1. Application in the Production of Food Packaging
The use of nanomaterials in food packaging alters the penetration properties, improves the barrier properties, increases thermal resistance and leads to strong antimicrobial activity [
77]. Nanoparticles are characterized by their large surface area and high surface energy. This creates strong interfacial interactions between the polymer bonds and the nanoparticles. This significantly improves the properties of the bio/polymers used for packaging [
78].
One of the main problems during food storage is its contact with oxygen. This agent is responsible for many degradation reactions in food, such as lipid oxidation, microbial increase, enzymatic browning and nutritional degradation. Changes in food as a result of oxygen exposure result in a significantly reduced shelf life, especially for oxygen-sensitive foods [
79]. In order to limit the access of oxygen to food, it is most often packaged using materials that provide an active barrier against oxygen penetration [
80]. One way in which food can be protected from oxygen is through the use of iron nanoparticles. Two strategies are possible for the use of nano-iron in packaging. The first is to insert sachets containing nZVI into the packaging, and the second method is to incorporate the nanoparticles into the packaging film using a monolayer or multilayer structure [
81,
82]. An example is bentonite and kaolinite modified with Fe
0. These substances have been approved by the European Food Safety Authority (EFSA) as non-nanoparticles [
83]. Unlike normal iron, Fe
0 reacts with oxygen under non-humid conditions [
80]. Khalay et al. [
84] investigated the suitability of PP nanocomposites having in their compositions montmorillonite (OMMT) with IONP modification for food packaging. They found that the nanoparticles were active as an active oxygen scavenger, and were able to capture and absorb oxygen by chemically reacting with it. Similarly, Busolo and Lagaron [
82] reported that iron kaolinite contained in active composites is a passive barrier due to its winding pathway, which impedes gas diffusion and captures and reacts with molecular oxygen.
The most important problem during food storage is spoilage by microorganisms. The use of IONPs and nanocomposites with antimicrobial functions is an effective means to minimize the impact of microorganisms on food during processing or storage. In this way, they provide an extended shelf life and improved food safety [
85]. Iron nanoparticles can be used to address food oxidation by applying antimicrobial coatings to the inner surface of the packaging. Such a coating gradually releases the antimicrobial substances, or they are immobilized on the active surface of the packaging [
86]. Song et al. [
87] produced composites of polydopamine with iron oxide nanoparticles (IONPs@pDA). Nisin was conjugated on the IONP@pDA nanoparticles. The newly synthesized material showed good efficiency in reducing Alicyclobacillus acidoterrestris, a food-spoilage-causing bacterium that is a serious problem in the food industry. Recent research has focused on producing IONP composites with surfaces modified with other nanometals. There is strong interest in silver-coated magnetic nanocomposites because of their unique antimicrobial properties [
88].
2. Edible Coatings on Food
The use of films and coatings produced from natural edible polymers (biopolymers) on food is increasingly being used to extend the shelf life of fresh produce. These films and coatings have similar functions to conventional protective packaging or synthetic coatings [
89]. The use of food-approved nanoparticles has enabled the creation of functional edible coatings. These coatings consist of nanoemulsions, polymer nanoparticles, nanofibers, solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs) and traditional polymer nanocomposites (inorganic–organic) [
90]. One of the most commonly used ingredients in edible films is chitosan. Chitosan is a natural polymer composed of (1,4)-linked 2-amino-deoxy-b-D-glucan. It has been shown in many publications that chitosan is non-toxic, biodegradable, biofunctional, biocompatible and has antimicrobial properties. Furthermore, due to its ability to form transparent films and the good mechanical properties of films that can meet various packaging needs, chitosan is an excellent ingredient for edible packaging [
91]. Combining chitosan with nanoparticles seems to be an excellent solution. Nehra et al. [
92] reported that chitosan coated with Fe
3O
4 nanoparticles is an antimicrobial agent against
E. coli,
B. subtilis,
Candida albicans,
Aspergillus niger and
Fusarium solani. Shrifian-Esfahni et al. [
93] found that under in vitro conditions, a chitosan–Fe
3O
4 nanoparticle film showed inhibitory activity against
E. coli and
P. aeruginosa. SPIONs composed of magnetite (Fe
3O
4) are characterized by such properties, which can be used for antimicrobial applications [
94], especially since the U.S. Food and Drug Administration has found that SPIONPs are biocompatible (BC) with the human body [
95].
3. Immobilization of Enzymes
Enzymes are used as biocatalysts for large-scale production in many areas of industry. Their great advantage is their limited environmental impact. In the food industry, they make it possible to modify the structures of foods. For example, this is used in the production of lactose-free milk and calorie-reduced fats [
96]. Placing enzymes on magnetic nanoparticle carriers ensures a strong covalent bond between the enzyme and the carrier. By doing so, it minimizes the leaching of the enzyme in the aqueous environment and prevents the contamination of the product with other proteins. An important advantage of this solution is that a magnetic field can be used to separate the biocatalyst from the reactants. This protects against particle losses and provides high mechanical strength, thermal stability and resistance to chemical and microbial degradation [
97]. Another area where iron SPIONs have found application is in protein/enzyme immobilization. Here, the characteristic magnetic properties of SPIONs and the ease of separation in a magnetic field have been exploited. However, the most important factor is the ability to orient proteins/enzymes in the carriers; this protects against internal diffusion, which is a problem in traditional methods [
98].
4. Artificial Enzymes
Nanomaterials that exhibit enzyme-like activity are proposed as a new generation of artificial enzymes and referred to as “nanozymes” [
99]. The main advantage of nanozymes over their natural counterparts is the ability to regulate activity by changing the size or morphology, or through doping or surface modification. The most typical nanozymes are iron oxide nanoparticles (Fe
3O
4 and Fe
2O
3). They mimic the activity of peroxidase and catalase [
100]. Peroxidase-like activity is possible under acidic conditions. Ferromagnetic nanoparticles can catalyze the reaction of hydrogen peroxide with hydroxyl radicals. This means that IONPs can be referred to as IONzymes. As reported by Qin et al. [
101], IONzymes are antiviral agents because they induce the peroxidation of membrane lipids in synthesized liposomes. Their large surface area enables them to exert strong catalytic activity. This activity matches and sometimes exceeds that of natural enzymes. IONzymes are more stable and less expensive than natural enzymes. In addition, proteolytic degradation and environmental factors (temperature, pH, ionic strength and heavy metals) are no longer an issue. One of the most important features is that they do not degrade during storage at room temperature. The disadvantages of IONzymes are that they only bind to a specific substrate (substrate specificity) and show different catalytic activity, which is determined by the size and structure [
102].
5. Food Analysis
Iron nanoparticles have been widely used in food analysis methods [
103]. Magnetic nanoparticles are usually integrated into detection techniques in two ways: as an electrode modifier and as a sample preconcentrator [
98]. An example is the method based on magnetic microparticles coated with protein A (a component of the cell wall produced by several strains of
Staphylococcus aureus). This has been used for the accurate (0.2 mg/kg) detection of the allergen Ara h3 (a heat-stable protein that comprises 19% of the total protein in peanut extracts) in food. The method has high repeatability and reproducibility [
104]. Another example is the use of magnetic nanoparticles in glucose biosensors. The biosensors use immobilized oxidase to convert the target analytes into electrochemically detectable products. This makes the determination of glucose in food easier [
105]. Magnetite nanoparticles enable food analysis for the detection of certain elements (e.g., silver, copper, lead, cadmium and mercury). Magnetite nanoparticles are coated with silanes. This allows them to be functionalized with ligands that are selective for metal ions [
103] (
Table 5).
Table 5. Examples of the use of IONPs in food analysis.
In a study by Yang et al. [
117], it was shown that SPIONs can accelerate the preconcentration process for the detection of bacteria in food. Target bacteria used for PCR detection are concentrated using submicron superparamagnetic anion exchangers. This enabled the PCR detection limit to be shifted from 10
4–10
5 CFU mL
−1 to 10
2 CFU mL
−1.
Escherichia coli and
Agrobacterium tumefaciens were used as models of bacteria. Wen et al. [
115] described a one-step assay for the detection
of Salmonella typhimurium by fluorescent identification using IONPs with functional additives. The work of Chen et al. in [
118] used silica-coated SPIONs to extract DNA from pathogenic bacteria. In this method, preincubation of the sample was avoided and the influence of the food matrix was eliminated.
6. Protein Purification
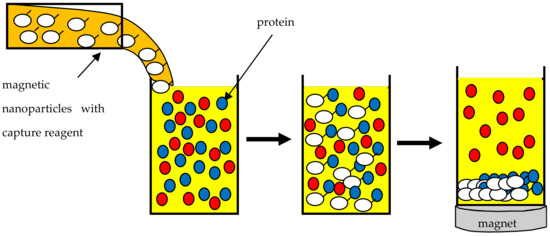
One increasingly used process in the food industry is the purification of proteins from isolates or concentrates. The resulting proteins can then provide the basis for new forms of food. For example, milk proteins fall into two distinct categories: casein micelles, which are often associated with cheese production, and serum proteins, which are involved in many functional properties, such as foaming, gelling and emulsification. In high-tech food production, it is necessary to use only one type of protein. Therefore, there is a need for a low-cost and efficient technique to obtain pure proteins [
119]. Traditionally, proteins are purified by chromatography, precipitation, ultrafiltration, centrifugation and dialysis. The inherent limitations of these methods are the long pretreatment times, equipment that is often expensive to purchase and operate and the need to hire experienced lab technicians. Magnetic separation, which has been known for many years, can be used for this purpose. It is characterized by several advantages. The technique using SPIONs is fast, scalable, sensitive and easy to automate and no special sample preparation is needed (
Figure 1).
Figure 1. Schematic illustration of a protein purification system based on magnetic nanoparticles.
SPIONs used for protein purification are immobilized with ligands. Primarily, these are ligands with confirmed affinity for the proteins to be purified [
105].
Another example is the use of SPIONs for the analysis and separation of hormones, drugs and contaminants from food. This technique is used in the study of food of animal origin. Traces of drugs may be present in this type of product. Such contaminated products, when consumed by humans, endanger their health [
103]. Estrogen hormones are administered as feed additives (called growth promoters) to accelerate growth and increase meat weight in cattle, poultry and pigs [
120]. As reported by Gao et al. [
100], functionalized Fe
3O
4 nanoparticles have been successfully used as sorbents for the specific separation and enrichment of proteins from milk. The authors report that they were able to recover estradiol in the range of 88.9 to 92.1%. Luo et al. [
121] used carbon nanotubes with SPIONs because of their excellent adsorption capacity against hydrophobic compounds. They were used as adsorbents in the extraction of phthalate acid esters. Esters such as phthalates and bisphenol A are derived from plastic packaging and are considered as substances with potential carcinogenic properties.
7. Iron Oxides as Ingredients in Foods and Dietary Supplements
The most important biological function of iron is oxygen transport, as it is part of the heme nucleus in the proteins hemoglobin and myoglobin. In living organisms, iron is involved in more than 200 enzyme systems that are essential for cellular function, particularly cellular energy utilization and DNA, RNA and protein synthesis. In addition, iron is involved in neurotransmitter metabolism, vitamin D activation, collagen metabolism and cholesterol catabolism. Therefore, iron is essential for the proper functioning of organisms, including humans [
122]. According to Dave and Gao [
33], ferritin is an intracellular protein found in the cells of the liver and immune system. It stores inactive iron in the body and releases it when needed. As reported by Kumari and Chauhan [
123], commonly used iron enrichment additives with high bioavailability (FeSO
4) are chemically reactive. These reactions cause color changes, a metallic aftertaste, rancidity in food products, etc. Due to these difficulties, FeSO
4 is replaced by water-insoluble compounds FePO
4, Fe
4(P
2O
7)
3 or ions of iron. These additives do not affect the properties of food but are poorly absorbed by the human body [
124]. Therefore, IONPs are an alternative to traditional iron supplements, preventing iron-deficiency-related diseases [
125], as similarly stated by von Moos et al. [
124] and Perfecto et al. [
126], using FePO
4 NPs as an iron source. In addition to iron oxides and phosphates, Hilty et al. [
127] used a Ca and Mg complex in iron oxide compounds. These minerals are essential for humans, so adding them to food poses no health risk. The authors showed that the addition of calcium and magnesium to Fe
2O
3 gives i.d.a. solubility > 85%, suggesting a high level of bioavailability. In addition, no caking was found during storage under various humidity conditions.
8. Colorants
Iron, in the form of various compounds, is used as a colorant (food additive E172). A range of colors can be obtained, such as black, red, yellow, blue, orange and brown, depending on the chemical composition. For example, magnetite (Fe
3O
4), with a particle size of 11.7 nm, is characterized by its black color. The red pigment is associated with hematite (α-Fe
2O
3) nanoparticles with a particle size of 39.5 nm, and the yellow pigment is attributed to goethite (α-FeOOH) nanoparticles with a particle size of 48.7 nm [
128]. Iron oxides are approved food pigments and have been shown to contain significant amounts of nanoparticles [
129].
9. Mycotoxin Removal
Mycotoxins are produced by filamentous fungi belonging to the Ascomycota cluster or molds and are toxic secondary metabolites. Their characteristic feature is their low molecular weight. The source of mycotoxins is usually infected food products. They are often carcinogenic and mutagenic; among other aspects, they inhibit DNA synthesis and cause changes in RNA metabolism [
130]. During various food processing technologies, including cooking, baking, frying, roasting and pasteurization, most mycotoxins remain chemically and thermally stable. Therefore, low-cost methods to eliminate these contaminants from food are still being sought [
131]. Iron oxide and graphene oxide nanostructures can be cheaply produced and are readily available. Mycotoxins interact with the surface oxygen functional groups of the nanostructures. Surface-active maghemite nanoparticles (γ-Fe
2O
3) have shown chelating properties for citrulline and ochratoxin A in the presence of iron(III). Another promising solution is IONP@chitosan complexes as patulin adsorbents. As reported Horky et al. [
132], after approximately 5 h, the patulin molecules were completely adsorbed. The adsorbent concentration was 400 μg, with a pH equal to the pH of apple juice. After adsorption, mycotoxins along with SPIONPs were removed in a magnetic field.
10. Anti-Allergic Effect
Histamine is found in some foods. In food, histamine is formed during storage as a result of the activity of bacteria—not only those intentionally added, but also those that contaminate it. Histamine content is considered one of the markers of food quality [
133]. This compound is one of the most important intermediates in the course of allergic reactions. Consumption of histamine-rich foods can cause nausea, headaches, diarrhea and asthma [
134]. According to Ghanbari, Adivi and Hashemi [
134], magnetic Fe
3O
4@ agarose/IDA@silica nanoparticles can be used to remove histamine from biological fluids. The histamine removal efficiency of real plasma samples was 92%.
11. Control of the Process Flow
Bacillus subtilis is used for the industrial production of a wide range of compounds in the fermentation process. One of the main problems of industrial fermentation is the formation of biofilms by this bacterium, which leads to many process and operational complications. Biofilms are an unfavorable phenomenon in industrial settings. Periodic cleaning of equipment is necessary. The use of SPIONs coated with aminopropyltriethoxysilane is a promising approach to control biofilm formation without losing the viability of B. subtilis cells [
135].
12. Preservation of Food in Supercooled State
Iron nanocrystals (Fe
3O
4) are a natural component of meat. They provide strong nucleation sites for heterogeneous ice crystallization. The ferromagnetism of magnetite nanoparticles allows an external oscillating magnetic field to induce magneto-mechanical motions. These movements can interfere with the formation of ice crystals in food by inducing a supercooled state. For this to occur, the magneto-mechanical rotation of the particles should be greater than the magnitude of Brownian motion [
136]. A study by Kang at al. [
137] found that fresh beef stored under refrigeration at −4 °C using an oscillating magnetic field was preserved for one week longer, without significant changes in weight loss, color or bacterial growth.
This entry is adapted from the peer-reviewed paper 10.3390/ma16020780