Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The typical two-dimensional layered structure materials, MXenes, are widely used in energy conversion and storage due to their high conductivity, ion transport ability, and rich surface structures. MXenes and their composites have been widely employed in secondary batteries, especially sodium-ion batteries (SIBs), with obvious performance improvement.
- Na-ion battery
- two-dimensional structure
- MXene-based materials
- energy storage device
- structure design
1. Introduction
The utilization of renewable energy is significant in achieving sustainable energy supplies. In particular, the use of clean energy, including wind, water, solar, and tidal energy, as well as other renewable energy sources, could effectively relieve carbon emissions and become a promising alternative for the future power industry [1][2]. However, the instability and discontinuity of renewable energy, which is significantly affected by environment and climate conditions, requires highly efficient energy conversion and storage devices (ECSDs). Electrochemical ECSDs, such as secondary batteries, supercapacitors, and fuel cells, have been widely used in recent decades [3][4][5][6][7][8]. Among them, rechargeable secondary batteries are the most effective electrochemical ECSD technologies, especially Li-ion batteries (LIBs), which are endowed with high energy density, long life cycles, environmental friendliness, and low self-discharge rates [9][10]. As a typical electrochemical ECSD, LIBs are used in electric vehicles, portable electronic devices, wearable energy devices, large-scale energy storage, and other fields [5][11][12]. However, the Li content in the Earth’s crust is only 0.0065%, and the need for lithium resources has also increased significantly with the large-scale use of LIBs, which has meant that it has gradually failed to meet the growing demand [2]. These reasons have further aggravated the rise in the cost of LIBs, making them gradually lose their advantages in low-speed electric vehicles, large-scale energy storage, and portable electronic devices.
Na and Li belong to the IA group, which is endowed with similar chemical and physical properties. In the crust, the Na content is 2.75%, more than 400 times that of Li, and it is rich and has a low cost [2]. Thus, SIBs with similar rocking-chair mechanisms based on Na+ reactions have attracted more attention [13]. In aprotic systems, the Na+/Na electrode potential is −2.93 V, close to that of Li+/Li (−3.05 V), which is conducive to improving battery voltage and energy density [14][15][16]. In addition, the desolvation energy of Na+ based on propylene carbonate solvent is 158.2 kJ/mol, obviously lower than that of Li+ (215.8 kJ/mol), which can form small solvated ions with faster diffusion kinetics in the battery reaction [17]. More importantly, the similar properties of Li and Na mean that the research and development of SIBs have many reference bases. For example, substituting many Li-containing cathodic materials with electrochemical activity with Na would lead to similar activity in SIBs, such as NaFePO4, NaCo1-x-yNixMnyO2, and other layered rock-salt structures [18]. The transition metal oxides, sulfides, and carbon materials, as well as silicon, have superior Li+ storage performance and also cause electrochemical activity in SIBs. This similar technical route has helped researchers make important progress in the research and development of SIBs, and the technologies have developed rapidly in a relatively short time [1][18][19][20][21][22][23]. However, large differences in the radius of movable ions (1.02 and 0.76 Å for Na+ and Li+) result in slow kinetics, serious volume expansion in discharge–charge processes, and capacity decay, as well as the poor rate performance and cyclability of SIBs. Thus, it is the main bottleneck in developing long-life electrode materials with the stable intercalation/deintercalation of Na+, which seriously restricts the practical application of SIBs in the future [19].
MXenes are novel two-dimensional (2D) materials with excellent conductivity and low ion diffusion barriers that are widely used in energy storage and conversion, electrolysis, adsorption, and other surface-related fields [24][25][26][27]. MXenes, such as Ti3C2, are fabricated by selectively etching Al layers in MAX Ti3AlC2 and have become one of the most important materials in electrochemical ESCDs. MXenes can mainly be obtained using two typical etching methods: wet chemical etching and molten salt etching. The resultant ultrathin 2D MXenes have large specific surface areas, high conductivity, rich active sites, and 2D ion transport channels [28], which can provide rich active sites for ion storage and enhanced reaction kinetics [27][28]. These superior properties endow them with highly applicable potential in SIBs, LIBs, Li-O2 batteries, Li-S batteries, aqueous Zn-ion batteries, and supercapacitors [29][30][31][32][33][34]. As electrode materials, their ultrathin structures have large specific areas, which are conducive to mass transport in the electrode, especially regarding the accessibility of electrolytes [35][36]. Simultaneously, with a typical layered structure, they have large layer spacing, which benefits the transfer of large ions in the interlayer and presents unobvious volume changes, ensuring structural stability. In addition, surface areas with functionalized group terminations could serve as active sites to adsorb or capture intermediators in discharge–charge processes. More importantly, this 2D structure has excellent electron conductivity, which causes fast electron transfer in the discharge–charge process and facilitates reaction kinetics [31][37]. In addition, MXenes have good adjustability, which can be converted from an accordion-like three-dimensional (3D) structure into nanodots, nanobelts, nanosheets, and porous 3D structures. They can also be functionalized using controllable surface modification via loading or the in situ generation strategy to construct new and highly efficient composites.
For MXenes, when used in batteries as active materials, their performance is limited by inevitable stacking, resulting in low capacity as well as poor durability and rate performances [26][38]. To effectively utilize MXenes, there has been extensive research carried out to optimize their electrochemical performance via surface structure functional modification, as well as the construction of heterostructures and hybrid structures. When MXenes are employed in compounding, their layered structures could provide more channels, and other materials adhering to MXenes or grown between MXene sheets could expand the layered space in MXenes and relieve the volume expansion in operation. Therefore, MXenes have good advantages in enhancing the stability and reversibility of sodium storage, and they can be developed into materials with high-capacity and good rate performance via reasonable structural design and combining their advantages with other sodium storage materials.
2. Structure and Synthesis of MXenes
2.1. Structure Characteristics
MXenes contain transition metal carbides and nitrides, marked as Mn+1XnTx. MXenes are obtained by etching MAX (Mn+1AXn), where M is the Ti, Mo, Zr, and Cr elements; A is the Al, Si, Ga, and Ge elements; X is the C and N elements; and T is the functional groups on the MXene’s surface [39]. MXenes have a hexagonal, closely packed structure with an ABABAB type. However, due to the influence of element proportions, face-centered cubic structures also exists in M3×2 and M4X3 with an ABCABC type [40]. Many MXene types have been explored and reported over the past decade, such as Ti2C, Ti3C2, Ti3CN, Ti4N3, V2C, Cr3TiC2, Mo2C, Mo3ScC2, Zr3C2, and Nb2C [39][40][41][42]. The structure of MXenes is highly dependent on the parent materials. Among these different MXenes, Ti3C2 has been widely investigated due to its accessibility, which is obtained from the hexagonal structure of Ti3AlC2, even with scale-up production [43]. With the removal of the Al layer from Ti3AlC2, the Ti3C2 phase presents high electron density at the Fermi level compared with the parent-phase Ti3AlC2, displaying metal conductivity. For MXenes, the majority of them have metal-like electron conductivity and hydrophilic natures due to conductive cores and rich surface-functionalized groups formed in the preparation process, which have good adjustability [44]. Benefitting from their unique structure, MXenes are considered promising 2D materials in electrochemical energy conversion and storage.
2.2. Controllable Synthesis
MXenes are usually prepared by selectively removing A from the Mn+1AXn phase with Lewis acid (an HF solution, a mixing fluoride solution, or molten salt-containing halogen ions) [27]. After etching, an MXene changes from the block structure of its precursor to an accordion-like structure, and the surface of the MXene sheet will produce rich functional groups, such as F and OH, which also lay a structural foundation for the MXene’s easily modified properties. To obtain high-quality MXenes, the accordion-like structure needs to be further intercalated and stripped to form ultrathin MXene nanosheets so as to assure the advantages of having large surface areas and high conductivity. In an HF solution system, after etching, the MXene is chemically intercalated with organic molecules (such as DMSO and TMAOH) to form monolayer or few-layer MXene nanosheets [45]. As for the mixing fluoride solution method (HCl + LiF/NaF/KF), which is a more environmentally friendly and safer strategy, the combination of HCl and fluoride salts generates the HF solution and intercalation solvent, so the etching and delamination processes can be carried out simultaneously, which is for better controlling the MXene’s size and quality [46]. MXenes can also be obtained in molten salt, avoiding oxidation and surface defects, as well as producing adjustable functional groups. For example, Ti3C2Br2 was successfully synthesized by halogen-compound-etching Ti3AlC2 in [47], while iodine in anhydrous acetonitrile benefited to oxygen-terminal Ti3C2Tx [48]. In addition, Sun et al. successfully realized MXenes exfoliated under fluoride-free action with LIB reactions by al-loying Li and Al layers at low potential, combined with the strategy of using a water-phase micro-explosion reaction, which is a relatively new and environmentally friendly method but may be limited by the output of products [49]. At present, this method is not widely used. Even so, the synthesis method, which used etchant, pH conditions, and treated temperatures, could affect the quality and productivity of MXenes, as well as the determination of the termination type and its distribution [44]. Therefore, in order to obtain an MXene with a specific structure and composition, it is very important to select appropriate preparation methods and conditions, which play an important role in the subsequent functionalization or further applications of MXenes. As new, ultrathin 2D materials, MXenes have many advantages and excellent characteristics, such as adjustable layer spacing, high conductivity, outstanding mechanical stability, rich polar functional groups, large surface areas, and modifiability. Therefore, they are widely used in electrochemical energy storage, such as SIBs, LIBs, Li-S batteries, Li-O2 batteries, and supercapacitors [31][32][34][49][50].
3. Anode Materials Based on MXenes and Their Composites
Sodium storage performance based on MXenes is restricted by inevitable stacking, low specific capacity, and high charge voltage [25][26][51]. To effectively utilize MXenes, many studies have been carried out. One of the main strategies is to effectively regulate the composition, structure, and surface characteristics of MXenes in order to achieve excellent sodium storage activities. Another important strategy is to construct MXene-based composite structures [1]. In composites, layered MXenes can provide more ion channels, and other sodium storage materials uniformly adhered to or grown on MXene sheets can expand the layered space. In particular, cheap, nonmetal materials such as Si, P, C, and low-cost oxides and sulfides have relatively high theoretical capacities [51][52][53][54]. However, as shown in Figure 1, most of them result in low electron conductivity and serious volume expansion in discharge–charge processes, which results in poor rate performances and cyclability. Reducing the particle size and simultaneously introducing a high-conductivity substrate are effective ways of improving performance. Thus, the construction of MXene-based composites with sodium storage materials could improve specific capacity, cyclability, and rate performance via enhanced conductivity, stable intercalation/deintercalation, and promoted kinetics, which is the current focus of research.
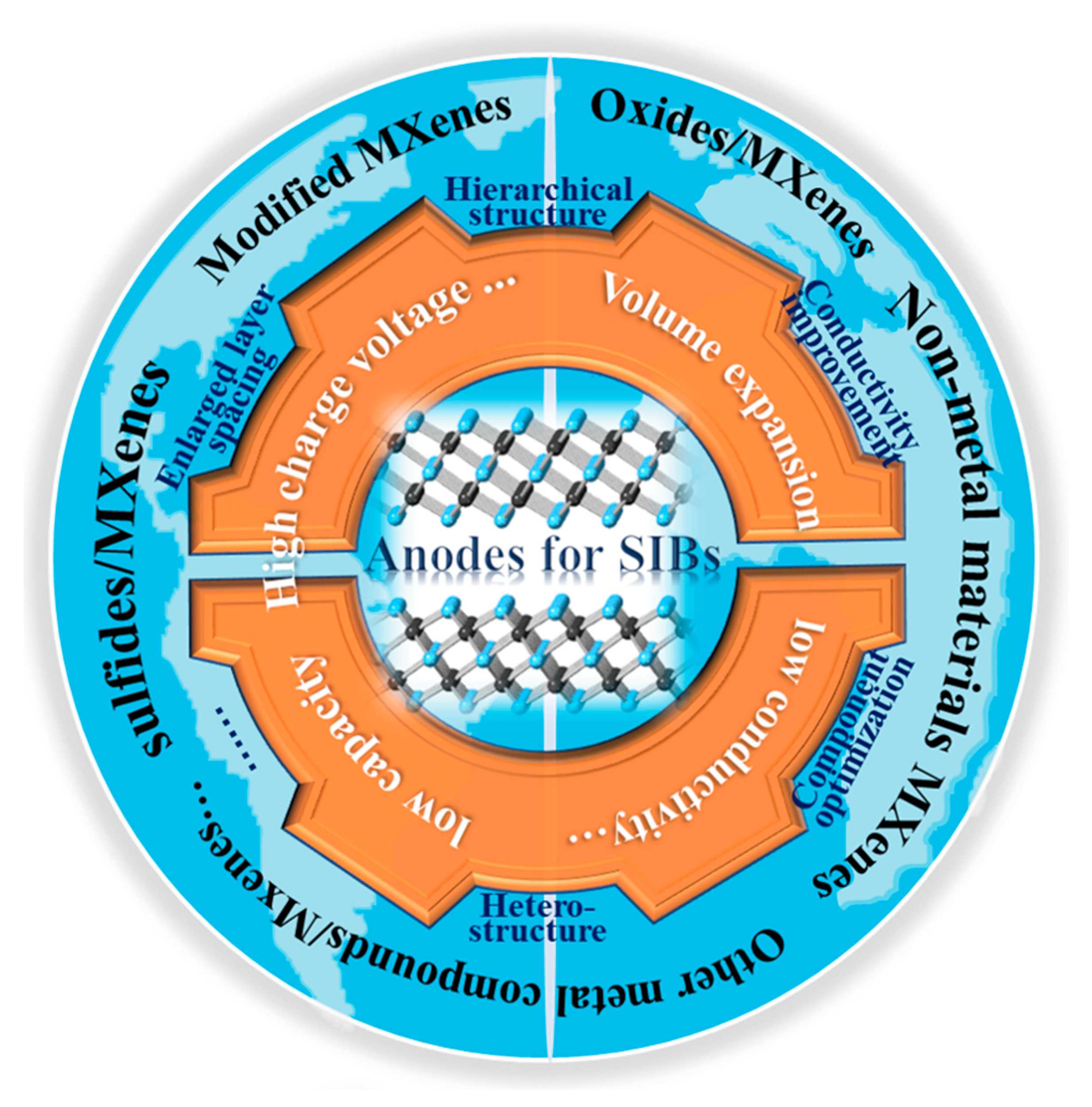
Figure 1. Problems in SIBs and the structural designs of MXene-based materials.
This entry is adapted from the peer-reviewed paper 10.3390/batteries9010048
References
- Ma, P.; Fang, D.; Liu, Y.; Shang, Y.; Shi, Y.; Yang, H.Y. MXene-Based Materials for Electrochemical Sodium-Ion Storage. Adv. Sci. 2021, 8, e2003185.
- Ni, Q.; Bai, Y.; Wu, F.; Wu, C. Polyanion-Type Electrode Materials for Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1600275.
- Ansari, M.Z.; Seo, K.M.; Kim, S.H.; Ansari, S.A. Critical Aspects of Various Techniques for Synthesizing Metal Oxides and Fabricating Their Composite-Based Supercapacitor Electrodes: A Review. Nanomaterials 2022, 12, 1873.
- Wang, W.; Zhang, J.; Li, C.; Kou, X.; Li, B.; Yu, D.Y.W. P2-Na2/3Ni2/3Te1/3O2 Cathode for Na-ion Batteries with High Voltage and Excellent Stability. Energy Environ. Mater. 2022.
- Liu, D.; Lu, S.; Xue, Y.; Guan, Z.; Fang, J.; Zhu, W.; Zhuang, Z. One-pot synthesis of core-shell nanoparticles as highly active hydrogen oxidation reaction electrocatalyst in alkaline electrolyte. Nano Energy 2019, 59, 26–32.
- Liu, B.; Sun, Y.; Liu, L.; Chen, J.; Yang, B.; Xu, S.; Yan, X. Recent advances in understanding Li–CO2 electrochemistry. Energy Environ. Sci. 2019, 12, 887–922.
- Liu, B.; Chen, J.; Yang, B.; Liu, L.; Sun, Y.; Hou, R.; Lin, Z.; Yan, X. Boosting the performance of lithium metal capacitors with a Li composite anode. J. Mater. Chem. A 2021, 9, 10722–10730.
- Zhao, D.; Qin, J.; Zheng, L.; Cao, M. Amorphous Vanadium Oxide/Molybdenum Oxide Hybrid with Three-Dimensional Ordered Hierarchically Porous Structure as a High-Performance Li-Ion Battery Anode. Chem. Mater. 2016, 28, 4180–4190.
- Tran, M.-K.; DaCosta, A.; Mevawalla, A.; Panchal, S.; Fowler, M. Comparative Study of Equivalent Circuit Models Performance in Four Common Lithium-Ion Batteries: LFP, NMC, LMO, NCA. Batteries 2021, 7, 51.
- Kuntz, P.; Raccurt, O.; Azaïs, P.; Richter, K.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Bardet, M.; Buzlukov, A.; Genies, S. Identification of Degradation Mechanisms by Post-Mortem Analysis for High Power and High Energy Commercial Li-Ion Cells after Electric Vehicle Aging. Batteries 2021, 7, 48.
- Zhang, S.; Liu, Y.; Hao, J.; Wallace, G.G.; Beirne, S.; Chen, J. 3D-Printed Wearable Electrochemical Energy Devices. Adv. Funct. Mater. 2021, 32, 2103092.
- He, X.-X.; Zhao, J.-H.; Lai, W.-H.; Yang, Z.; Gao, Y.; Zhang, H.; Qiao, Y.; Li, L.; Chou, S.-L. Challenges and Applications of Flexible Sodium Ion Batteries. Mater. Lab 2022, 1, 210001-1.
- Wu, X.; Ru, Y.; Bai, Y.; Zhang, G.; Shi, Y.; Pang, H. PBA composites and their derivatives in energy and environmental applications. Coordin. Chem. Rev. 2022, 451, 214260.
- Wang, N.; Chu, C.; Xu, X.; Du, Y.; Yang, J.; Bai, Z.; Dou, S. Comprehensive New Insights and Perspectives into Ti-Based Anodes for Next-Generation Alkaline Metal (Na+, K+) Ion Batteries. Adv. Energy Mater. 2018, 8, 1801888.
- Wang, F.; Liu, X.; Chen, L.; Chen, C.; Liu, Y.; Fan, L. Recent progress in key materials for room-temperature sodium-ion battery. J. Electrochem. 2019, 25, 55–76.
- Chen, J.; Zhong, X.; He, C.; Wang, X.; Xu, Q.; Li, J. Synthesis and Raman Study of Hollow Core-Shell Ni1.2Co0.8 as an Anode Material for Sodium-Ion Batteries. J. Electrochem. 2020, 26, 328–337.
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-Ion and Na-Ion Batteries as “Beyond Li-Ion”. Chem. Rec. 2018, 18, 459–479.
- Mu, L.-Q.; Hu, Y.-S.; Chen, L.-Q. New layered metal oxides as positive electrode materials for room-temperature sodium-ion batteries. Chin. Phys. B 2015, 24, 038202.
- Xu, X.; Liu, J.; Liu, J.; Ouyang, L.; Hu, R.; Wang, H.; Yang, L.; Zhu, M. A General Metal-Organic Framework (MOF)-Derived Selenidation Strategy for In Situ Carbon-Encapsulated Metal Selenides as High-Rate Anodes for Na-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1707573.
- Wei, X.; Li, Y.; Peng, H.; Zhou, M.; Ou, Y.; Yang, Y.; Zhang, Y.; Xiao, P. Metal-organic framework-derived hollow CoS nanobox for high performance electrochemical energy storage. Chem. Eng. J. 2018, 341, 618–627.
- Zhao, C.; Lu, Y.; Chen, L.; Hu, Y.-S. Ni-based cathode materials for Na-ion batteries. Nano Res. 2019, 12, 2018–2030.
- Mohsin, I.U.; Ziebert, C.; Rohde, M.; Seifert, H.J. Thermophysical Characterization of a Layered P2 Type Structure Na0.53MnO2 Cathode Material for Sodium Ion Batteries. Batteries 2021, 7, 16.
- Nisa, S.S.; Rahmawati, M.; Yudha, C.S.; Nilasary, H.; Nursukatmo, H.; Oktaviano, H.S.; Muzayanha, S.U.; Purwanto, A. A Fast Approach to Obtain Layered Transition-Metal Cathode Material for Rechargeable Batteries. Batteries 2022, 8, 4.
- Zhang, T.; Zhang, L.; Hou, Y. MXenes: Synthesis strategies and lithium-sulfur battery applications. eScience 2022, 2, 164–182.
- Kajiyama, S.; Szabova, L.; Sodeyama, K.; Iinuma, H.; Morita, R.; Gotoh, K.; Tateyama, Y.; Okubo, M.; Yamada, A. Sodium-Ion Intercalation Mechanism in MXene Nanosheets. ACS Nano 2016, 10, 3334–3341.
- Aslam, M.K.; Xu, M. A Mini-Review: MXene composites for sodium/potassium-ion batteries. Nanoscale 2020, 12, 15993–16007.
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581.
- Li, M.; Mullaliu, A.; Passerini, S.; Giorgetti, M. Titanium Activation in Prussian Blue Based Electrodes for Na-ion Batteries: A Synthesis and Electrochemical Study. Batteries 2021, 7, 5.
- Mateen, A.; Ansari, M.Z.; Abbas, Q.; Muneeb, A.; Hussain, A.; Eldin, E.T.; Alzahrani, F.M.; Alsaiari, N.S.; Ali, S.; Javed, M.S. In Situ Nitrogen Functionalization of 2D-Ti3C2Tx-MXenes for High-Performance Zn-Ion Supercapacitor. Molecules 2022, 27, 7446.
- Cao, Z.-J.; Zhang, Y.-Z.; Cui, Y.-L.-S.; Li, B.; Yang, S.-B. Harnessing the unique features of MXenes for sulfur cathodes. Tungsten 2020, 2, 162–175.
- Zheng, X.; Yuan, M.; Guo, D.; Wen, C.; Li, X.; Huang, X.; Li, H.; Sun, G. Theoretical Design and Structural Modulation of a Surface-Functionalized Ti3C2Tx MXene-Based Heterojunction Electrocatalyst for a Li-Oxygen Battery. ACS Nano 2022, 16, 4487–4499.
- Aslam, M.K.; AlGarni, T.S.; Javed, M.S.; Shah, S.S.A.; Hussain, S.; Xu, M. 2D MXene Materials for Sodium Ion Batteries: A review on Energy Storage. J. Energy Storage 2021, 37, 102478.
- Meng, J.; Zhang, F.; Zhang, L.; Liu, L.; Chen, J.; Yang, B.; Yan, X. Rolling up MXene sheets into scrolls to promote their anode performance in lithium-ion batteries. J. Energy Chem. 2020, 46, 256–263.
- Wen, C.; Zheng, X.; Li, X.; Yuan, M.; Li, H.; Sun, G. Rational design of 3D hierarchical 3/Ni(OH)2 nanohybrid for high-performance lithium-sulfur batteries. Chem. Eng. J. 2021, 409, 128102.
- Hussain, I.; Lamiel, C.; Sahoo, S.; Ahmad, M.; Chen, X.; Javed, M.S.; Qin, N.; Gu, S.; Li, Y.; Nawaz, T.; et al. Factors affecting the growth formation of nanostructures and their impact on electrode materials: A systematic review. Mater. Today Phys. 2022, 27, 100844.
- Hussain, I.; Lamiel, C.; Sufyan Javed, M.; Ahmad, M.; Chen, X.; Sahoo, S.; Ma, X.; Bajaber, M.A.; Zahid Ansari, M.; Zhang, K. Earth- and marine-life-resembling nanostructures for electrochemical energy storage. Chem. Eng. J. 2023, 454, 140313.
- Xie, X.; Kretschmer, K.; Anasori, B.; Sun, B.; Wang, G.; Gogotsi, Y. Porous Ti3C2Tx MXene for Ultrahigh-Rate Sodium-Ion Storage with Long Cycle Life. ACS Appl. Nano Mater. 2018, 1, 505–511.
- Li, H.; Liu, A.; Ren, X.; Yang, Y.; Gao, L.; Fan, M.; Ma, T. A black phosphorus/Ti3C2 MXene nanocomposite for sodium-ion batteries: A combined experimental and theoretical study. Nanoscale 2019, 11, 19862–19869.
- Tahir, M.; Khan, A.A.; Tasleem, S.; Mansoor, R.; Sherryna, A.; Tahir, B. Recent advances in titanium carbide MXene-based nanotextures with influential effect of synthesis parameters for solar CO2 reduction and H2 production: A critical review. J. Energy Chem. 2023, 76, 295–331.
- Salim, O.; Mahmoud, K.A.; Pant, K.K.; Joshi, R.K. Introduction to MXenes: Synthesis and characteristics. Mater. Today Chem. 2019, 14, 100191.
- Hong, L.-F.; Guo, R.-T.; Yuan, Y.; Ji, X.-Y.; Li, Z.-S.; Lin, Z.-D.; Pan, W.-G. Recent progress of two-dimensional MXenes in photocatalytic applications: A review. Mater. Today Energy 2020, 18, 100521.
- Yuan, Z.; Wang, L.; Li, D.; Cao, J.; Han, W. Carbon-Reinforced Nb2CTx MXene/MoS2 Nanosheets as a Superior Rate and High-Capacity Anode for Sodium-Ion Batteries. ACS Nano 2021, 15, 7439–7450.
- Shuck, C.E.; Sarycheva, A.; Anayee, M.; Levitt, A.; Zhu, Y.; Uzun, S.; Balitskiy, V.; Zahorodna, V.; Gogotsi, O.; Gogotsi, Y. Scalable Synthesis of Ti3C2Tx MXene. Adv. Eng. Mater. 2020, 22, 1901241.
- Ibragimova, R.; Puska, M.J.; Komsa, H.P. pH-Dependent Distribution of Functional Groups on Titanium-Based MXenes. ACS Nano 2019, 13, 9171–9181.
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1716.
- Lipatov, A.; Alhabeb, M.; Lukatskaya, M.R.; Boson, A.; Gogotsi, Y.; Sinitskii, A. Effect of Synthesis on Quality, Electronic Properties and Environmental Stability of Individual Monolayer Ti3C2 MXene Flakes. Adv. Electron. Mater. 2016, 2, 1600255.
- Jawaid, A.; Hassan, A.; Neher, G.; Nepal, D.; Pachter, R.; Kennedy, W.J.; Ramakrishnan, S.; Vaia, R.A. Halogen Etch of Ti3AlC2 MAX Phase for MXene Fabrication. ACS Nano 2021, 15, 2771–2777.
- Shi, H.; Zhang, P.; Liu, Z.; Park, S.; Lohe, M.R.; Wu, Y.; Shaygan Nia, A.; Yang, S.; Feng, X. Ambient-Stable Two-Dimensional Titanium Carbide (MXene) Enabled by Iodine Etching. Angew. Chem. Int. Ed. 2021, 60, 8689–8693.
- Sun, Z.; Yuan, M.; Lin, L.; Yang, H.; Nan, C.; Li, H.; Sun, G.; Yang, X. Selective Lithiation–Expansion–Microexplosion Synthesis of Two-Dimensional Fluoride-Free Mxene. ACS Mater. Lett. 2019, 1, 628–632.
- Han, Q.; Zhou, Y.; Du, R.; Xiao, B.; Cheng, J.; Zhang, M.; Dong, C.; Sun, X.; Jiang, F.; Yang, J. Ti3C2Tx with a hydroxyl-rich surface for metal sulfides as high performance electrode materials for sodium/lithium storage. J. Mater. Chem. A 2021, 9, 14013–14024.
- Gou, L.; Jing, W.; Li, Y.; Wang, M.; Hu, S.; Wang, H.; He, Y.-B. Lattice-Coupled Si/MXene Confined by Hard Carbon for Fast Sodium-Ion Conduction. ACS Appl. Energy Mater. 2021, 4, 7268–7277.
- Zhang, W.; Pan, Z.-Z.; Lv, W.; Lv, R.; Shen, W.; Kang, F.; Yang, Q.-H.; Weng, Y.; Huang, Z.-H. Wasp nest-imitated assembly of elastic rGO/p-Ti3C2Tx MXene-cellulose nanofibers for high-performance sodium-ion batteries. Carbon 2019, 153, 625–633.
- Zhang, S.; Li, X.Y.; Yang, W.; Tian, H.; Han, Z.; Ying, H.; Wang, G.; Han, W.Q. Novel Synthesis of Red Phosphorus Nanodot/Ti3C2Tx MXenes from Low-Cost Ti3SiC2 MAX Phases for Superior Lithium- and Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 42086–42093.
- Meng, R.; Huang, J.; Feng, Y.; Zu, L.; Peng, C.; Zheng, L.; Zheng, L.; Chen, Z.; Liu, G.; Chen, B.; et al. Black Phosphorus Quantum Dot/Ti3C2 MXene Nanosheet Composites for Efficient Electrochemical Lithium/Sodium-Ion Storage. Adv. Energy Mater. 2018, 8, 1801514.
This entry is offline, you can click here to edit this entry!
