1. Endocannabinoids: Synthesis, Release, and Metabolism
With the discovery of cannabinoid receptors, there has been interest in finding endogenous ligands that are responsible for their modulation. An evaluation of purified porcine brain fractions led to the identification of a new compound that binds to CB1R. Arachidonylethanolamide, an arachidonic acid derivative in the porcine brain, was characterized and named anandamide (AEA), a word derived from the Sanskrit word ananda, which means extreme happiness [
18,
77,
80,
81,
82].
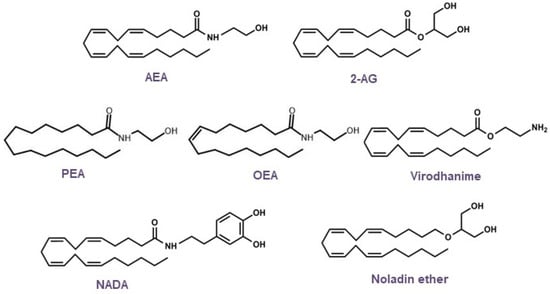
Based on the structural elucidation of AEA, other endogenous lipid molecules were identified (
Figure 4) and are generally called
N-acylethanolamines (NAEs), such as 2-arachidonoylglycerol (2-AG),
N-oleoylethanolamine (OEA), 2-arachidonyl glyceryl ether (noladin, 2-AGE), virodhamine,
N-arachidonoyldopamine (NADA), and
N-palmitoylethanolamine (PEA). AEA and 2-AG are the most studied endogenous ligands; however, research on endocannabinoids has since been conducted, and additional receptors, along with their lipid mediators and signaling pathways, have been revealed [
81,
83,
84,
85,
86,
87,
88,
89,
90,
91,
92,
93].
Figure 4. Chemical structures of the main endocannabinoids.
Endocannabinoids, unlike classical neurotransmitters, are considered atypical messengers because of the modulation of information from postsynaptic terminals to presynaptic terminals, which is known as the retrograde signaling mechanism. Endogenous ligands are synthesized on demand or by activity dependent on the cleavage of the phospholipid membrane and are released immediately after their biosynthesis to act as pro-homeostatic factors through interactions with specific receptors [
77,
94,
95].
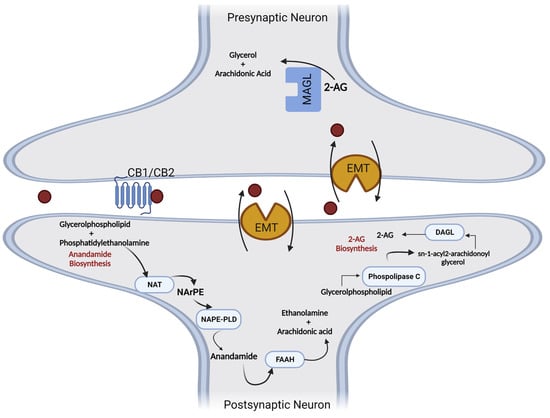
The synthesis and degradation of endogenous cannabinoid receptor ligands involve different enzymatic reactions. AEA biosynthesis occurs through its release from membrane phospholipids and can follow the Ca
2+-dependent
N-acyltransferase (NAT) or Ca
2+-independent
N-acyltransferase (iNAT) pathways. Therefore,
N-arachidonoyl-phosphatidylethanolamine (NArPE) is formed, and by the action of
N-Acyl-phosphatidylethanolamine-specific phospholipase D (NAPE-PLD), NArPE is converted to
N-arachidonoylethanolamine (AEA) [
55,
96,
97].
Another endogenous ligand, 2-AG, is formed via a two-step mechanism. Initially, 1,2-diacylglycerol (DAG) is synthesized after the cleavage of a membrane phospholipid by the phospholipase C (PLC) enzyme. DAG is subsequently esterified by the enzyme diacylglycerol lipase (DAGL), creating 2-AG [
98,
99].
Endogenous cannabinoids become inactive through a cellular reuptake mechanism involving membrane transporters (EMT), followed by intracellular degradation through the action of hydrolytic enzymes. Anandamide is mainly metabolized by the fatty acid amide hydrolase enzyme (FAAH), and 2-AG is a substrate of monoacylglycerol lipase (MAGL), which produces arachidonic acid (AA) and glycerol [
100,
101] (
Figure 5).
Figure 5. Metabolic pathways involved in the synthesis and degradation of anandamide (5) and 2-arachidonyl glycerol (6). AA—arachidonic acid; 2-AG-2—arachidonylglycerol; DAGL—diacylglycerol lipase; EMT—membrane transporters; FAAH—fatty acid amide hydrolase; MAGL—monoacylglycerol lipase; NAPE-PLD—N-arachidonylphosphatidylethanolamine phospholipase D; NArPE—N-acylphosphatidylethanolamine; NAT—N-acyltransferase; PLC—phospholipase C.
Furthermore, AEA and 2-AG may be susceptible to oxidative mechanisms catalyzed by cyclooxygenases (COXs), lipoxygenases (LOXs), and enzymes involved in the oxidation of arachidonic acid (AA), which is biotransformed into prostaglandins (PG), eicosanoids, and hydroxy-peroxy-anandamide, among other compounds derived from this metabolic reaction [
55,
77].
The endocannabinoid deficiency theory is based on the concept that many brain disorders are associated with a deficiency of neurotransmitters, such as acetylcholine in Alzheimer’s disease (AD), dopamine in Parkinsonian syndromes, and serotonin and norepinephrine in depression, and a comparable deficiency in endocannabinoid levels might similarly manifest in certain disorders that exhibit predictable clinical features as sequelae of this deficiency [
102,
103,
104].
In 2004, Professor Dr. Ethan Russo and his coworkers proposed clinical endocannabinoid deficiency syndrome (CDS), suggesting that an endocannabinoid depletion (hypofunctional eCB) could cause many diseases, such as migraine, a highly complex disease that involves signaling between different areas of the brain and various neurochemical transmitters. The exact cause of migraine is not fully understood, although genetic predisposition is considered a primary contributor to its genesis and modulation [
102,
104]. The possible relationship between migraine and the endocannabinoid system has been highlighted by several studies [
105,
106].
Fibromyalgia is also related to deficiencies in the endocannabinoid system and is characterized by acute and chronic widespread musculoskeletal pain throughout the body. This pain is more often accompanied by other conditions such as insomnia, migraine, mood swings, memory problems, irritable bowel syndrome (IBS), and chronic fatigue. The presence of characteristic painful nodules, known as trigger points, is notable and particularly prevalent in the shoulders and neck. Similar to migraine, fibromyalgia is associated with hyperalgesia, a lowered pain threshold associated with central endocannabinoid hypofunction in the spinal cord. According to Russo et al., the approved drugs for fibromyalgia, duloxetine, milnacipran (serotonin and adrenergic inhibitors, respectively), and pregabalin (an anticonvulsant used to treat neuropathic pain) showed little efficacy in treating fibromyalgia compared to
Cannabis [
106,
107,
108].
IBS, also known as spastic colon, is a functional disorder characterized by GIT pain, spasm, discomfort, and altered bowel movements, predominantly diarrhea. GIT propulsion, secretion, and inflammation in the gut are modulated by the endocannabinoid system, providing a rationale for the inclusion of cannabinoids in IBS treatment [
109]. Studies have shown that increased capsaicin receptor TRPV1 expressing sensory fibers may contribute to visceral hypersensitivity and pain in IBS and provide a new therapeutic target. Cannabidiol could be used for therapeutic interventions because of its effect on vanilloid VR1 receptors; it also enhances anandamide signaling. Its analogs have been shown to be potent inhibitors of anandamide cellular uptake [
110,
111,
112,
113,
114,
115].
Neurodegenerative disorders may lead to the development of Parkinson’s disease (PD) and AD. Normally, they are characterized by cognitive impairment and other neurological defects. Currently, the endocannabinoid system is studied as a drug target in PD and AD because of the overexpression of endocannabinoid system receptors, which exert neuroprotection against PD and reduce neuroinflammation in AD. Increased levels of AEA were found in the cerebrospinal fluid of untreated patients with PD, which was suggested to be a possible compensatory mechanism. Cognitive deficits in AD patients correlate with cerebral disturbances in sensitive brain areas, largely in the frontal cortex and hippocampal regions, which are rich in CB1Rs. Δ9-THC and CBD showed neuroprotection in PD and AD animal models; however, they triggered toxic effects in patients when administered directly. Studies are necessary to determine the therapeutic efficacy of targeting the endocannabinoid system in neurodegenerative diseases [
101,
116,
117,
118].
In some cases, eCB might be hyperfunctional, promoting cognitive deficits that may be noticeable in fragile X syndrome (FXS), Down syndrome, and Williams–Beuren syndrome (WBS). In addition to the genetic causes of these syndromes, it is believed that eCB is overactivated. In an animal model of FXS, knockout mice with fragile X mental retardation protein (Fmr1) recapitulate the main features of the disease. BlockingCB1R and CB2R in male Fmr1 knockout mice normalized the cognitive impairment and anxiolytic-like behavior, respectively [
119]. In a preclinical model of Down syndrome, the segmental trisomic Ts65Dn mouse model showed that CB1R expression was enhanced, and its function increased in hippocampal excitatory terminals. The knockdown and inhibition of CB1R repaired memory deficits in male Ts65Dn mice [
120]. To evaluate a model mimicking WBS, mice with the same genetic deletion found in patients with WBS were used. Male mice showed hypersocial behaviors, memory deficits, enlarged hearts, and differences in the function of CB1R. These mutant mice received JZL184, an MAGL inhibitor, which improved their social and memory symptoms and cardiovascular function [
121]. These studies show that the modulation of eCB hyperactivity is a promising therapeutic approach for cognitive deficits associated with genetic syndromes.
2. Molecules That Modulate the Endocannabinoid System
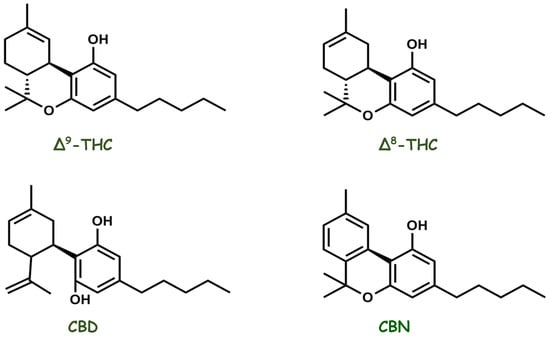
Cannabis, an herbal medicine, is a complex mixture of several compounds, including cannabinoid phenols, non-cannabinoid phenols (simple phenols, spiro-indans, dihydrophenanthrenes, and dihydrostilbenes), flavonoids, terpenoids, alcohols, aldehydes, n-alkanes, wax esters, steroids, and alkaloids. In 1899, Wood isolated cannabinol (CBN), the first compound purified from the plant. Currently, more than 500 different substances have been isolated and reported from
Cannabis plants belonging to different classes, among which the class of cannabinoid compounds is the most representative because it has more than 120 identified compounds, such as delta-eight and delta-nine tetrahydrocannabinol (Δ8-THC and Δ9-THC), CBD, and CBN (
Figure 6). Diverse classes of secondary metabolites from different parts of the plant have been identified, with a wide range of applications (nutraceuticals, cosmetics, aromatherapy, and pharmacotherapy) that are beneficial to humans. However, in the past, studies were focused on the two most abundant phytocannabinoids, THC and CBD, thus resulting in greater knowledge about their pharmacological activities and increasing interest in the numerous possibilities of the medicinal actions of the plant [
109,
122,
123,
124,
125,
126,
127,
128]. CBD has been gaining prominence in pharmacological research since the 1970s. Epidiolex
®, a purified oral CBD medicine, is currently approved by the U.S. Food and Drug Administration for the treatment of intractable childhood-onset seizures [
129,
130].
Figure 6. Chemical structures of the main pharmacologically active cannabinoid compounds isolated from Cannabis sativa.
However, the biochemical basis of the pharmacological activity of cannabinoids has remained an enigma for many years. The highly lipophilic molecular structure of cannabinoids suggests that they exert their effects by penetrating cell membranes and acting in the CNS. Currently, important insights into the physicochemical properties of cannabinoids are available. Novel selective ligands for cannabinoid receptors can have specific substituents that increase binding kinetics and decrease side effects [
131,
132,
133,
134,
135].
3. Endocannabinoid System Emerging as a Pharmacotherapy Target for Chronic Pain and Mood Disorders
Pain is described as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or in terms of such damage. When pain persists or recurs for longer than three months, it is defined as chronic pain and has a major impact on society. An estimated 20% of the global population suffers from chronic pain. Importantly, depression and anxiety are significantly observed in such patients [
136,
137,
138]. Pain therapy includes both pharmacological and non-pharmacological treatment options. Antidepressants, anticonvulsants, and drugs that act on the CNS are commonly recommended for chronic pain treatment. Therapeutic agents are considered adjuvant analgesics, medications that were not primarily developed as analgesics but have pain-relieving properties, and are the first-line drugs for neuropathic pain treatment and psychiatric problems [
139]. However, some patients do not show pain alleviation and seek other therapies to reverse their condition. Pain relief was already described by the Chinese in the third millennium BC due to the use of extracts of the hemp plant (
Cannabis sativa) to cause a variety of medicinal effects. Recently, interest in the medicinal properties of
Cannabis sativa has resurged with the emergence of the eCB, offering not only new insights into the mechanisms underlying the therapeutic actions of cannabinoid-like molecules and phytocannabinoids but also novel molecular targets for the pharmacotherapy of pain [
2,
140]. Studies in animal models of acute pain showed that Δ9-THC, CBD, AEA, and synthetic cannabinoids such as CP55,940 and WIN 55,212-2 had antinociceptive actions [
141,
142,
143,
144,
145,
146,
147,
148]. In a model of chronic pain, AEA and cannabinoid ligands were effective treatments [
147,
149,
150,
151,
152]. The combination of endocannabinoids and synthetic cannabinoids with nonsteroidal anti-inflammatory drugs promotes synergistic antinociceptive effects and may be useful in the pharmacotherapy of pain. In addition, studies of paracetamol (acetaminophen) activity, the most widely used painkiller, suggest that its analgesic efficacy is, in part, mediated by CB1R stimulation [
144,
153,
154,
155,
156,
157]. Natural and synthetic cannabinoids, such as dronabinol and nabilone, have been studied in humans for chronic pain relief, and therapeutic efficacy for pain management and quality of life improvement in patients was observed. The eCB is distributed throughout the spinal and supraspinal regions, thus can effectively regulate nociceptive processing [
158,
159,
160,
161,
162,
163].
CB1Rs may be activated by THC, producing analgesia and adverse events (e.g., headache, numbness, cough, burning sensation, dizziness, feeling high, somnolence, and dry eyes and mouth) [
164,
165]. Many of the psychoactive events depend on THC concentrations and might become important disadvantages of its use as a pharmacological therapy [
166]. A previous study demonstrated that CB1R activation causes memory impairment [
167,
168]. In addition, THC and other cannabinoids activate serotonin 2A receptors (5-HT2AR), modulating memory deficits, anxiolytic-like effects, and social interaction [
169]. Previous studies have shown that CB1R and 5-HT2AR form heteromers that are expressed and functionally active in specific brain regions involved in memory impairment, such as the hippocampus and prefrontal cortex [
167,
170,
171,
172]. However, memory deficits and anxiety were abrogated in wild-type mice with the use of a 5-HT2AR antagonist or the selective disruption of the CB1R/5-HT2AR heteromers by an infusion of synthetic interference peptides without losing the antinociceptive effect [
167]. Ongoing studies on the use of
Cannabis show that it promotes pain relief and dissociated memory impairment, reducing drawbacks for the use of cannabinoids as therapeutic agents [
168]. CB2R plays an important role in modulating analgesia via two pathways. The first mechanism occurs in the peripheral immune system, where CB2Rs mediate analgesia by modifying the cytokine profile and preventing adverse effects on the CNS. Secondly, CB2Rs present in glial cells and neurons contribute to pain relief [
173,
174]. Additionally, studies have shown that selective CB2 agonists promote antinociception [
175,
176].
Anxiety and panic disorders, major depressive conditions, and bipolar disorder (manic–depressive illness) are mood complications that are often serious and potentially life-threatening. More than 20% of the adult population experience mood disorders at some point in their lives [
177]. Many advances have been made in mood disorder treatment over the past decades. Approximately 30% of the population does not respond to current therapies, and the search for novel pharmacological approaches continues [
178,
179].
The psychoactive effects of
Cannabis include calming, anxiolytic, sleep-inducing, and euphoric effects. Some of these factors positively affect moods. However, symptoms such as paranoia, irritation, dysphoria, depression, depersonalization, and demotivation may appear in some individuals with such adverse effects [
51,
180]. The reactions depend on the patient’s endocannabinoid activity, the dose used (normally stimulating action at a low dose and inhibitory action at a high dose), the proportion of phytocannabinoids, and the terpenoid composition [
179,
181]. Evidence is increasing regarding the role of eCB in mood regulation. Clinical studies have shown altered endocannabinoid signaling in psychiatric patients [
182]. Genetic polymorphisms in CB1R and CB2R are associated with major depression, bipolar disorder, and resistance to therapy, which has been observed in depressed patients who have a single-nucleotide polymorphism in CB1R [
183,
184,
185,
186]. Moreover, eCB might modulate the functions of all hypothalamic–pituitary axes via CB1R, and chronic stress seems to reduce the eCB system’s ability to suppress stress and may induce psychopathology, including depression and anxiety [
187]. In this sense, it is important to remember the trajectory of the drug rimonabant, the first cannabinoid receptor blocker to be approved for metabolic syndrome treatment, obesity, and smoking. However, due to important adverse effects that became evident in patients following chronic exposure to rimonabant, Sanofi-Aventis withdrew it from the market. This drug mainly exerts its beneficial effects by blocking CB1 receptors in the periphery. However, due to its lipophilic nature, rimonabant can cross the blood–brain barrier and enter the CNS, and it is linked to the development of depression, suicidal feelings, and anxiety disorders [
188,
189,
190]. The mood-elevating properties of cannabinoids have long been known and are considered non-toxic. Some
Cannabis constituents or mixtures may have antidepressant and/or anxiolytic effects. Many patients who are nonresponsive to the usual pharmacological treatments for depression may benefit from medicinal
Cannabis use. Cannabinoids may have therapeutic potential for both depression and bipolar disorder. This is related to some patients adding
Cannabis to ongoing treatment since this association might improve the efficacy of such medication and/or reduce its side effects.
Cannabis may be a mood stabilizer in bipolar disorder and an adjuvant to lithium treatment. Patients experiencing a mood disorder may not be objective in assessing their condition and cannot decide on their own to modify the treatment. Thus, professional care and control are essential [
177,
179,
191,
192,
193,
194].
This entry is adapted from the peer-reviewed paper 10.3390/ph16020148