation of Salt Stress by Halotolerant and Halophilic Plant Growth-Promoting Bacteria in Wheat (Triticum aestivum). Braz. J. Microbiol. 2016, 47, 621.
- Al-Yassin, A. Adverse Effects of Salinity on Citrus. A review paper. J. Cent Eur. Agric. 2004, 4, 263–272.
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791.
- Mishra, A.; Tanna, B. Halophytes: Potential Resources for Salt Stress Tolerance Genes and Promoters. Front. Plant Sci. 2017, 8, 829.
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Rahman, M.A.; Tran, L.S.-P. Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 10733.
- Nguyen, H.M.; Sako, K.; Matsui, A.; Suzuki, Y.; Mostofa, M.G.; Ha, C.V.; Tanaka, M.; Tran, L.-S.P.; Habu, Y.; Seki, M. Ethanol Enhances High-Salinity Stress Tolerance by Detoxifying Reactive Oxygen Species in Arabidopsis thaliana and Rice. Front. Plant Sci. 2017, 8, 1001.
- Dong, O.X.; Ronald, P.C. Genetic Engineering for Disease Resistance in Plants: Recent Progress and Future Perspectives. Plant Physiol. 2019, 180, 26–38.
- Singh, R.; Singh, P.; Sharma, R. Microorganism as a Tool of Bioremediation Technology for Cleaning Environment: A Review. Proc. Int. Acad. Ecol. Environ. Sci. 2014, 4, 1–6.
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.L.; Krishnamurthy, L. Plant Growth Promoting Rhizobia: Challenges and Opportunities. 3 Biotech 2015, 5, 355–377.
- Abbas, R.; Rasul, S.; Aslam, K.; Baber, M.; Shahid, M.; Mubeen, F.; Naqqash, T. Halotolerant PGPR: A Hope for Cultivation of Saline Soils. J. King Saud Univ. Sci. 2019, 31, 1195–1201.
- Sharma, K.; Sharma, S.; Vaishnav, A.; Jain, R.; Singh, D.; Singh, H.B.; Goel, A.; Singh, S. Salt-Tolerant PGPR Strain Priestia Endophytica SK1 Promotes Fenugreek Growth under Salt Stress by Inducing Nitrogen Assimilation and Secondary Metabolites. J. Appl. Microbiol. 2022, 133, 2802–2813.
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104.
- Sunita, K.; Mishra, I.; Mishra, J.; Prakash, J.; Arora, N.K. Secondary Metabolites From Halotolerant Plant Growth Promoting Rhizobacteria for Ameliorating Salinity Stress in Plants. Front. Microbiol. 2020, 11, 567768.
- Hernández-Canseco, J.; Bautista-Cruz, A.; Sánchez-Mendoza, S.; Aquino-Bolaños, T.; Sánchez-Medina, P.S. Plant Growth-Promoting Halobacteria and Their Ability to Protect Crops from Abiotic Stress: An Eco-Friendly Alternative for Saline Soils. Agronomy 2022, 12, 804.
- Ruginescu, R.; Gomoiu, I.; Popescu, O.; Cojoc, R.; Neagu, S.; Lucaci, I.; Batrinescu-Moteau, C.; Enache, M. Bioprospecting for Novel Halophilic and Halotolerant Sources of Hydrolytic Enzymes in Brackish, Saline and Hypersaline Lakes of Romania. Microorganisms 2020, 8, 1903.
- Zhang, H.; Kim, M.-S.; Sun, Y.; Dowd, S.E.; Shi, H.; Paré, P.W. Soil Bacteria Confer Plant Salt Tolerance by Tissue-Specific Regulation of the Sodium Transporter HKT1. Mol. Plant Microbe Interact. 2008, 21, 737–744.
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509.
- Pan, J.; Peng, F.; Xue, X.; You, Q.; Zhang, W.; Wang, T.; Huang, C. The Growth Promotion of Two Salt-Tolerant Plant Groups with PGPR Inoculation: A Meta-Analysis. Sustainability 2019, 11, 378.
- Huang, X.-F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere Interactions: Root Exudates, Microbes, and Microbial Communities. Botany 2014, 92, 267–275.
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Nawaz, S. Mitigation of Salinity-Induced Negative Impact on the Growth and Yield of Wheat by Plant Growth-Promoting Rhizobacteria in Naturally Saline Conditions. Ann. Microbiol. 2013, 63, 225–232.
- Phour, M.; Sindhu, S.S. Amelioration of Salinity Stress and Growth Stimulation of Mustard (Brassica juncea L.) by Salt-Tolerant Pseudomonas Species. Appl. Soil Ecol. 2020, 149, 103518.
- Aslam, F.; Ali, B. Halotolerant Bacterial Diversity Associated with Suaeda fruticosa (L.) Forssk. Improved Growth of Maize under Salinity Stress. Agronomy 2018, 8, 131.
- Zahir, Z.A.; Ghani, U.; Naveed, M.; Nadeem, S.M.; Asghar, H.N. Comparative Effectiveness of Pseudomonas and Serratia Sp. Containing ACC-Deaminase for Improving Growth and Yield of Wheat (Triticum aestivum L.) under Salt-Stressed Conditions. Arch. Microbiol. 2009, 191, 415–424.
- Kasotia, A.; Varma, A.; Tuteja, N.; Choudhary, D.K. Amelioration of Soybean Plant from Saline-Induced Condition by Exopolysaccharide Producing Pseudomonas-Mediated Expression of High Affinity K + -Transporter (HKT1) Gene. Curr. Sci. 2016, 111, 1961–1967.
- Kwon, S.-W.; Park, J.-Y.; Kim, J.-S.; Kang, J.-W.; Cho, Y.-H.; Lim, C.-K.; Parker, M.A.; Lee, G.-B. Phylogenetic Analysis of the Genera Bradyrhizobium, Mesorhizobium, Rhizobium and Sinorhizobium on the Basis of 16S RRNA Gene and Internally Transcribed Spacer Region Sequences. Int. J. Syst. Evol. Microbiol. 2005, 55, 263–270.
- Kwon, Y.S.; Ryu, C.-M.; Lee, S.; Park, H.B.; Han, K.S.; Lee, J.H.; Lee, K.; Chung, W.S.; Jeong, M.-J.; Kim, H.K.; et al. Proteome Analysis of Arabidopsis Seedlings Exposed to Bacterial Volatiles. Planta 2010, 232, 1355–1370.
- Baek, D.; Rokibuzzaman, M.; Khan, A.; Kim, M.C.; Park, H.J.; Yun, D.; Chung, Y.R. Plant-Growth Promoting Bacillus Oryzicola YC7007 Modulates Stress-Response Gene Expression and Provides Protection From Salt Stress. Front. Plant Sci. 2020, 10, 1646.
- Chu, T.N.; Tran, B.T.H.; Van Bui, L.; Hoang, M.T.T. Plant Growth-Promoting Rhizobacterium Pseudomonas PS01 Induces Salt Tolerance in Arabidopsis thaliana. BMC Res. Notes 2019, 12, 11.
- Rabhi, N.E.H.; Silini, A.; Cherif-Silini, H.; Yahiaoui, B.; Lekired, A.; Robineau, M.; Esmaeel, Q.; Jacquard, C.; Vaillant-Gaveau, N.; Clément, C.; et al. Pseudomonas Knackmussii MLR6, a Rhizospheric Strain Isolated from Halophyte, Enhances Salt Tolerance in Arabidopsis thaliana. J. Appl. Microbiol. 2018, 125, 1836–1851.
- Chen, L.; Liu, Y.; Wu, G.; Veronican Njeri, K.; Shen, Q.; Zhang, N.; Zhang, R. Induced Maize Salt Tolerance by Rhizosphere Inoculation of Bacillus Amyloliquefaciens SQR9. Physiol. Plant 2016, 158, 34–44.
- Pinedo, I.; Ledger, T.; Greve, M.; Poupin, M.J. Burkholderia Phytofirmans PsJN Induces Long-Term Metabolic and Transcriptional Changes Involved in Arabidopsis thaliana Salt Tolerance. Front. Plant Sci. 2015, 6, 466.
- Sukweenadhi, J.; Kim, Y.-J.; Choi, E.-S.; Koh, S.-C.; Lee, S.-W.; Kim, Y.-J.; Yang, D.C. Paenibacillus Yonginensis DCY84T Induces Changes in Arabidopsis thaliana Gene Expression against Aluminum, Drought, and Salt Stress. Microbiol. Res. 2015, 172, 7–15.
- Kim, Y.-J.; Sukweenadhi, J.; Seok, J.W.; Kang, C.H.; Choi, E.-S.; Subramaniyam, S.; Yang, D.C. Complete Genome Sequence of Paenibacillus Yonginensis DCY84T, a Novel Plant Symbiont That Promotes Growth via Induced Systemic Resistance. Stand. Genom. Sci. 2017, 12, 63.
- Kim, K.; Jang, Y.-J.; Lee, S.-M.; Oh, B.-T.; Chae, J.-C.; Lee, K.-J. Alleviation of Salt Stress by Enterobacter Sp. EJ01 in Tomato and Arabidopsis Is Accompanied by Up-Regulation of Conserved Salinity Responsive Factors in Plants. Mol. Cells 2014, 37, 109–117.
- Qurashi, A.W.; Sabri, A.N. Bacterial Exopolysaccharide and Biofilm Formation Stimulate Chickpea Growth and Soil Aggregation under Salt Stress. Braz. J. Microbiol. 2012, 43, 1183–1191.
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Rhizobacteria AK1 Remediates the Toxic Effects of Salinity Stress via Regulation of Endogenous Phytohormones and Gene Expression in Soybean. Biochem. J. 2019, 476, 2393–2409.
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.-M.; Lee, I.-J. Halotolerant Rhizobacterial Strains Mitigate the Adverse Effects of NaCl Stress in Soybean Seedlings. Biomed. Res. Int. 2019, 2019, 9530963.
- Vaishnav, A.; Kumari, S.; Jain, S.; Varma, A.; Tuteja, N.; Choudhary, D.K. PGPR-Mediated Expression of Salt Tolerance Gene in Soybean through Volatiles under Sodium Nitroprusside: PGPR-Mediated Amelioration of Soybean under Salt Stress. J. Basic Microbiol. 2016, 56, 1274–1288.
- Vaishnav, A.; Kumari, S.; Jain, S.; Varma, A.; Choudhary, D.K. Putative Bacterial Volatile-Mediated Growth in Soybean (Glycine max L. Merrill) and Expression of Induced Proteins under Salt Stress. J. Appl. Microbiol. 2015, 119, 539–551.
- Ma, Y.; Rajkumar, M.; Oliveira, R.S.; Zhang, C.; Freitas, H. Potential of Plant Beneficial Bacteria and Arbuscular Mycorrhizal Fungi in Phytoremediation of Metal-Contaminated Saline Soils. J. Hazard. Mater. 2019, 379, 120813.
- Kiani, M.Z.; Ali, A.; Sultan, T.; Ahmad, R.; Hydar, S.I. Plant Growth Promoting Rhizobacteria Having 1-Aminocyclopropane-1-Carboxylic Acid Deaminase to Induce Salt Tolerance in Sunflower (Helianthus annus L.). Nat. Resour. 2015, 6, 391–397.
- Mahmoud, M.B.; Hidri, R.; Talbi-Zribi, O.; Taamalli, W.; Abdelly, C.; Djébali, N. Auxin and Proline Producing Rhizobacteria Mitigate Salt-Induced Growth Inhibition of Barley Plants by Enhancing Water and Nutrient Status. S. Afr. J. Bot. 2020, 128, 209–217.
- Sultana, S.; Paul, S.C.; Parveen, S.; Alam, S.; Rahman, N.; Jannat, B.; Hoque, S.; Rahman, M.T.; Karim, M.M. Isolation and Identification of Salt-Tolerant Plant-Growth-Promoting Rhizobacteria and Their Application for Rice Cultivation under Salt Stress. Can. J. Microbiol. 2020, 66, 144–160.
- Chauhan, P.S.; Lata, C.; Tiwari, S.; Chauhan, A.S.; Mishra, S.K.; Agrawal, L.; Chakrabarty, D.; Nautiyal, C.S. Transcriptional Alterations Reveal Bacillus Amyloliquefaciens-Rice Cooperation under Salt Stress. Sci. Rep. 2019, 9, 11912.
- Nghia, N.K.; Tien, T.T.M.; Oanh, N.T.K.; Nuong, N.H.K. Isolation and Characterization of Indole Acetic Acid Producing Halophilic Bacteria from Salt Affected Soil of Rice–Shrimp Farming System in the Mekong Delta, Vietnam. Agric. For. Fish. 2017, 6, 69.
- Jha, Y.; Subramanian, R.B.; Patel, S. Combination of Endophytic and Rhizospheric Plant Growth Promoting Rhizobacteria in Oryza Sativa Shows Higher Accumulation of Osmoprotectant against Saline Stress. Acta Physiol. Plant 2011, 33, 797–802.
- Niu, S.-Q.; Li, H.-R.; Paré, P.W.; Aziz, M.; Wang, S.-M.; Shi, H.; Li, J.; Han, Q.-Q.; Guo, S.-Q.; Li, J.; et al. Induced Growth Promotion and Higher Salt Tolerance in the Halophyte Grass Puccinellia Tenuiflora by Beneficial Rhizobacteria. Plant Soil 2016, 407, 217–230.
- Kang, S.-M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.-G.; Lee, K.-E.; Asaf, S.; Khan, M.A.; Lee, I.-J. Indole-3-Acetic-Acid and ACC Deaminase Producing Leclercia Adecarboxylata MO1 Improves Solanum lycopersicum L. Growth and Salinity Stress Tolerance by Endogenous Secondary Metabolites Regulation. BMC Microbiol. 2019, 19, 80.
- Vaishnav, A.; Singh, J.; Singh, P.; Rajput, R.S.; Singh, H.B.; Sarma, B.K. Sphingobacterium Sp. BHU-AV3 Induces Salt Tolerance in Tomato by Enhancing Antioxidant Activities and Energy Metabolism. Front. Microbiol. 2020, 11, 443.
- Yan, J.; Smith, M.D.; Glick, B.R.; Liang, Y. Effects of ACC Deaminase Containing Rhizobacteria on Plant Growth and Expression of Toc GTPases in Tomato (Solanum lycopersicum) under Salt Stress. Botany 2014, 92, 775–781.
- Han, Q.-Q.; Lü, X.-P.; Bai, J.-P.; Qiao, Y.; Paré, P.W.; Wang, S.-M.; Zhang, J.-L.; Wu, Y.-N.; Pang, X.-P.; Xu, W.-B.; et al. Beneficial Soil Bacterium Bacillus Subtilis (GB03) Augments Salt Tolerance of White Clover. Front. Plant Sci. 2014, 5, 525.
- Shah, D.; Khan, M.S.; Aziz, S.; Ali, H.; Pecoraro, L. Molecular and Biochemical Characterization, Antimicrobial Activity, Stress Tolerance, and Plant Growth-Promoting Effect of Endophytic Bacteria Isolated from Wheat Varieties. Microorganisms 2021, 10, 21.
- Vaishnav, A.; Kumar, R.; Singh, H.B.; Sarma, B.K. Extending the Benefits of PGPR to Bioremediation of Nitrile Pollution in Crop Lands for Enhancing Crop Productivity. Sci. Total Environ. 2022, 826, 154170.
- Safdarian, M.; Askari, H.; Shariati, J.V.; Nematzadeh, G. Transcriptional Responses of Wheat Roots Inoculated with Arthrobacter Nitroguajacolicus to Salt Stress. Sci. Rep. 2019, 9, 1792.
- Singh, R.P.; Jha, P.N. The Multifarious PGPR Serratia Marcescens CDP-13 Augments Induced Systemic Resistance and Enhanced Salinity Tolerance of Wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026.
- Pirhadi, M.; Enayatizamir, N.; Motamedi, H.; Sorkheh, K. Screening of Salt Tolerant Sugarcane Endophytic Bacteria with Potassium and Zinc for Their Solubilizing and Antifungal Activity. Biosci. Biotech. Res. Comm. 2016, 9, 530–538.
- Ramadoss, D.; Lakkineni, V.K.; Bose, P.; Ali, S.; Annapurna, K. Mitigation of Salt Stress in Wheat Seedlings by Halotolerant Bacteria Isolated from Saline Habitats. Springerplus 2013, 2, 6.
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alzahrani, S.M.; Ali, H.M.; Alayafi, A.A.; Ahmad, M. Serratia Liquefaciens KM4 Improves Salt Stress Tolerance in Maize by Regulating Redox Potential, Ion Homeostasis, Leaf Gas Exchange and Stress-Related Gene Expression. Int. J. Mol. Sci. 2018, 19, 3310.
- Alla, M.M.N.; Hassan, N.M. A Possible Role for C4 Photosynthetic Enzymes in Tolerance of Zea mays to NaCl. Protoplasma 2012, 249, 1109–1117.
- Habib, S.H.; Kausar, H.; Saud, H.M. Plant Growth-Promoting Rhizobacteria Enhance Salinity Stress Tolerance in Okra through ROS-Scavenging Enzymes. Biomed. Res. Int. 2016, 2016, 6284547.
- Etesami, H.; Beattie, G.A. Mining Halophytes for Plant Growth-Promoting Halotolerant Bacteria to Enhance the Salinity Tolerance of Non-Halophytic Crops. Front. Microbiol. 2018, 9, 148.
