1. Silver Nanoparticles (Ag NPs)
Ag NPs can damage the extracellular membrane of bacteria and their intracellular components, exhibiting a broad-spectrum antimicrobial effect [
4]. Many Ag NP synthesis strategies have been developed to allow specific Ag NP surface properties, which, in turn, strongly depend on the characteristics of the reducing agent and the type of stabilizer used during their synthesis [
4,
73,
74,
75,
76,
77,
78,
79].
According to Sondi et al. [
80], Ag NPs can cause harm to
E. coli by forming pits in the cell wall, which could increase its permeability and affect the membrane vesicles. Such damage has also been observed in other bacteria, such as
Scrub typhus,
P. aeruginosa, and
Vibrio cholerae [
81], which is attributed to the ability of Ag NPs to interact with some of its components, such as lipopolysaccharides (LPSs), and phosphatidylethanolamines (PEs) [
82].
Mirzajani et al. [
83] suggested that the ability of Ag NPs to harm the bacterial cell wall may result from their interaction with the peptidoglycan layer, since Ag NPs attack the β-1-4 bonds of N-acetylglucosamine and N-acetylmuramic acid of the glycan chain in the cell membrane of
S. aureus. Additionally, Ag NPs may produce free radicals, such as reactive oxygen species (ROS), inside and outside the bacteria [
84,
85]. Elevated ROS levels are known to damage cell DNA, proteins, and enzymes, which could, in turn, interfere with the normal metabolism of bacteria [
86]. It was found that Ag
+ ions released from Ag NPs can damage bacterial membrane function. In particular, the differences in Ag
+ concentration can induce a difference between the pH and the electrical potential inside and outside the membrane vesicles in
Vibro cholerae, leading to the failure of membrane respiration and H
+ leakage [
87].
The effect of Ag NPs on the bacterial membrane is related to their physicochemical properties, such as size, shape, surface area, surface charge, oxidation state, and surface chemistry. It has been reported that Ag NPs with small size and colloidal stability are preferred rather than those susceptible to aggregation [
4,
13,
22,
66,
73,
88]. The size of NPs is one of the most critical aspects determining their interaction with cells. Actually, Ag NP interaction is size-dependent [
81,
89]. Several works have shown that Ag NPs with a diameter of 3–10 nm, are the most effective in killing bacteria due to their preferential direct interaction with the bacterial membrane [
52] and how fast the bacterial killing took place after their interaction [
89].
The shape of Ag NPs can directly influence the available contact area needed to facilitate interactions of Ag NPs with the bacterial membrane. A comparative study using polyvinylpyrrolidone (PVP)-coated Ag NPs with different shapes suggested a strong correlation between the shape of the Ag NPs and their bactericidal properties. For example, Ag nanoplates (two-dimensional structure, 2D) showed the highest antimicrobial activity against
S. aureus and
E. coli, when compared to Ag nanorods (one-dimensional structure, 1D) and spherical Ag NPs (zero-dimensional structure, 0D). Sadeghi et al. [
90] showed that Ag nanoplates exhibited the largest surface area, providing the most significant contact area to interact with the bacterial cell wall.
The Ag NP surface charge is also important. It was observed that positively charged Ag NPs using capping agents such as poly(amide amine) dendrimers (PAMAM) [
91], poly(ethyleneimine) (PEI) [
92], poly(ethylene glycol) (PEG), and polyvinylpyrrolidone (PVP) [
67] facilitated the interaction between the particles and the negatively charged bacterial membrane [
91]. Ag NPs with a negative surface charge have shown lower antimicrobial activity [
93] due to the strong repulsion between the particles and the bacterial wall. This limits the interaction between Ag NPs and bacteria and considerably weakens their antimicrobial effect.
Ag NPs have been also combined with antibiotics (ampicillin, amoxicillin, chloramphenicol, erythromycin, among others) [
94] by chelation of the active groups. The combination of Ag NPs with other materials, such as polycationic chitosan, has shown promising results by facilitating the attachment of Ag NPs to the negatively charged bacterial wall [
95]. Mishra et al. [
96] developed a multifunctional system of Ag NPs embedded in the chitosan-polyethylene glycol (CS-PEG) hydrogel. This implantable device inhibited biofilm formation and the released the drug payload at the same time. Chen et al. [
97] prepared a chitosan sponge containing Ag NPs and used it as a tissue for wound healing. Both in vitro and in vivo composite tests showed excellent antibacterial activity against drug-resistant pathogenic bacteria.
Recently, researchers discovered that Ag nanoclusters (NCs) are effective for this type of application [
98,
99]. NCs are NPs whose sizes are smaller than 2 nm and contain “countable” Ag atoms as a nucleus protected by organic ligands [
4]. Ag NCs have shown promising results for biomedical applications, such as bioimaging, biosensing, and antimicrobial agents [
100,
101]. These NPs have also been used to functionalize natural cellulose nanofibers [
102], silk fibers [
103], textiles [
104], and natural or synthetic polymer-based hydrogels to exhibit antimicrobial activity. Although there are many studies of antimicrobial Ag NPs embedded into hydrogels as platforms for delivering metallic nanostructures as alternative to standard drugs; their mechanism of action has not been entirely elucidated. Nevertheless, all the above examples demonstrate this as a promising strategy in preventing and eradicating infections [
7,
105].
2. Antibacterial Activity of Ag NPs Loaded into Hydrogels
Ag NPs incorporated into hydrogels have shown antibacterial properties and the ability to control infections [
37]. The NPs are incorporated into a hydrogel with a porous structure by in situ polymer synthesis or by adding the NP colloid to the polymer. Additionally, microwave radiation is another approach to produce NPs within hydrogels. The polymer-based hydrogels help to control the morphology and size of the nanostructures and participate as a stabilizing medium for nucleation sites to produce silver seeds [
13,
66]. Biocompatible polymers, such as chitosan [
27], konjac glucomannan [
23], carboxymethyl cellulose [
13], carboxymethyl chitosan [
62], polyvinyl alcohol [
37], carbopol-934 [
63], graphene [
41,
70,
71,
72], gelatin methacrylate [
65], polyacrylamide [
40], polyethyleneimine [
66], and polyvinylpyrrolidone [
40,
63,
67,
68,
69], have been used to fabricate antibacterial and antibiofilm materials.
These polymeric biomaterials have helped to treat and prevent infections caused by pathogenic bacteria and are capable of improving the healing and regeneration of the skin. For example, Ag/chitosan/hydrogel has been shown to help the healing process, reduced inflammation at skin wounds, and accelerated the re-epithelization rate to treat post-operative infection [
22].
Chitosan (CS) is derived from chitin, the second most abundant biopolymer in nature, after cellulose. It has been used in the synthesis of hydrogels due to its biodegradability, biocompatibility, and antibacterial activity [
22]. Chemical crosslinking, the addition of nanofillers, blending with other polymers, and using alkali–urea solutions, are some of the methods used to improve chitosan processability. To improve the water solubility of chitosan, quaternization method has been used, in which a quaternary ammonium moiety was introduced into the chitosan structure by chemical reactions, thus producing quaternate chitosan. Some studies have reported the use of chitosan as a matrix to incorporate Ag nanoparticles. Ag NPs were also synthesized in situ within oxidized dextran (ODex), adipic dihydrazide-grafted hyaluronic acid (HA-ADH), and quaternized chitosan (HACC), resulting in the Ag–ODex/HA-ADH/HACC hydrogel [
27]. The Ag NPs had a particle distribution size of around 50–190 nm. The hydrogel displayed antibacterial properties against
E. coli ATCC 8739,
S. aureus ATCC 14458, and
P. aeruginosa CMCCB10104, and the inhibition zones were 24, 24, and 27 mm, respectively. These results were associated with the hydrogel’s positive charge due to the quaternate chitosan’s cationic group, that favor the interaction with the negatively charged bacterial cell walls. This system reduced the wound area in rats up to 41.3% after 7 days, decreased inflammation, and improved re-epithelialization [
27].
In a similar study, Xie et al. [
22] prepared an Ag/chitosan hydrogel using an alkali–urea solution, LiOH (4.5% wt.)/KOH (7% wt.)/CH
4N
2O (8% wt.) by the freeze/thaw process, AgNO
3, and Na
3C
6H
5O
7. The silver concentration in the hydrogel increased, leading to spherical and ellipsoidal Ag NPs with a size distribution of 4.45 nm ± 0.37 nm to 9.22 ± 0.54 nm. The hydrogel composite had large tensile mechanical properties (15.95 ± 1.95 MPa). The antimicrobial activity was 99.86 ± 0.12% against
E. coli and 99.94 ± 0.10% against
S. aureus tested on rats for 14 days. The wound contraction was 70.5% on the 4th day and 99.75% on the 14th day. Thus, Ag NPs coated with chitosan accelerated the healing process. The authors determined that Ag NPs destroyed the bacterial cell wall due to interactions between the NPs and the lipid layer of the bacterial cell membrane. The Ag NPs would merge with bacterial DNA damaging bacterial replication and impairing bacterial respiratory function.
Furthermore, carboxymethyl chitosan is a derivative of chitosan, non-toxic, and also capable of forming gels [
62]. Carboxymethyl chitosan-based hydrogels have shown enhanced physicochemical, and biological properties, including antimicrobial, antioxidant, and antifungal activities. This hydrogel has been used in applications such as wound healing, drug-carrying, smart tissue, and biomedical nanodevices [
106]. Additionally, it has been well explored in the cosmetic and food industry [
62].
Ag/chitosan-carboxymethyl β-cyclodextrin hydrogel (CM-βCD) is an alternative approach to inhibit the growth of bacteria. It has been shown to display antibacterial activity against
E. coli and
S. aureus [
25]. The interactions between Ag
+ ions and bacteria were improved through ions exchange between Ag
+ and H
+ from the carboxylic and amino groups within the Ag NPs-CM-βCD hydrogel. The inhibition zone increased when the concentration of CM-βCD was increased in the hydrogel [
25].
Pandian et al. [
37] fabricated a Ag/N, O-carboxymehtyl chitosan (N, O-CMC) hydrogel with self-healing properties. The ethylenediaminetetraacetic acid (EDTA, C
10H
16N
2O
8) and ferric ions (Fe
3+, FeCl
3, 2%) were used in the synthesis process to produce a self-healing hydrogel. The size distribution of Ag NPs was 25 ± 14 nm according to TEM images. The hydrogel displayed an antibacterial activity against ATCC and clinical strains of
E. coli ATCC 25922,
S. aureus ATCC 35556, MRSA ATCC 43300,
P. aeruginosa ATCC 47085, and
K. pneumonia ATCC 700603. The minimum inhibitory concentration (MIC) for
P. aeruginosa was 48.5 mg/mL, 32.5 mg/mL for MRSA, and 32 mg/mL for
S. aureus. The Ag NPs/N, O-CMC hydrogel was more efficient against
E. coli and
K. pneumonia with MIC values of 17.5 and 23.0 mg/mL, respectively. At the same time, the minimum bactericidal concentration (MBC) values were 55 and 71 mg/mL, respectively. The authors described that the interaction between Fe
3+ (metal) and -COOH (ligand) was responsible for the self-healing property of the Ag NPs/N, O-CMC hydrogel [
37].
The carboxymethyl chitosan (CMCS) has been mixed with oxidized konjac glucomannan (OKGM). The OKGM is a natural polysaccharide, soluble in water, that was shown to improve the microstructure and mechanical properties of chitosan [
107,
108], gelatin [
109], and oxidized hyaluronic acid [
110], acting as a macromolecular cross-linker [
108]. The OKGM-based hydrogel exhibited self-healing characteristics in a recent study, where Ag NPs/OKGM/CMCS hydrogel demonstrated antibacterial properties against
E. coli and
S. aureus [
23]. This hydrogel was tested on rats’ skin. The hydrogel pore size distribution was in the range of 59.4 to 230 µm, increasing as the concentration of OKGM increased, but the swelling capacity decreased. Higher concentrations of polymers accelerated the gelation time from 600 to 57 s [
23]. Similar to a previous study, Ag/konjac glucomannan hydrogel was tested against
S. aureus and
E. coli showing good antibacterial efficiency on rabbit skin infections [
111].
Hydrogels based on carboxymethyl cellulose (CMC), polyvinyl alcohol (PVA), and C
8H
14O
4 (EDGE) has been prepared using microwave radiation as a carrier of Ag NPs (8–14 nm). The Ag release rate from this hydrogel was 85% over five days [
13]. Ag
+ ions are bound to the hydrogel composite via electrostatic interactions. This Ag/hydrogel acted as a bactericide against pathogenic microorganisms of the urinary tract, such as
E. coli,
K. pneumoniae,
P. aeruginosa,
P. vulgaris,
S. aureus, and
P. mirabilis [
13]. The Ag/hydrogels with 5 mg/mL of Ag presented a growth inhibition diameter of 16.6 mm against
E. coli, 15.8 mm against
K. pneumoniae, 15.6 mm against
P. aeruginosa, and 15.2 mm against
P. vulgaris.
Hydrogels based on carbopol-934 and
Aloe vera supported Ag spherical NPs encapsulating quercetin (QCT) [
63]. This system was designed to take advantage of (i) the properties of QCT as an anti-inflammatory and antioxidant; (ii) of carbopol-934, as a biodegradable and bioadhesive polymer with good tensile strength; (iii)
Aloe vera that stimulates collagen production; and finally, (iv) of Ag NPs that have broad antimicrobial activity. The QCT-Ag/carbopol-
Aloe vera hydrogel presented antibacterial activity against
S. aureus MTCC 3160 and
E. coli BL-21 with inhibition zone values of about 19.0 and 17.0 mm, respectively. Ag NPs improved the release rate of quercetin from the hydrogel for the treatment of wounds in diabetic patients.
Some studies have explored the incorporation of graphene into hydrogel structures due to its high thermal and electrical conductivity, and sizeable mechanical strength [
66,
70,
71,
72]. The graphene embedded in hydrogel reduced hydrogel breaking and reinforced its mechanical properties. The Ag/graphene composite hydrogel was prepared using acrylic acid and N,N′-methylene bisacrylamide (C
7H
10N
2O
2), with a mass ratio of 5:1 silver to graphene [
41]. The Ag NPs of an average size of 39 nm were deposited onto the surface of graphene nanosheets. The Ag NPs/graphene hydrogel was evaluated against
E. coli and
S. aureus using the shaking flask method. The antimicrobial activity was enhanced as the Ag NP concentration increased. Larger nanoparticle sizes displayed better antimicrobial activity than smaller ones. The graphene promoted the incorporation of a higher number of NPs and avoided their aggregation onto its surface.
Another approach that has been explored is to combine chitosan and graphene to produce an antibacterial hydrogel with enhanced durability [
71]. For instance, Nešović et al. prepared Ag/poly(vinyl alcohol)/chitosan/graphene hydrogels [
70,
71,
72] by electrochemical synthesis of nanoparticles in a hydrogel network. The hydrogel displayed better mechanical characteristics, such as tensile strength and elastic modulus. The Ag NPs size distribution was from 6.38 to 10.00 nm depending on the chitosan content. The antimicrobial activity was evaluated against
E. coli ATCC 25922 and
S. aureus TL. The number of bacteria colonies decreased quickly in 15 min, when the AgNO
3 concentration was 0.25 mM and 0.5% wt. of chitosan, during the composite hydrogel preparation (0.25Ag/PVA/0.5CHI/Gr). Increasing the chitosan content resulted in a slower Ag release rate from the hydrogel. Nešović et al. [
71] found that Ag NPs prevented adenosine 5′-triphosphate (ATP) formation within the microorganism.
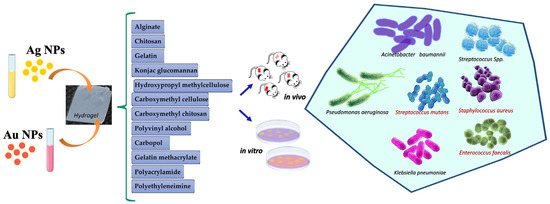
Figure 1 summarizes the hydrogels embedded with Ag and Au NPs against multidrug-resistant bacteria.
Figure 1. Silver and gold NPs loaded into hydrogels for antibacterial application.
A Ag-polyethyleneimine (PEI)-graphene oxide (GO) hydrogel was produced using Pluronic F127 gel [
66]. In this case, Pluronic F-127 was used to create a sustained antimicrobial effect, presenting reverse thermal gelation properties. PEI decreased the aggregation of nanostructures within the hydrogel. The antimicrobial activity against
E. coli was 99.86%, and 99.94% against
C. albicans, using 10 µg/mL of the hydrogel. The Ag release rate from the hydrogel was 72% in 7 days. The authors proposed that the graphene oxide nanosheets damaged the bacterial cell wall due to the sharp edges leading to a faster disruption of the plasmatic membrane by the Ag NPs.
Furthermore, Ag NPs have been incorporated into nanotubes/polymer hydrogels to explore NP delivery. For instance, aluminosilicate nanotubes (NTs) are platforms with a great capacity to store and carry molecules and drugs. They also help to reduce the hydrogel degradation rate and can be loaded, as additives, into the hydrogels, such as gelatin methacrylate (GelMA), a biocompatible hydrogel with many biological characteristics [
112]. For example, Ag NPs were loaded into aluminosilicate nanotubes (NTs) and then within a methacrylate gelatin [
65] matrix, to produce an antibacterial hydrogel capable of improving bone regeneration. The hydrogel was prepared using photopolymerization by UV irradiation of 365 nm and 400 W. According to the inhibition zone results, the Ag/NTs/GelMA hydrogel showed higher antibacterial activity against
E. coli ATCC 8739 than
S. aureus ATCC 29213.
In addition, the morphology of Ag NPs is another relevant aspect that influences a hydrogel’s antibacterial efficiency. Different NP shapes may present a distinct surface area to interact with bacterial membranes, leading to diverse antibacterial activity [
40,
113,
114]. In this context, Ag NPs with different morphologies (spherical, triangular, and rod) were incorporated into polyacrylamide (PPA) and N-mehtylene bisacrylamide (MBA) hydrogels, named PAA-MBA [
40]. The mechanical strength of the Ag NPs-PAA-MBA hydrogel (4 to 5 KPa) did not depend on Ag NP shape. Rod-shaped nanoparticles were poorly absorbed within the hydrogel network due to the formation of aggregates on the hydrogel surface. However, these NPs showed antibacterial activity. The hydrogel doped with spherical NPs of 12.7 ± 5.9 nm and triangular NPs of 37.1 ± 15.0 nm demonstrated high antimicrobial activity against
E. coli.Table 2 summarizes the hydrogels embedded with Ag NPs for antibacterial application.
Table 2. Ag NPs loaded into hydrogel for antibacterial application.
3. Antibiofilm Activity of Hydrogels Loaded with Ag NPs
Taking the usefulness of non-invasive therapy into consideration, and the elimination of drug-resistant biofilms in oral infections and wound healing, hydrogels loaded with Ag NPs is an alternative method of infection management [
115,
116,
117]. In this scenario, Haidari et al. [
43] investigated the effectiveness of applied Ag NP hydrogels in mature
S. aureus biofilms, both in vitro and in vivo. In vitro tests were performed by flow cytometry, where bacterial cells with compromised membranes were stained red by propidium iodide, whereas cells with intact membranes were stained green by SYTO9. The test showed that after treating the
S. aureus biofilm with the Ag NP hydrogel, most of the cells were stained in a high red fluorescence intensity, associated with a substantially lower biofilm biomass, indicating severe disruption of the mature biofilm. For in vivo tests, an established
S. aureus mouse model of a mature biofilm wound infection was utilized. The antibiofilm treatment started after biofilms had been fully established. IVIS bioluminescent imaging was used to track 10 days of Ag NP hydrogel treatment in real-time. The Ag NP hydrogel treatment gradually decreased the
S. aureus biofilm starting on day 4.
From 5 to 10 days after the infection, there was a statistically significant decrease in the concentration of bacterial cells, showing the high efficiency of the Ag NPs in eradicating established mature biofilms in wounds. This study demonstrated the use of an Ag NP hydrogel as a valid therapeutic approach for the effective and safe elimination of mature S. aureus biofilms in wounds.
Consistent with these results, Imran et al. [
118], also reported the antibiofilm activity of a Ag NP-loaded hydrogel against
B. subtilis and
E. coli. It was revealed that the hydrogel showed a dose-dependent biofilm inhibition activity, with a minimum biofilm inhibition of approximately 27% when the Ag NPs were used at a concentration of 10 ppm and a maximum inhibition of 97% when the Ag NPs were used at a concentrations of 100 ppm. Additionally, the half maximal inhibitory concentration (IC
50) values obtained were 29.88 and 27.36 for
E. coli and
B. subtilis, respectively. Pandian et al. [
37], in turn, evaluated the antibiofilm activity of in situ Ag NPs incorporated in an N, O-carboxymethyl chitosan self-healing hydrogel. After 48 h, a decrease of 68.86 ± 0.05%, 75.07 ± 0.02%, and 83.22 ± 0.01% was observed in
E. coli-,
S. aureus-, and
P. aeruginosa-treated biofilms, respectively.
Alfuraydi et al. [
119] described the preparation of novel cross-linked chitosan and PVA hydrogels impregnated with Ag NPs, as well as its activity against different strains of fungi, Gram-positive and Gram-negative bacteria. In their results, The minimal biofilm inhibition concentration (MBIC) for the chitosan hydrogels alone ranged from 15.63 to 125 µg/mL, differing from the MBIC values of the hydrogel containing Ag NPs at 1 and 3%, which ranged from 1.95 to 7.81 µg/mL. These data demonstrated how the dispersion of Ag NPs inside the matrix of the chitosan hydrogel significantly improved its ability to prevent the formation of biofilms.
Similarly, the antibiofilm action of the chitosan hydrogel containing Ag NPs was previously explored by Pérez-Díaz et al. [
120]. In their work, the hydrogels demonstrated a great impact on the multi-species biofilm of oxacillin-resistant
S. aureus (ORSA), achieving a 6 Log
10 reduction at a Ag NP concentration of 100 ppm. The antibiofilm activity against
P. aeruginosa was lower, with a Log
10 decrease of 3.3 at a concentration of 1000 ppm. As stated in the study conducted by Arinah et al. [
121], the different results on the tested drugs’ antibiofilm activity could be attributed to structural variations in the bacterial membrane walls, which differ in Gram-negative or Gram-positive bacteria. In their work, the authors incorporated
Pleurotus ostreatus-biosynthesized Ag NPs into a genipin-crosslinked gelatin hydrogel to investigate the antibiofilm properties against the biofilms of
S. aureus,
P. aeruginosa,
Bacillus sp., and
E. coli. Stronger biofilm inhibition of about 58 ± 4% was observed in Gram-negative strains. For Gram-positive bacteria, the percentage of inhibition was 55 ± 5% for
S. aureus and 38 ± 1% for
Bacillus spp.
Furthermore, many recent studies have reported antibacterial and antibiofilm activity improvement of drugs when they are associated with metallic nanoparticles, such as Ag NPs [
122,
123]. Thus, in the research conducted by Lopez-Carrizales et al. [
124], chitosan hydrogel loaded with Ag NPs and the antibiotic ampicillin (AMP) were tested against resistant bacterial pathogens, evaluating its capacity to prevent the early formation of biofilms by the colony biofilm model. The biofilm produces thick, layered structures, and the counting of colony-forming units (CFU) was Log
10-transformed. The antibiofilm action of the hydrogel changed depending on the Ag NP and ampicillin concentrations and the tested strain. The biofilms of
A. baumannii,
E. faecium, and
S. epidermidis, were significantly inhibited by the hydrogel with the lowest concentration of Ag NPs and ampicillin (25 ppm Ag NPs/50 ppm AMP), exhibiting Log
10 reductions of 10 ± 0.01, 8.9 ± 0.02, and 7.8 ± 0.13, respectively. However, the
E. cloacae biofilm was only inhibited by a higher antimicrobial dose (250 ppm Ag NPs/500 ppm AMP), resulting in a Log
10 reduction of 9.9 ± 0.11.
Recently, Wunno et al. [
125] investigated a potentially new sustainable delivery system of Ag NPs for, among other activities, antibiofilm action. In their work, an ex situ thermosensitive hydrogel based on poloxamers loaded with biosynthesized Ag NPs from
Eucalyptus camaldulensis was created and tested against Gram-positive (
S. aureus and
S. epidermidis) and Gram-negative bacterial (
A. baumannii and
P. aeruginosa) biofilms. At a ½ minimum inhibitory concentration (MIC), the proportion of biofilm inhibition reached 83%. When the mature biofilms were exposed to the Ag NP hydrogel and analyzed by confocal laser scanning, loosening of the biofilm architecture and cell death were revealed after 4 h of co-incubation with the hydrogel formulation at a 2 MIC (μg/mL) concentration. Based on the presented results, it is clear that the tested hydrogel formulation successfully interrupted biofilm formation and eradicated cell viability within the mature biofilms.
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics12010104