Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Classical risk factors play a major role in the initiation and development of atherosclerosis. Efforts have been made to identify biomarkers that indicate ongoing atherosclerosis. Among important circulating biomarkers associated with peripheral arterial disease (PAD) are inflammatory markers which are determined by the expression of different genes and epigenetic processes. Among these proinflammatory molecules, interleukin-6, C-reactive protein, several adhesion molecules, CD40 ligand, osteoprotegerin and others are associated with the presence and progression of PAD.
- peripheral arterial disease
- biomarkers
- inflammation
- prothrombotic
1. Introduction
Peripheral artery disease (PAD) is a common manifestations of atherosclerosis affecting more than 200 million people worldwide [1][2]. The presence of PAD represents a powerful and independent risk of cardiac and cerebral ischemic events [3]. It is well-established that hypertension, smoking, diabetes mellitus and hypercholesterolemia play a major role in the initiation and development of atherosclerosis and cardiovascular (CV) events [4]. However, the prognostic potency of each of these factors in atherogenesis differs in various arterial beds and estimation of risk for atherosclerotic CV events based only on classical risk factors is often insufficient [4]. In ancient times when classical risk factors were less prevalent, atherosclerosis was already widespread as evidenced by Egyptian mummies [5].
Plaque formation is influenced by the expression of different genes [4][6][7]. Oxidized low density lipoprotein (LDL) particles which accumulate in the intima strongly modulate inflammation-related gene expression through various signaling pathways [8]. Epigenetic processes are also involved in cytokine expression that strongly affects atherogenesis [7]. MicroRNAs (mi-RNAs) which are single-stranded, non-coding RNAs that modulate gene-expression at the posttranscriptional level, have emerged as promising novel biomarkers for different aspects PAD, including its detection, prediction of progression and revascularization outcomes [9].
Biomarkers refer to a broad category of quantifiable biological characteristics that predict CV events. Besides providing diagnostic and prognostic information, some biomarkers might also be helpful in assessing the efficacy of therapeutic interventions [10].
2. Inflammation and PAD
The role of inflammation in the atherogenic process was established as a basic pathophysiological process, which is responsible for the initiation, clinical manifestation and CV risk of atherosclerotic diseases [11]. The vascular inflammatory process is related to systemic inflammatory response and patients with preclinical or clinical atherosclerotic disease have increased circulatory inflammatory markers. Increased levels of inflammatory markers are associated with the development and progression of PAD, and the risk of developing cardiac and cerebrovascular ischemic events [12]. A number of cross-sectional and longitudinal studies demonstrated a close link between inflammation and PAD [13].
Inflammatory molecules are not simply markers of inflammation, they also play an active role in peripheral atherogenesis [14][15]. In a prospective case-control study in apparently healthy men enrolled in the Physicians Health Study (PHS) the relative risk of developing PAD increased significantly with each quartile of baseline C-reactive protein (CRP) and this increase was independent of other risk factors [16]. The PHS also showed that elevated levels of soluble intercellular adhesion molecule-1 (sICAM-1) are independently associated with the development of symptomatic PAD in men [17]. Similar findings were reported in the Women Health Study [18].
Among inflammatory biomarkers, the pro-inflammatory cytokine interleukin-6 (IL-6) was shown to be the strongest predictor of PAD and was independently associated with disease progression [13]. Circulating IL-6 levels significantly increase after exercise in patients with PAD, and higher IL-6 levels have been associated with lower functional capacity [19]. The Edinburg Artery Study also showed that interleukin-1 (IL-1) has an important predictive role in outcome of patients with PAD [20]. Interleukins, E-selectin and metalloproteinases predicted major events in patients with severe limb ischemia and allowed for the creation of a biomarker-model [21].
There are conflicting data on the role of interleukin-8 (IL-8). Some studies showed that PAD patients who were submitted to vascular surgical procedures had a higher production of IL-8 in polymorphonuclear leucocytes [22]. The anti-inflammatory interleukin-10 (IL-10), which is associated with reduced apoptosis of cells of the lipid core and thereby with the reduced risk of plaque rupture, has been related to reduced risk of developing atherosclerosis. However, the correlation between PAD and levels of IL-10 remains uncertain [23].
Several other inflammatory markers have been examined in a limited number of studies. In the Atherosclerotic Risk in Communities (ARIC) Study, monocyte chemoattractant protein-1 (MCP-1) was associated with the ankle-brachial index (ABI) [24]. Further, CD40 ligand was associated with an angiographic severity of PAD [25]. Serum osteoprotegerin was associated with a pathological ABI in a cohort study [26]. Wilson et al. reported that serum levels of of β2-microglobulin were higher in patient with PAD than in non-PAD patients and were independently associated with ABI [27].
2.1. Inflammatory Markers as Mediators of Harmful Effects of Other Risk Factors for PAD
Risk factors of atherosclerosis often trigger their atherogenic effects through an inflammatory mechanism (Figure 1). Cigarette smoking and diabetes mellitus, the strongest predictors of developing PAD, promote oxidative stress, which directly and indirectly stimulates inflammatory pathways [28].
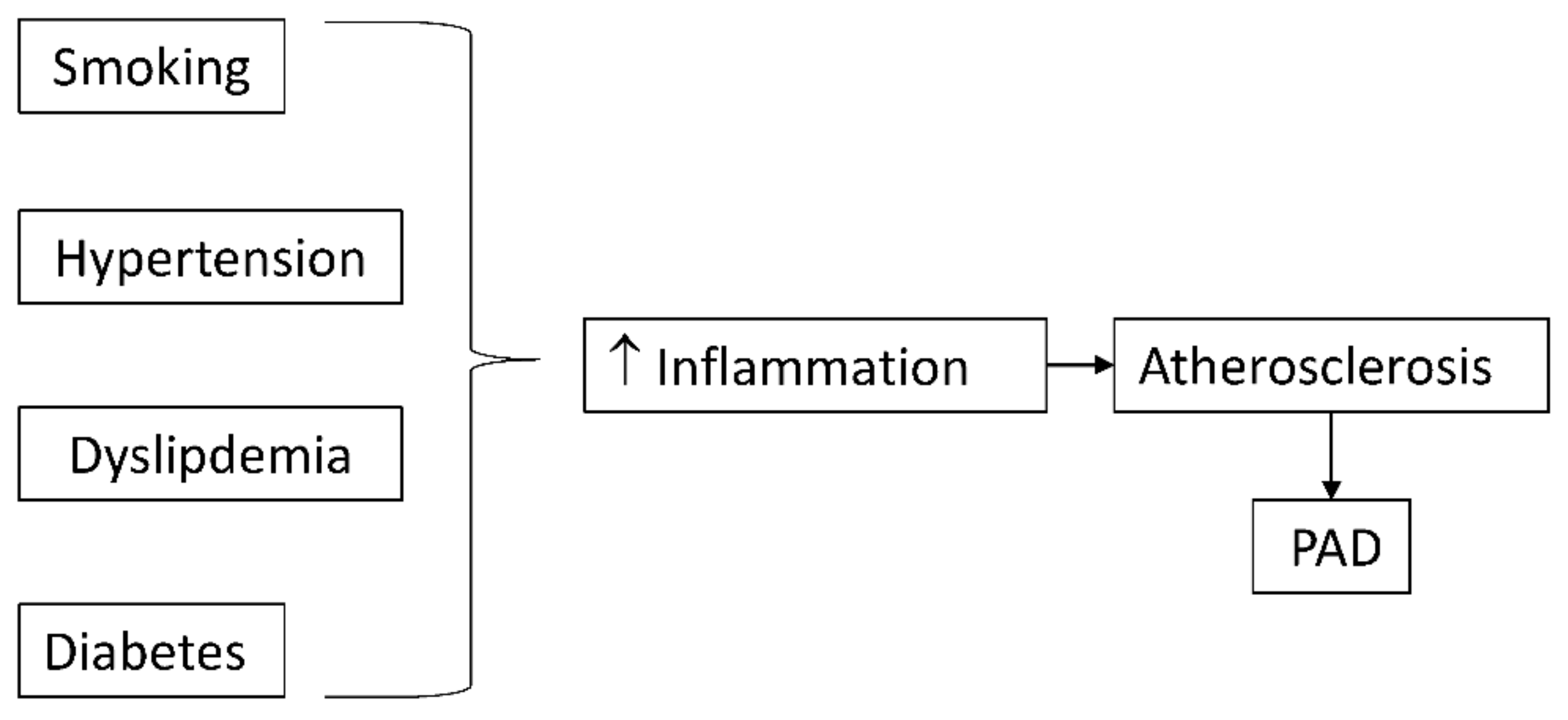
Figure 1. Classical risk factors for atherosclerosis promote inflammation which accelerates atherosclerosis and peripheral arterial disease (PAD).
Smoking is undoubtedly one of the most influential risk factors for PAD. The mechanisms associated with smoking include the activation of inflammation, dysregulation of lipid metabolism, increase of oxidative stress and endothelial dysfunction [29]. Smoking promotes inflammation through elevated white blood cell count, CRP, fibrinogen and von Willebrand factor which are elevated in patients with PAD [30]. Smoking also promotes activation of monocytes and production of various chemokines and cytokines [31].
Hypertension which is present in about 80% of PAD patients, also promotes inflammation [32]. Angiotensin II, which is involved in pathogenesis of arterial hypertension, elicits the production of reactive oxygen species and modifies the oxidation of LDL, stimulates the expression of vascular cell adhesion molecules and increases the expression of proinflammatory cytokines such as IL-6 [33] and dysregulates circulating miRNAs, which are associated with the presence of PAD and its progression [29]. Smoking cessation can correct abnormalities related to smoking including vascular inflammation, dyslipidemia, endothelial dysfunction, arterial stiffness and insulin resistance, but the success rates of smoking cessation are relatively low [34].
Diabetes also ranks among the strongest risk factors for PAD. Several biomarkers of PAD were identified in diabetic patients. A significant association was shown between HbA1c levels and the incidence of PAD [35]. Ischemia-modified albumin in type 2 diabetic patients which is associated with HbA1c, was also shown to be a risk marker of PAD [36]. Further, copeptin, B-type of natriuretic peptide and cystatin C is associated with the incidence of symptomatic PAD [37]. In diabetic patients, amputations re-amputations represent frequent complication. Guelcu et al. showed that CRP, together with lower albumin, higher HbA1c, and higher creatinine levels is associated with poor prognosis and re-amputation [38]. Diabetes induces dysregulation of miRNA expression that is associated with the development of macrovascular complications, including PAD [39] and dysregulates miRNAs expression related to atherosclerosis [40].
Dyslipidemia also plays a pivotal role in the activation of inflammatory pathways, increasing the production of inflammatory cytokines, mainly tumor necrosis factor alpha (TNF-α) and IL-6 [41] and inducing miRNA dysregulation [40].
2.2. Effects of Preventive and Therapeutic Measures on Inflammatory Biomarkers
Determination of inflammatory biomarkers can be used as an indicator of effects of preventive and therapeutic measures in patients with atherosclerotic disease including PAD.
2.2.1. Physical Exercise
Patients with PAD regularly experience ischemia of the tissue distant to arterial occlusions during exercise. The transient exercise-induced leg ischemia is related to increased release of inflammatory markers [42] and impairs vasodilator function of distant arteries in correlation with increased circulatory levels of interleukins, particularly IL-6 [43]. This could be one of the reasons that because of repeated ischemia-related release of inflammatory markers, PAD patients experience advanced systemic atherosclerosis including coronary heart disease (CHD) and other CV disease (CVD).
In contrast to the acute deleterious effects of ischemia, regular moderate exercise training not only improves walking capacity but also decreases vascular and inflammatory biomarkers [44]. Chronic exercise improves anti-oxidant capacity and decreases inflammatory response without increasing oxidative stress in symptomatic PAD patients [42][44]. Acute exercise usually leads to robust inflammatory response mainly characterized by the mobilization of leukocytes and an increase in circulating inflammatory mediators produced by immune cells and directly from active muscle tissue [44].
Moderate physical exercise training results in improvements in systemic inflammation, evident by reduction in acute phase proteins [45]. Therefore, repeated moderate physical exercise in patients with intermittent claudication reinforces antioxidant capacity, reduces oxidative stress and inflammation and regulates the immune response [46].
Overall, physical training is one of the most effective treatment options for PAD patients, which prevents progression of local disease and CV events. These benefits are most probably based on reducing the inflammatory response and improving immune function.
2.2.2. Statins
The anti-inflammatory properties of statins represent one of the basic anti-atherosclerotic mechanisms, involving a reduction in the release of CRP, chemokines, cytokines, and adhesion molecules [47]. Furthermore, statins inhibit the transendothelial migration of leukocytes [48]. In addition, statins have been shown to decrease the number of inflammatory cells in atherosclerotic plaques and to possess other anti-inflammatory properties [47]. This could be the consequence of inhibition of adhesion molecules or cytokines (IL-6, IL-8), which are involved in the accumulation of inflammatory cells. The importance of inhibition of inflammation by statins was shown in the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study [49]. Besides stabilization and regression of atherosclerotic plaques, statins were shown to reduce inflammation [lowering of levels of CRP, fibrinogen, neutrophils], which, in patients with PAD correlates with improved survival [50][51]. Therefore, an important mechanism by which statins improve outcomes in atherosclerotic patients, including PAD, may be the reduction of vascular inflammation [52].
Among patients with PAD in the National Veterans Affairs cohort, any statin use reduced mortality and high intensity statin use also reduced limb amputations [53][54].
2.2.3. “Novel” Anti-inflammatory Agents and Approaches
Several novel anti-inflammatory agents are being tested in prevention of atherosclerosis. Canakinumab, a monoclonal anti-IL-1beta antibody effectively reduced atherosclerotic CV events in the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) study independent of lipid-lowering, also reduced cancer mortality, especially of lung cancer, but increased the incidence of fatal infections [55]. On the other hand, low-dose methotrexate did not result in a clinical benefit in very high risk patients with previous myocardial infarction or multivessel coronary disease who also had either type 2 diabetes or metabolic syndrome [56].
Colchicine is an ancient herbal drug with powerful anti-inflammatory potency. Colchicine reduces the levels of pro-inflammatory cytokines and stabilizes the coronary plaques, leading to a reduction of recurrent coronary events after acute coronary syndromes and better outcomes in patients with chronic coronary disease [57][58]. The efficacy of colchicine in reducing cardiovascular and limb events in patients with symptomatic PAD is being tested in the on-going LEADER-PAD (Low dose Colchicine in Patients with peripheral Artery DiseasE) trial (ClinicalTrials.gov: NCT04774159).
The use of immunosuppressive drugs targeting chronic inflammation could assume an important role in the future. Further, drugs with anti-inflammatory capacity, currently used for other indications, might be reprogrammed for use in PAD. Among them, sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists are particularly interesting [59]. Overall, new anti-inflammatory drugs and approaches are already on the horizon. It seems likely that in the future anti-inflammatory treatment will be guided by a personalized approach, based on the individual risk profile.
This entry is adapted from the peer-reviewed paper 10.3390/ijms231912054
References
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. ESC Scientific Document Group. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816.
- Shu, J.; Santulli, G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis 2018, 275, 379–381.
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143.
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533.
- Allam, A.H.; Thompson, R.C.; Wann, L.S.; Miyamoto, M.I.; El-Din, A.E.-H.N.; El-Maksoud, G.A.; Soliman, M.A.-T.; Badr, I.; Amer, H.A.E.-R.; Sutherland, M.L.; et al. Atherosclerosis in ancient Egyptian mummies: The Horus study. JACC Cardiovasc. Imaging 2011, 4, 315–327.
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325.
- McLaren, J.E.; Michael, D.R.; Ashlin, T.G.; Ramji, D.P. Cytokines, macrophage lipid metabolism and foam cells: Implications for cardiovascular disease therapy. Prog. Lipid Res. 2011, 50, 331–347.
- Leonarduzzi, G.; Gamba, P.; Gargiulo, S.; Biasi, F.; Poli, G. Inflammation-related gene expression by lipid oxidation-derived products in the progression of atherosclerosis. Free Radic. Biol. Med. 2012, 52, 19–34.
- Menghini, R.; Stöhr, R.; Federici, M. MicroRNAs in vascular aging and atherosclerosis. Ageing Res. Rev. 2014, 17, 68–78.
- Wang, J.; Tan, G.J.; Han, L.N.; Bai, Y.Y.; He, M.; Liu, H.B. Novel biomarkers for cardiovascular risk prediction. J. Geriatr. Cardiol. 2017, 14, 135–150.
- Brevetti, G.; Giugliano, G.; Brevetti, L.; Hiatt, W.R. Inflammation in peripheral artery disease. Circulation 2010, 122, 1862–1875.
- Brevetti, G.; Schiano, V.; Chiariello, M. Endothelial dysfunction: A key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis 2008, 197, 1–11.
- Tzoulaki, I.; Murray, G.D.; Lee, A.J.; Rumley, A.; Lowe, G.D.; Fowkes, F.G. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation 2005, 112, 976–983.
- Galkina, E.; Ley, K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2292–2301.
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581.
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation 1998, 97, 425–428.
- Pradhan, A.D.; Rifai, N.; Ridker, P.M. Soluble intercellular adhesion molecule-1, soluble vascular adhesion molecule-1, and the development of symptomatic peripheral arterial disease in men. Circulation 2002, 106, 820–825.
- Pradhan, A.D.; Shrivastava, S.; Cook, N.R.; Rifai, N.; Creager, M.A.; Ridker, P.M. Symptomatic peripheral arterial disease in women: Nontraditional biomarkers of elevated risk. Circulation 2008, 117, 823–831.
- Nylaende, M.; Kroese, A.; Stranden, E.; Morken, B.; Sandbaek, G.; Lindahl, A.K.; Arnesen, H.; Seljeflot, I. Markers of vascular inflammation are associated with the extent of atherosclerosis assessed as angiographic score and treadmill walking distances in patients with peripheral arterial occlusive disease. Vasc. Med. 2006, 11, 21–28.
- Tzoulaki, I.; Murray, G.D.; Lee, A.J.; Rumley, A.; Lowe, G.D.; Fowkes, F.G. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur. Heart J. 2007, 28, 354–362.
- Gremmels, H.; Teraa, M.; de Jager, S.C.A.; Pasterkamp, G.; de Borst, G.J.; Verhaar, M.C. A pro-inflammatory biomarker-profile predicts amputation-free survival in patients with severe limb ischemia. Sci. Rep. 2019, 9, 10740.
- Mallat, Z.; Besnard, S.; Duriez, M.; Deleuze-Marquès, V.; Emmanuel, F.; Bureau, M.F.; Soubrier, F.; Esposito, B.; Duez, H.; Fievet, C.; et al. Protective role of interleukin-10 in atherosclerosis. Circ. Res. 1999, 85, e17–e24.
- Signorelli, S.S.; Fiore, V.; Malaponte, G. Inflammation and peripheral arterial disease: The value of circulating biomarkers. Int. J. Mol. Med. 2014, 334, 777–783.
- Hoogeveen, R.C.; Morrison, A.; Boerwinkle, E.; Miles, J.S.; Rhodes, C.E.; Sharrett, A.R.; Ballantyne, C.M. Plasma MCP-1 level and risk for peripheral arterial disease and incident coronary heart disease: Atherosclerosis Risk in Communities study. Atherosclerosis 2005, 183, 301–307.
- Lee, W.-J.; Sheu, W.H.-H.; Chen, Y.-T.; Liu, T.-J.; Liang, K.-W.; Ting, C.-T. Circulating CD40 ligand is elevated only in patients with more advanced symptomatic peripheral arterial diseases. Thromb. Res. 2006, 118, 619–626.
- Ali, Z.; Ellington, A.A.; Mosley, T.H., Jr.; Kullo, I.J. Association of serum osteoprotegerin with ankle-brachial index and urine albumin: Creatinine ratio in African-Americans and non-Hispanic whites. Atherosclerosis 2009, 206, 575–580.
- Wilson, A.M.; Kimura, E.; Harada, R.K.; Nair, N.; Narasimhan, B.; Meng, X.-Y.; Zhang, F.; Beck, K.R.; Olin, J.W.; Fung, E.T.; et al. Beta2-microglobulin as a biomarker in peripheral arterial disease: Proteomic profiling and clinical studies. Circulation 2007, 116, 1396–1403.
- Fowkes, F.G.R.; Housley, E.; Riemersma, R.A.; MacIntyre, C.C.A.; Cawood, E.H.H.; Prescott, R.J.; Ruckley, C.V. Smoking, lipids, glucose intolerance, and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh Artery Study. Am. J. Epidemiol. 1992, 135, 331–340.
- Pereira-Da-Silva, T.; Napoleão, P.; Costa, M.; Gabriel, A.; Selas, M.; Silva, F.; Enguita, F.; Ferreira, R.; Carmo, M. Cigarette smoking, miR-27b downregulation, and peripheral artery disease: Insights into the mechanisms of smoking toxicity. J. Clin. Med. 2021, 10, 890.
- Liu, J.; Liang, Q.; Frost-Pineda, K.; Muhammad-Kah, R.; Rimmer, L.; Roethig, H.; Mendes, P.; Sarkar, M. Relationship between biomarkers of cigarette smoke exposure and biomarkers of inflammation, oxidative stress, and platelet activation in adult cigarette smokers. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 1760–1769.
- Walters, M.J.; Paul-Clark, M.J.; McMaster, S.K.; Ito, K.; Adcock, I.M.; Mitchell, J.A. Cigarette smoke activates human monocytes by an oxidant-AP-1 signaling pathway: Implications for steroid resistance. Mol. Pharmacol. 2005, 68, 1343–1353.
- Hirsch, A.T.; Criqui, M.H.; Treat-Jacobson, D.; Regensteiner, J.G.; Creager, M.A.; Olin, J.W.; Krook, S.H.; Hunninghake, D.B.; Comerota, A.J.; Walsh, M.E.; et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001, 286, 1317–1324.
- Tummala, P.E.; Chen, X.L.; Sundell, C.L.; Laursen, J.B.; Hammes, C.P.; Alexander, R.W.; Harrison, D.G.; Medford, R.M. Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: A potential link between the renin-angiotensin system and atherosclerosis. Circulation 1999, 100, 1223–1229.
- Athyros, V.G.; Katsiki, N.; Doumas, M.; Karagiannis, A.; Mikhailidis, D.P. Effect of tobacco smoking and smoking cessation on plasma lipoproteins and associated major cardiovascular risk factors: A narrative review. Curr. Med. Res. Opin. 2013, 29, 1263–1274.
- Yang, C.-P.; Lin, C.-C.; Li, C.-I.; Liu, C.-S.; Lin, C.-H.; Hwang, K.-L.; Yang, S.-Y.; Li, T.-C. Fasting plasma glucose variability and HbA1c are associated with peripheral artery disease risk in type 2 diabetes. Cardiovasc. Diabetol. 2020, 19, 4.
- Ma, S.G.; Wei, C.L.; Hong, B.; Yu, W.N. Ischemia-modified albumin in type 2 diabetic patients with and without peripheral arterial disease. Clinics 2011, 66, 1677–1680.
- Fatemi, S.; Acosta, S.; Gottsäter, A.; Melander, O.; Engström, G.; Dakhel, A.; Zarrouk, M. Copeptin, B-type natriuretic peptide and cystatin C are associated with incident symptomatic PAD. Biomarkers 2019, 24, 615–621.
- Gülcü, A.; Etli, M.; Karahan, O.; Aslan, A. Analysis of routine blood markers for predicting amputation/re-amputation risk in diabetic foot. Int. Wound J. 2020, 17, 1996–2004.
- Bielska, A.; Niemira, M.; Kretowski, A. Recent highlights of research on miRNAs as early potential biomarkers for cardiovascular complications of type 2 diabetes mellitus. Int. J. Mol. Sci. 2021, 22, 3153.
- Quiat, D.; Olson, E.N. MicroRNAs in cardiovascular disease: From pathogenesis to prevention and treatment. J. Clin. Investig. 2013, 123, 11–18.
- Lira, F.S.; Rosa Neto, J.C.; Antunes, B.M.; Fernandes, R.A. The relationship between inflammation, dyslipidemia and physical exercise: From the epidemiological to molecular approach. Curr. Diabetes Rev. 2014, 10, 391–396.
- Pellegrin, M.; Bouzourène, K.; Mazzolai, L. Exercise prior to lower extremity peripheral artery disease improves endurance capacity and hindlimb blood flow by inhibiting muscle inflammation. Front. Cardiovasc. Med. 2021, 8, 706491.
- Joras, M.; Poredos, P. The association of acute exercise-induced ischaemia with systemic vasodilator function in patients with peripheral arterial disease. Vasc. Med. 2008, 13, 255–262.
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S. Changes in vascular and inflammatory biomarkers after exercise rehabilitation in patients with symptomatic peripheral artery disease. J. Vasc. Surg. 2019, 70, 1280–1290.
- Allen, J.; Sun, Y.; Woods, J.A. Exercise and the regulation of inflammatory responses. Prog. Mol. Biol. Transl. Sci. 2015, 135, 337–354.
- Scheffer, D.D.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165823.
- Diamantis, E.; Kyriakos, G.; Quiles-Sanchez, L.V.; Farmaki, P.; Troupis, T. The anti-inflammatory effects of statins on coronary artery disease: An updated review of the literature. Curr. Cardiol. Rev. 2017, 13, 209–216.
- Greenwood, J.; Mason, J.C. Statins and the vascular endothelial inflammatory response. Trends Immunol. 2007, 28, 88–98.
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207.
- Youssef, F.; Seifalian, A.M.; Jagroop, I.A.; Myint, F.; Baker, D.; Mikhailidis, D.P.; Hamilton, G. The early effect of lipid-lowering treatment on carotid and femoral intima media thickness (IMT). Eur. J. Vasc. Endovasc. Surg. 2002, 23, 358–364.
- Schillinger, M.; Exner, M.; Mlekusch, W.; Amighi, J.; Sabeti, S.; Muellner, M.; Rumpold, H.; Wagner, O.; Minar, E. Statin therapy improves cardiovascular outcome of patients with peripheral artery disease. Eur. Heart J. 2004, 25, 742–748.
- Crismaru, I.; Diaconu, C.C. Lipid-lowering therapy in patients with peripheral artery disease. EJ. Cardiol. Pract. 2015, 13. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-13/lipid-lowering-therapy-in-patients-with-peripheral-artery-disease (accessed on 9 September 2022).
- Arya, S.; Khakharia, A.; Binney, Z.O.; DeMartino, R.R.; Brewster, L.P.; Goodney, P.P.; Wilson, P.W.F. Association of statin dose with amputation and survival in patients with peripheral artery disease. Circulation 2018, 137, 1435–1446.
- Hsu, C.Y.; Chen, Y.T.; Su, Y.W.; Chang, C.C.; Huang, P.H.; Lin, S.J. Statin therapy reduces future risk of lower-limb amputation in patients with diabetes and peripheral artery disease. J. Clin. Endocrinol. Metab. 2017, 102, 2373–2381.
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131.
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 2019, 380, 752–762.
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 2019, 381, 2497–2505.
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. LoDoCo2 Trial Investigators. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 2020, 383, 1838–1847.
- Schubert, M.; Hansen, S.; Leefmann, J.; Guan, K. Repurposing antidiabetic drugs for cardiovascular disease. Front. Physiol. 2020, 11, 568632.
This entry is offline, you can click here to edit this entry!
