You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Intraspecific heterogeneity describes the phenotypic and genetic diversity within the population of a species. Intraspecific heterogeneity is derived from microevolution and is a strong driving force for the expansion of invasive species such as the bloom-forming cyanobacterium Raphidiopsis raciborskii.
- cylindrospermopsin
- microbial invasion
- microevolution
- nutrient fluctuations
1. Introduction
Cyanobacterial blooms are severe environmental problems in eutrophic freshwater ecosystems [1][2]. Raphidiopsis raciborskii (previously known as Cylindrospermopsis raciborskii) is a filamentous bloom-forming cyanobacterium belonging to Nostocales with heterocysts and akinetes (Figure 1A). Upon the occurrence of an R. raciborskii bloom, a significant amount of cell filaments usually distributes evenly in the water column (Figure 1B). As a major producer of hepatotoxic cylindrospermopsin (CYN) and its analogs (Figure 1C) [3][4][5][6], R. raciborskii often proliferates in lakes or reservoirs in tropical and subtropical zones [7][8], posing a significant threat to ecological safety. In the past decades, the occurrence frequency of this species has been significantly increased over the world, including both subtropical and temperate zones of the globe [7][9][10]. Therefore, R. raciborskii was suggested to be an invasive cyanobacterium, and much attention has been paid to its dispersion routes and adaptation mechanisms [9][11][12].
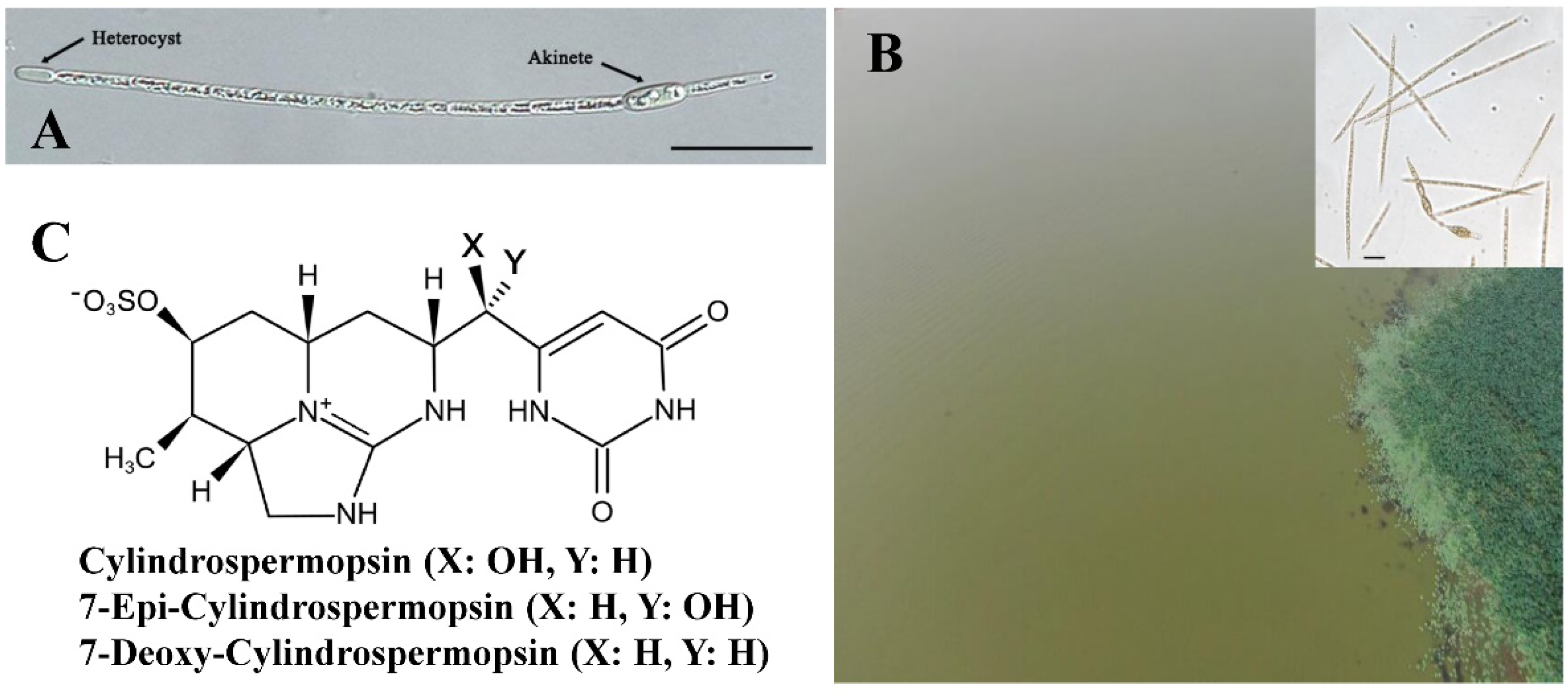
Figure 1. Cell differentiation of R. raciborskii filament (A), aerial view of the R. raciborskii bloom (B), and chemical structure of cylindrospermopsins (C). Inset in (B) is a microscopic graph of R. raciborskii bloom. Scale bars in (A,B), 10 µm.
2. Phenotypic and Genetic Diversity
In previous studies on the environmental adaptability of R. raciborskii, it has often been regarded as a homogenous population because of the highly similar 16S rRNA genes of different strains. For example, most present studies suggest that R. raciborskii is a mesophile with a tolerance to low temperatures [7][13][14][15]. However, laboratory experimentation has found that several strains maintain a high growth rate at 15 °C, while the growth of some strains is completely inhibited under low-temperature conditions [16][17]. These findings indicate that low-temperature tolerance is not an intrinsic characteristic of R. raciborskii and that significant intraspecific heterogeneity exists for the temperature adaptability of this species. Similarly, the intraspecific heterogeneity of R. raciborskii has also been found in its response to light intensity [9][18][19] and conductivity [20], as well as its strategies for the utilization of N [21][22] and P [23]. In addition, varied growth rates, morphologies, and toxicities have been observed in genetically similar isolates of R. raciborskii [24][25].
In contrast to the 16S rRNA gene, the RNA polymerase C1 (rpoC1) gene and ITS-L sequence, which is the larger fragment of the inter-transcribed sequence of rRNA, are useful molecular markers for discriminating different R. raciborskii strains [26][27]. The strains were classified into nontoxic, CYN-producing, and PSP-producing clusters based on the phylogenetic analysis of rpoC1 and ITS-L [26]. This result is supported by the genomic variations between R. raciborskii strains [28][29][30]. The relationship between genetic diversity and phenotypic variation remains to be clarified and requires further systematic investigation.
The presence of cyr genes is the main genetic difference between CYN-producing and non-CYN-producing strains [31]. However, genomic variations were also found in genes associated with stress and adaptation, which are probably related to the physiological role of CYN [29]. In fact, CYN has been shown to contribute to the successful invasion and survival of R. raciborskii [4][5]. For example, CYN-producing strains have a competitive advantage under nutrient-replete conditions [32]. In comparison to nontoxic R. raciborskii, toxic strains have a more efficient response to inorganic phosphorus and could become dominant in the community [33]. A recent study also found that toxic strains were more competitive under Fe-starved conditions [34].
Previous investigations have revealed that the cell quotas of CYN varied significantly between R. raciborskii strains [24][25][35]. However, the genetic basis of this finding remains unknown. As aforementioned, sequence variations were observed for cyrI and cyrJ genes, which encodes a hydroxylase and a sulfotransferase, respectively [31][36][37][38]. These two enzymes catalyze tailoring reactions in the last two steps of CYN biosynthesis [39]. An inactivated mutation of cyrI inhibits the synthesis of CYN, leading to an accumulation in the intermediate product 7-deoxy-CYN [40]. Therefore, variations in cyr genes may change the activity of the enzymes encoded by them and further affect CYN synthesis efficiency.
3. Competition between R. raciborskii and M. aeruginosa
M. aeruginosa is the dominant species in most eutrophic water bodies, both in China and worldwide [41][42]. Competition against M. aeruginosa is inevitable for R. raciborskii during invasion. However, recent culture experiments have produced inconsistent results regarding the competition between R. raciborskii and M. aeruginosa (Table 1). Under light- or P-limited conditions, both R. raciborskii and M. aeruginosa are likely to dominate the mixed culture of these two species with equal starting biovolumes [43]. R. raciborskii, with N-fixation capability, was a more successful invader than M. aeruginosa when N was depleted in batch culture [44]. Likewise, the model prediction of species competition outcomes is strongly affected by the growth variability of strains [45]. These findings demonstrate the intraspecific heterogeneity for R. raciborskii and M. aeruginosa [46].
Table 1. Results of competition experiments using R. raciborskii and M. aeruginosa.
| Strain Pair and Culture Condition | Dominant Strain | Reference | |
|---|---|---|---|
| R. raciborskii FACHB 1096 vs. M. aeruginosa strain 205 | |||
| P substrates | K2HPO4, β-glycerol phosphate, (2-aminoethyl)-phosphinic acid, P-free | R. raciborskii FACHB 1096 | [47] |
| Glyphosate | M. aeruginosa strain 205 | ||
| R. raciborskii CS vs. M. aeruginosa LEA | |||
| P and light | P-limitation | R. raciborskii CS | [43] |
| Light-limitation | R. raciborskii CS | ||
| R. raciborskii CP vs. M. aeruginosa MIRF | |||
| P and light | P-limitation | M. aeruginosa MIRF | [43] |
| Light-limitation | M. aeruginosa MIRF | ||
| R. raciborskii N8 vs. M. aeruginosa FACHB905 | |||
| Temperature | 16 °C, 24 °C, 32 °C | M. aeruginosa FACHB905 | [46] |
| R. raciborskii N8 vs. M. aeruginosa (FACHB469 and 915) with a biovolume ratio of 30:1 | |||
| Temperature | 16 °C, 24 °C, 32 °C | R. raciborskii N8 maintained initial advantages at 16 °C and 32 °C | [46] |
| M. aeruginosa strains at 24 °C | |||
| R. raciborskii ITEP-A1 vs. M. aeruginosa NPLJ-4 | |||
| pH and inorganic carbon | Aeration | R. raciborskii ITEP-A1 | [48] |
| Bicarbonate | M. aeruginosa NPLJ-4 | ||
| R. raciborskii NW-R vs. M. aeruginosa NW-M | |||
| Growth capability | Multiple inoculation ratios | R. raciborskii NW-R | [44] |
| Four M. aeruginosa vs. eight R. raciborskii strains | |||
| Growth capability | Simulated using a deterministic model | No absolute winner | [45] |
4. Ecotype and Microevolution
In several studies, R. raciborskii was assumed to have evolved into different ecotypes with special physiological characteristics in its original habitats, and the geographic dispersion of this species is a dynamic selection process for existing ecotypes [14][15][35]. The concept of an ecotype is reasonable for understanding the intraspecific heterogeneity of R. raciborskii in different environments but not for explaining the coexistence of different phenotypes and genotypes in the same environment [24][28]. Heterogeneity between coexisting strains is better defined as microevolution, the concept of which refers to minor variations within species [49]. Microevolution is the source of adaptive variation for organisms, and the accumulation of microevolution may further lead to new speciation. The ecotypes of R. raciborskii were presumably established by the natural selection of heterogeneous strains generated during microevolution.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph20031984
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483.
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670.
- La Cruz, A.A.D.; Hiskia, A.; Kaloudis, T.; Chernoff, N.; Hill, D.; Antoniou, M.G.; He, X.; Loftin, K.A.; Oshea, K.E.; Zhao, C. A review on cylindrospermopsin: The global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environ. Sci. Process Impacts 2013, 15, 1979–2003.
- Yang, Y.; Yu, G.; Chen, Y.; Jia, N.; Li, R. Four decades of progress in cylindrospermopsin research: The ins and outs of a potent cyanotoxin. J. Hazard. Mater. 2020, 406, 124653.
- Rzymski, P.; Poniedzialek, B. In search of environmental role of cylindrospermopsin: A review on global distribution and ecology of its producers. Water Res. 2014, 66, 320–337.
- Scarlett, K.R.; Kim, S.; Lovin, L.M.; Chatterjee, S.; Scott, J.T.; Brooks, B.W. Global scanning of cylindrospermopsin: Critical review and analysis of aquatic occurrence, bioaccumulation, toxicity and health hazards. Sci. Total Environ. 2020, 738, 139807.
- Antunes, J.T.; Leao, P.N.; Vasconcelos, V.M. Cylindrospermopsis raciborskii: Review of the distribution, phylogeography, and ecophysiology of a global invasive species. Front. Microbiol. 2015, 6, 473.
- Lei, L.; Peng, L.; Huang, X.; Han, B. Occurrence and dominance of Cylindrospermopsis raciborskii and dissolved cylindrospermopsin in urban reservoirs used for drinking water supply, South China. Environ. Monit. Assess. 2014, 186, 3079–3090.
- Burford, M.A.; Beardall, J.; Willis, A.; Orr, P.T.; Magalhaes, V.F.; Rangel, L.M.; Azevedo, S.; Neilan, B.A. Understanding the winning strategies used by the bloom-forming cyanobacterium Cylindrospermopsis raciborskii. Harmful Algae 2016, 54, 44–53.
- Sinha, R.; Pearson, L.A.; Davis, T.W.; Burford, M.A.; Orr, P.T.; Neilan, B.A. Increased incidence of Cylindrospermopsis raciborskii in temperate zones–Is climate change responsible? Water Res. 2012, 46, 1408–1419.
- Moreira, C.; Fathalli, A.; Vasconcelos, V.; Antunes, A. Phylogeny and biogeography of the invasive cyanobacterium Cylindrospermopsis raciborskii. Arch. Microbiol. 2015, 197, 47–52.
- Ribeiro, K.F.; Ferrero, A.P.; Duarte, L.; Turchetto-Zolet, A.C.; Crossetti, L.O. Comparative phylogeography of two free-living cosmopolitan cyanobacteria: Insights on biogeographic and latitudinal distribution. J. Biogeogr. 2020, 47, 1106–1118.
- Bonilla, S.; Aubriot, L.; Soares, M.C.S.; Gonzalezpiana, M.; Fabre, A.; Huszar, V.L.M.; Lurling, M.; Antoniades, D.; Padisak, J.; Kruk, C. What drives the distribution of the bloom-forming cyanobacteria Planktothrix agardhii and Cylindrospermopsis raciborskii? FEMS Microbiol. Ecol. 2012, 79, 594–607.
- Chonudomkul, D.; Yongmanitchai, W.; Theeragool, G.; Kawachi, M.; Kasai, F.; Kaya, K.; Watanabe, M.M. Morphology, genetic diversity, temperature tolerance and toxicity of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) strains from Thailand and Japan. FEMS Microbiol. Ecol. 2004, 48, 345–355.
- Piccini, C.; Aubriot, L.; Fabre, A.; Amaral, V.; González-Piana, M.; Giani, A.; Figueredo, C.C.; Vidal, L.; Kruk, C.; Bonilla, S. Genetic and eco-physiological differences of South American Cylindrospermopsis raciborskii isolates support the hypothesis of multiple ecotypes. Harmful Algae 2011, 10, 644–653.
- Soares, M.C.S.; Lürling, M.; Huszar, V.L.M. Growth and temperature-related phenotypic plasticity in the cyanobacterium Cylindrospermopsis raciborskii. Psychol. Res. 2013, 61, 61–67.
- Jia, N.; Wang, Y.; Guan, Y.; Chen, Y.; Li, R.; Yu, G. Occurrence of Raphidiopsis raciborskii blooms in cool waters: Synergistic effects of nitrogen availability and ecotypes with adaptation to low temperature. Environ. Pollut. 2021, 270, 116070.
- Briand, J.-F.; Leboulanger, C.; Humbert, J.-F.; Bernard, C.; Dufour, P. Cylindrospermopsis raciborskii (cyanobacteria) invasion at mid-latitudes: Selection, wide physiological tolerance, or global warming? J. Phycol. 2004, 40, 231–238.
- Pierangelini, M.; Stojkovic, S.; Orr, P.T.; Beardall, J. Photosynthetic characteristics of two Cylindrospermopsis raciborskii strains differing in their toxicity. J. Phycol. 2014, 50, 292–302.
- Neves de Lima, D.V.; Furlanetto Pacheco, A.B.; Goulart, C.L.; de Oliveira e Azevedo, S.M.F. Physiological responses of Raphidiopsis raciborskii (Cyanobacteria) strains to water conductivity: Effect of sodium and magnesium ions. Hydrobiologia 2020, 847, 2449–2464.
- Lu, Z.; Ye, J.; Chen, Z.; Xiao, L.; Lei, L.; Han, B.-p.; Paerl, H.W. Cyanophycin accumulated under nitrogen-fluctuating and high-nitrogen conditions facilitates the persistent dominance and blooms of Raphidiopsis raciborskii in tropical waters. Water Res. 2022, 214, 118215.
- Willis, A.; Chuang, A.W.; Burford, M.A. Nitrogen fixation by the diazotroph Cylindrospermopsis raciborskii (Cyanophyceae). J. Phycol. 2016, 52, 854–862.
- Xiao, M.; Hamilton, D.P.; Chuang, A.; Burford, M.A. Intra-population strain variation in phosphorus storage strategies of the freshwater cyanobacterium Raphidiopsis raciborskii. FEMS Microbiol. Ecol. 2020, 96, fiaa092.
- Willis, A.; Chuang, A.W.; Woodhouse, J.N.; Neilan, B.A.; Burford, M.A. Intraspecific variation in growth, morphology and toxin quotas for the cyanobacterium, Cylindrospermopsis raciborskii. Toxicon 2016, 119, 307–310.
- Saker, M.L.; Neilan, B.A. Varied diazotrophies, morphologies, and toxicities of genetically similar isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from Northern Australia. Appl. Environ. Microbiol. 2001, 67, 1839–1845.
- Jiang, Y.; Chen, Y.; Yang, S.; Li, R. Phylogenetic relationships and genetic divergence of paralytic shellfish toxin- and cylindrospermopsin- producing Cylindrospermopsis and Raphidiopsis. Harmful Algae 2020, 93, 101792.
- Li, X.; Li, S.; Kong, R.; Li, R. Molecular separation of two long taxonomically debated cyanobacterial genera Cylindrospermopsis and Raphidiopsis (Nostocales) based on the ITS-L phylogeny. Harmful Algae 2016, 57, 88–97.
- Willis, A.; Woodhouse, J.N.; Ongley, S.E.; Jex, A.R.; Burford, M.A.; Neilan, B.A. Genome variation in nine co-occurring toxic Cylindrospermopsis raciborskii strains. Harmful Algae 2018, 73, 157–166.
- Sinha, R.; Pearson, L.A.; Davis, T.W.; Muenchhoff, J.; Pratama, R.; Jex, A.; Burford, M.A.; Neilan, B.A. Comparative genomics of Cylindrospermopsis raciborskii strains with differential toxicities. BMC Genom. 2014, 15, 83.
- Abreu, V.A.C.; Popin, R.V.; Alvarenga, D.O.; Schaker, P.D.C.; Hoff-Risseti, C.; Varani, A.M.; Fiore, M.F. Genomic and genotypic characterization of Cylindrospermopsis raciborskii: Toward an intraspecific phylogenetic evaluation by comparative genomics. Front. Microbiol. 2018, 9, 306.
- Jiang, Y.; Xiao, P.; Yu, G.; Shao, J.; Liu, D.; Azevedo, S.M.F.O.; Li, R. Sporadic distribution and distinctive variations of cylindrospermopsin genes in cyanobacterial strains and environmental samples from Chinese freshwater bodies. Appl. Environ. Microbiol. 2014, 80, 5219–5230.
- Lei, L.; Lei, M.; Cheng, N.; Chen, Z.; Xiao, L.; Han, B.; Lin, Q. Nutrient regulation of relative dominance of cylindrospermopsin-producing and non-cylindrospermopsin-producing Raphidiopsis raciborskii. Front. Microbiol. 2021, 12, 793544.
- Burford, M.A.; Davis, T.W.; Orr, P.T.; Sinha, R.; Willis, A.; Neilan, B.A. Nutrient-related changes in the toxicity of field blooms of the cyanobacterium, Cylindrospermopsis raciborskii. FEMS Microbiol. Ecol. 2014, 89, 135–148.
- D’Agostino, P.M.; Yeung, A.C.Y.; Poljak, A.; David Waite, T.; Neilan, B.A. Comparative proteomics of the toxigenic diazotroph Raphidiopsis raciborskii (cyanobacteria) in response to iron. Environ. Microbiol. 2021, 23, 405–414.
- Willis, A.; Adams, M.P.; Chuang, A.W.; Orr, P.T.; O’Brien, K.R.; Burford, M.A. Constitutive toxin production under various nitrogen and phosphorus regimes of three ecotypes of Cylindrospermopsis raciborskii ((Woloszyriska) Seenayya et Subba Raju). Harmful Algae 2015, 47, 27–34.
- Mazmouz, R.; Chapuis-Hugon, F.; Pichon, V.; Mejean, A.; Ploux, O. The last step of the biosynthesis of the cyanotoxins cylindrospermopsin and 7-epi-cylindrospermopsin is catalysed by CyrI, a 2-Oxoglutarate-dependent iron oxygenase. ChemBioChem 2011, 12, 858–862.
- Mazmouz, R.; Essadik, I.; Hamdane, D.; Méjean, A.; Ploux, O. Characterization of CyrI, the hydroxylase involved in the last step of cylindrospermopsin biosynthesis: Binding studies, site-directed mutagenesis and stereoselectivity. Arch. Biochem. Biophys. 2018, 647, 1–9.
- Méjean, A.; Ploux, O. Biosynthesis of cylindrospermopsin in cyanobacteria: Characterization of CyrJ the sulfotransferase. J. Nat. Prod. 2021, 84, 408–416.
- Mihali, T.K.; Kellmann, R.; Muenchhoff, J.; Barrow, K.D.; Neilan, B.A. Characterization of the gene cluster responsible for cylindrospermopsin biosynthesis. Appl. Environ. Microbiol. 2008, 74, 716–722.
- Jiang, Y.; Xiao, P.; Yu, G.; Sano, T.; Pan, Q.; Li, R. Molecular basis and phylogenetic implications of deoxycylindrospermopsin biosynthesis in the cyanobacterium Raphidiopsis curvata. Appl. Environ. Microbiol. 2012, 78, 2256–2263.
- Huo, D.; Gan, N.; Geng, R.; Cao, Q.; Song, L.; Yu, G.; Li, R. Cyanobacterial blooms in China: Diversity, distribution, and cyanotoxins. Harmful Algae 2021, 109, 102106.
- Xiao, M.; Li, M.; Reynolds, C.S. Colony formation in the cyanobacterium Microcystis. Biol. Rev. 2018, 93, 1399–1420.
- Marinho, M.M.; Souza, M.B.G.; Lurling, M. Light and phosphate competition between Cylindrospermopsis raciborskii and Microcystis aeruginosa is strain dependent. Microb. Ecol. 2013, 66, 479–488.
- Jia, N.; Yang, Y.; Yu, G.; Wang, Y.; Qiu, P.; Li, H.; Li, R. Interspecific competition reveals Raphidiopsis raciborskii as a more successful invader than Microcystis aeruginosa. Harmful Algae 2020, 97, 101858.
- Xiao, M.; Adams, M.P.; Willis, A.; Burford, M.A.; O’Brien, K.R. Variation within and between cyanobacterial species and strains affects competition: Implications for phytoplankton modelling. Harmful Algae 2017, 69, 38–47.
- Lei, L.; Dai, J.; Lin, Q. Competitive dominance of Microcystis aeruginosa against Raphidiopsis raciborskii is strain- and temperature-dependent. Knowl. Manag. Aquat. Ecosyst. 2020, 421, 36.
- Bai, F.; Shi, J.; Yang, S.; Yang, Y.; Wu, Z. Interspecific competition between Cylindrospermopsis raciborskii and Microcystis aeruginosa on different phosphorus substrates. Environ. Sci. Pollut. Res. 2020, 27, 42264–42275.
- da Silva Brito, M.T.; Duarte-Neto, P.J.; Reis Molica, R.J. Cylindrospermopsis raciborskii and Microcystis aeruginosa competing under different conditions of pH and inorganic carbon. Hydrobiologia 2018, 815, 253–266.
- Hendry, A.P.; Kinnison, M.T. An introduction to microevolution: Rate, pattern, process. Genetica 2001, 112, 1–8.
This entry is offline, you can click here to edit this entry!
