Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
SNX27 belongs to the sorting nexin (SNX) family of proteins that play a critical role in protein sorting and trafficking in the endocytosis pathway. This protein family is characterized by the presence of a Phox (PX) domain; however, SNX27 is unique in containing an additional PDZ (post-synaptic density 95/discs large/zonula occludens-1) domain.
- autophagy
- colon cancer
- endosomal pathway
- endocytic recycling
1. SNX27 and Its Special Domains
As shown in Figure 1, SNX27 (sorting nexin (SNX)) contains three distinct domains: a central Phox (PX) domain, a C-terminal FERM (4.1/ezrin/radixin/moesin) domain, and an N-terminal PDZ (post-synaptic density 95/discs large/zonula occludens-1) domain (only in SNX27) [1] (Figure 1). Despite carrying a common PX and FERM domain, SNX27 behaves functionally distinct from the other members of the SNX-FERM subfamily [2]. While both SNX17 and SNX31 depend on their FERM domain and have strong affinity towards targeting and recycling cargoes carrying a NPxY/NxxY motif, SNX27 mediates endocytic recycling of cargoes in a PDZ-domain dependent manner [2]. The SNX27-FERM domain associates with an N-terminal DLF motif found on the SNX1/2 homodimer, which has not been reported in either SNX17 or SNX31 [2]. This was further supported by structural analysis data, which reported very low sequence identity between SNX27-FERM domain compared with that of SNX17 and SNX31 [2]. Therefore, the FERM domain has a distinct role in SNX27 than in the other SNX-FERM members, such that it only promotes SNX27 interaction with the SNX-BAR (Bin/Amphiphysin/Rvs) proteins. This structure mediates the recruitment of SNX27 to the membranes of early endosomes and initiates membrane remodeling to assist in the endocytic recycling and trafficking of PDZ-domain containing cargoes [3].
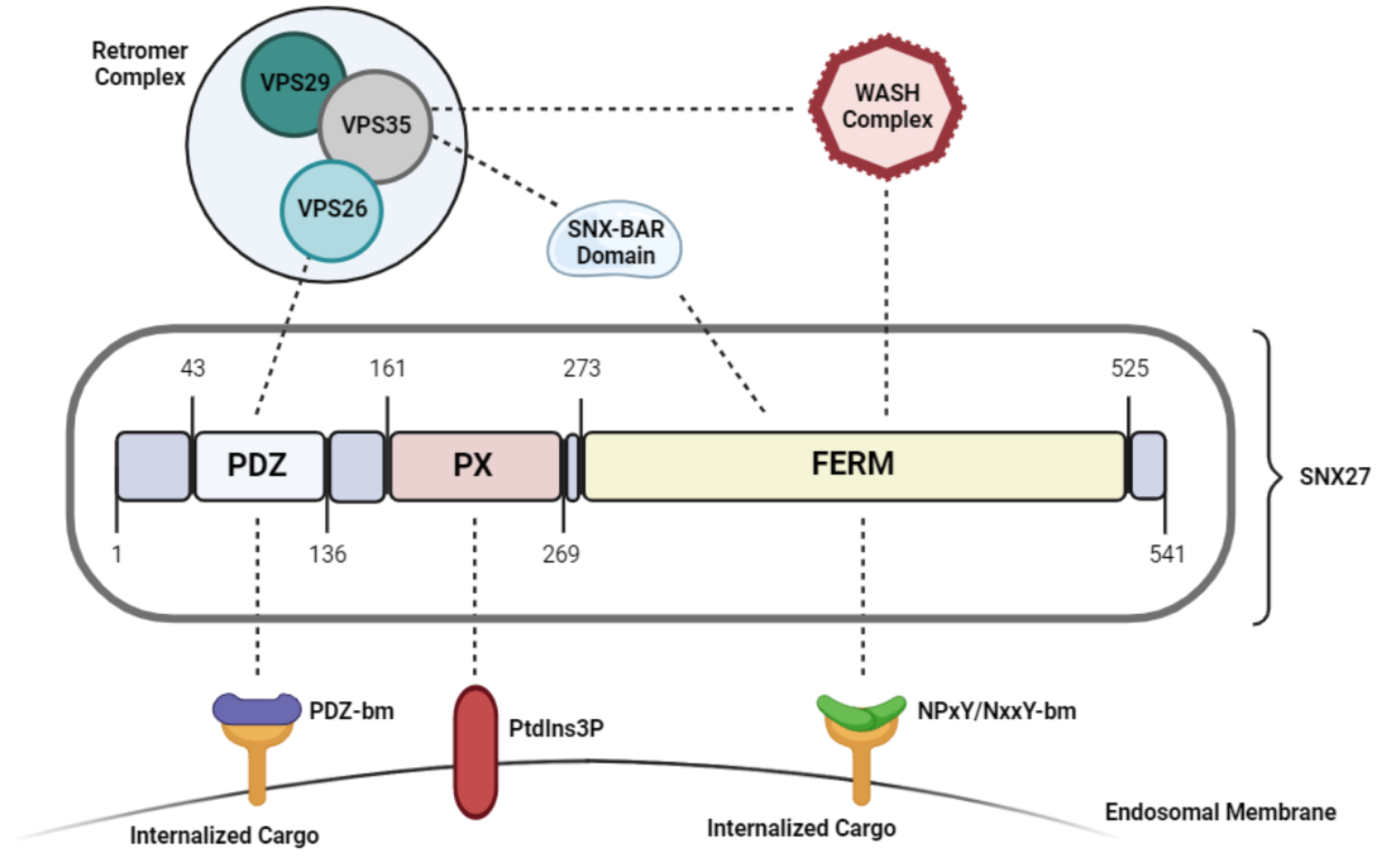
Figure 1. Structure and functional domains of SNX27.
SNX27 was initially discovered in the brain tissue where it was found to be interacting with 5-HT4.a/R, a G-protein coupled receptor (GPCR) [4]. Structural analyses later revealed that 5-HT4.a/R carries a C-terminal class-I PDZ-binding motif and its association with SNX27 was found to be dependent on its interaction with the PDZ-domain [5]. Therefore, the canonical basis for the PDZ domain to recognize and bind to cargoes was predisposed to the presence of a class-I PDZ-binding motif [S/T]-x-Φ (where x represents any amino acid and Φ represents any hydrophobic amino acid) at the C-termini, often present in the cytosolic tails of transmembrane proteins [6]. However, presence of certain sequences upstream of the C-terminus PDZ-binding motif was also shown to enhance binding specificity [7]. Studies have revealed that acidic side chains are often found alongside the PDZ-binding motifs, however several SNX27 cargoes have replaced these upstream acidic side chain sequences with conserved serine/threonine phosphorylation sites which was later shown to mimic the acidic side chain residues, thus promoting binding affinity in a similar manner [7]. With the advent of proteomics and sequence quantitative analysis, over hundreds of transmembrane proteins have been identified to be interacting with SNX27-PDZ domain. These include β-2-adrenergic receptor, GLUT1 glucose transporter, and the Menkes copper transporter ATP7A [8].
2. SNX27 Mediated Endosomal Recycling of Proteins
Endocytosed transmembrane proteins require to go through a complex network of the endomembrane system that is subdivided into the early, late, and recycling endosome vesicles. While the cargo sorted in late endosomes proceed for lysosomal degradation, those present in the recycling endosomes further interact with the components of the secretory pathway in order to be transported to either the trans-Golgi network (TGN) or delivered back to the cell surface [9]. Therefore, improper sorting of endocytosed cargo can disrupt physiological homeostasis and give rise to various health problems and diseases [10].
Sorting and recycling of the transmembrane proteins from early/recycling endosomal compartments directly to the plasma membrane is heavily dependent on the association of the SNX27/SNX-BAR/retromer complex on Rab4-positive endosomes [11]. In this model, the PX domain is critical for the recruitment and binding to the endosomal membrane; the FERM domain recruits SNX-BAR heterodimers which initiates membrane remodeling and tubule formation necessary for transporting the cargo [12], and the PDZ domain binds to VPS26 retromer subunit and acts as the cargo adaptor [8]. Studies have shown that the disruption of either of these components impairs SNX27 dependent cargo binding and trafficking.
While the PX domain drives SNX27 membrane recruitment, structure-based analyses have shown that the synergistic interaction of the FERM domain with the endosomal membranes also enhances localization of the SNX27-retromer complex to the endosomes, as disrupting the FERM domain decreased the affinity between SNX27 and the endosomal compartments [2].
The actin remodeling WASH complex, which consists of the WASH1, WASHC3, WASHC4, WASHC5, and FAM21 subunits [13], was identified as another SNX27-retromer interacting partner, which synergistically binds to the SNZ27-FERM domain as well as the VPS35 subunit of retromer via FAM21 [14]. Upon its recruitment, the WASH complex has shown to initiate the formation of F-actin filaments on the endosomal membranes which promotes trafficking of cargo from endosomes to the plasma membrane directly [15]. This has been particularly observed in the SNX27 PDZ-domain-dependent cell surface recycling of GLUT1, as disrupting the binding of the WASH component to the SNX27-retromer complex directed the recycling of GLUT1 to the TGN instead of the plasma membrane [16]. Therefore, this interaction may be critical for the assembly and/or regulation of the SNX27-retromer complex.
Among the many identified PDZ-domain-containing cargoes dependent on the SNX27-retromer-mediated endosome-to-plasma membrane recycling (see Table 1), β-2-andrenergic receptor was the very first transmembrane protein [8]. Some other cargoes include Ras, a monomeric small GTPase [2]; zonula occludens 2 (ZO2), an epithelial tight junction protein [8]; and AMPA receptor [17]. More recently identified cargoes include OTULIN, a deubiquitinating enzyme, which contains a class-1 PDZ-binding motif [18]. However, via X-ray crystallography it was observed that OTULIN has a high affinity to the PDZ-VPS26 binding site in addition to the canonical PDZ domain-binding motif interaction site [18]. SNX27 interactome analysis also revealed NHE3, which depends on the PDZ-domain interaction with SNX27 for its recycling from early endosomes to the plasma membrane [19]. In addition to maintaining NHE3 surface expression levels, SNX27 was also found to be necessary for brush border stability as SNX27 deletion in intestinal epithelial cells reduced NHE3 basal activity. Similarly, DRA was found to be dependent on SNX27 for its recycling to the apical plasma membrane, as shown in CaCo2 intestinal epithelial cells [20]. These studies have revealed a novel function of SNX27 mediated endocytic recycling of transmembrane proteins in the gastrointestinal tract which may be of importance in studying disease pathogenesis.
Table 1. Identified binding partners of SNX27.
| Target ID | Target Name | Ref. |
|---|---|---|
| β2-ar | β2 adrenergic receptor | [12] |
| 5-HT4.a/R | 5-hydroxytryptamine type 4 receptor | [4] |
| GIRK2 | G protein-gated inwardly rectifying potassium 2 | [21] |
| GIRK3 | G protein-gated inwardly rectifying potassium 3 | [22] |
| PTHR | Parathyroid hormone 1 receptor | [23] |
| mGluR5 | Metabotropic glutamate receptor 5 | [24] |
| FZD7 | Frizzled receptor 7 | [25] |
| GLUT1 | Glucose transporter 1 | [26] |
| ATP7A | ATPase copper transporting alpha | [26] |
| ASCT2 | Alanine-, serine-, cysteine-preferring transporter 2 | [27] |
| Ras | Ras GTPase | [2] |
| OTULIN | OTU Deubiquitinase With Linear Linkage Specificity | [18] |
| NHE3 | Sodium (Na+)/hydrogen (H+) exchanger 3 | [19] |
| DRA | Downregulated in adenoma | [20] |
| MT1-MMP | Membrane type 1 matrix metalloproteinase | [28] |
| DGKζ | Diacylglycerol kinase zeta | [6] |
| ZO-2 | Zonula occludens 2 | [29] |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor | [30] |
| β1-ar | β1 adrenergic receptor | [31] |
| SSTR5 | mouse Somatostatin receptor subtype 5 | [32] |
| CASP | Cytohesin associated scaffolding protein | [33] |
| NR2C | N-methyl-D-aspartate (NMDA) receptor 2C | [34] |
| AQP2 | Aquaporin 2 | [35] |
| β-Pix | β-PAK-interacting exchange factor | [36] |
| Git | G-protein receptor kinase interacting target | [36] |
| MRP4 | Multidrug resistance-associated protein 4 | [37] |
| SorLA | Sorting-related receptor with A-type repeats | [38] |
| GRP17 | Gadd related protein, 17 kDa | [39] |
In contrast to the canonical mechanism of class-1 PDZ-binding motifs necessary for interaction with SNX27, it was recently observed that MT1-MMP, which lacks a class-1 PDZ-binding motif on its C-terminus, is still able to bind to the SNX27-retromer complex and is recycled to the cell surface in a SNX27-retomer-dependent manner [28]. Structural analysis revealed that this interaction is mediated via the DKV motif present in the cytosolic tail of MT1-MMP, which has shown to possess the features similar to a class-III PDZ-binding motif, however further biophysical studies are deemed necessary to fully understand this association [40]. This observation provides novel insights into the possibilities of non-canonical mechanisms of SNX27 PDZ-domain interactions with its cargoes.
Overall, SNX27’s interacts with various transmembrane proteins for the maintenance of their cell surface levels and activity. It suggests that loss of SNX27 may lead to an array of cellular dysfunction, which has been reciprocated in vivo as SNX27 whole-body knockout mice have been shown to be embryonically lethal [41].
3. SNX27 and Activation of T-cells
SNX27 is found to be expressed in almost every cell type, based on the data from Human Atlas (https://www.proteinatlas.org/ENSG00000143376-SNX27/tissue, accessed on 21 December 2022). Primarily, SNX27 was studied in the brain–nervous system and related diseases. However, it can be believed that its role in other organs could be equally critical.
SNX27 was found to be localized at the immune synapse (IS) in a T-cell receptor (TCR) activation dependent manner [42]. Cells of the immune system give a great example of an intricate molecular network that depends on the endocytic recycling pathway to maintain constant communication between the intercellular components and the extracellular environment. The plasma membrane of T-cell lymphocytes expresses several important receptors, including TCR, which is necessary for the recognition of antigens presented by an antigen-presenting cell (APC) [43]. The binding of the TCR and a recognized antigenic peptide triggers a rapid morphological change within the T-cells causing actin remodeling and vesicular polarized trafficking of organelles towards the T cell-APC interface, thus forming the immune synapse (IS) [44]. This event is critical for sustaining intact communication between T-cell and the APC which ensures the activation of T-cell and downstream immune responses [45]. Extensive studies revealed that T-cells maintain surface level TCR expression and other signaling components necessary for IS via endosomal recycling pathways [46][47].
Under unstimulated conditions, SNX27 is predominantly expressed on the PtdIns[1]P-rich membranes of early and recycling endosomes, which then rapidly polarizes towards the IS upon TCR activation [6]. Structural studies have revealed that this redistribution is mediated by the interactions between the PX and FERM domains of SNX27 with PtdIns[1]P and PtdIns(4,5)P2/PtdIns(3,4,5)P3-enriched membranes, respectively [48][49]. This was further supported by Tello-Lafoz et al., as they showed that disruption of the FERM domain impaired SNX27 spatial distribution during IS initiation [42].
Proteomic analysis of the SNX27 interactome in IS forming activated T-cell lymphocytes has not only confirmed the participation of retromer and WASH complexes in SNX27-mediated polarized trafficking, but also revealed several SNX27 interacting cargoes that are presented at the IS in a PDZ-dependent manner, e.g., diacylglycerol kinase-ζ (DGKζ) [6][50]. TCR activation leads to the generation and accumulation of diacylglycerols (DAGs) at the IS which facilitates the recruitment of other signaling proteins involved in IS formation and maintenance [51]. While DGKζ is a negative regulator of DAG, its association with SNX27 prevents degeneration of DAG, thereby regulating IS stability via SNX27-mediated DAG metabolism [6]. Likewise, disrupting the SNX27-DGKζ interaction in activated T-cells affected downstream signaling pathways, as indicated by increased ERK phosphorylation and NF-κB hyperactivation upon either SNX27 or DGKζ silencing [6][52][53]. However, silencing of SNX27 does not affect protein expression levels of DGKζ, which suggests that the association of DGKζ with SNX27 is only necessary for its spatial distribution and trafficking during IS formation [53].
The identification of DGKζ and its association with SNX27 provides novel insights into DAG associated T-cell activation. It also suggests the tissue specific role of SNX27 in immune system. Further investigation is required to determine the extent of SNX27-mediated regulation of IS assembly and participation of other SNX27-interacting cargoes in this process. Underlying the importance of SNX27 mediated endocytic recycling of a wide array of transmembrane proteins with distinct functions, SNX27 is necessary for proper functioning of human health [54].
4. SNX27 in Neurodegenerative Disorders
The PDZ binding motif is most commonly found in proteins involved in excitatory synapses, and thus located within regions of postsynaptic densities in the neurons [55]. In the brain itself, SNX27 is found to be localized primarily within dendrites and has been shown to regulate synaptic plasticity [56]. SNX27 was first identified in the brain in an experiment wherein metamphetamine induced stimulation of dopamine receptors caused an upregulation of SNX27 [57]. Additionally, in vivo studies have shown that SNX27 is also critical for postnatal growth and survival as SNX27-/- mice die shortly after birth [34]. Therefore, dysregulated SNX27 functioning has been reported in several neurological and degenerative diseases, such as Alzheimer’s disease.
Multiple studies have shown enlargement of early endosomes and multivesicular bodies in brain tissues and neurons isolated from human as well murine models of Alzheimer’s disease (AD), overall suggesting defects in endocytic trafficking and recycling pathways [58][59]. SNX27 loss-of-function proteomic analysis has also demonstrated that SNX27 mediates recycling of internalized AD-related protein APP, as deletion of SNX27 decreased cell surface expression levels of APP [38][60]. However, the authors of this study were unable to detect direct binding of SNX27 to APP, suggesting the involvement of an intermediate molecule promoting SNX27/APP interaction [26]. Previously, an intracellular sorting receptor, SorLA, was identified as an APP binding protein, and reduction in SorLA expression levels caused APP cellular redistribution [61]. Therefore, functional studies looking into the interaction between SNX27, APP and SorLA were conducted, and the results revealed that SorLA binds to APP as well as SNX27 and forms a ternary structural protein complex, thereby acting as the molecular link in SNX27 mediated trafficking and recycling of APP [38].
SNX27 has also been reported to contribute towards impairment in neuronal and learning abilities in Down’s Syndrome (DS) [55]. The overall expression levels of SNX27 are shown to be downregulated in brain tissues from human patients and murine models of DS. Interestingly, rescue studies in DS mouse model have shown that overexpression of SNX27 reverses or corrects DS-related cognitive and synaptic impaired phenotypes [55]. Further analysis revealed that expression of SNX27 is regulated by C/EBPβ, a transcription factor targeted by miR-155. It was identified that miR-155 is a micro-RNA encoded on chromosome 21; therefore, trisomy of chromosome 21 in DS drives up miR-155 which causes silencing of C/EBPβ, thereby downregulating SNX27 [55]. More recently SNX27 was shown to recycle the myelination-related protein, GRP17, which is crucial for the differentiation and maturation of oligodendrocytes [39]. Improper functioning and distribution of oligodendrocytes has been reported in human and mouse DS as it leads to abnormalities in the white matter of the brain which increases defects in cognitive and motor skills [62]. Therefore, these studies suggest an underlying mechanism of SNX27 mediated neuropathogenesis of DS through oligodendrocyte dysfunction.
Lastly, SNX27 has been shown to recycle AMPA and NMDA glutamate receptors [34][56]. Glutamate serves as the primary neurotransmitter in the brain and therefore plays a critical role in mediating cognitive function and excitatory synapses, both of which promote learning and motor abilities [63]. Consequently, heterozygous SNX27-/+ mice appear to have learning and memory disabilities associated with a reduction in cell surface expression of AMPA or NMDA receptors [41]. Therefore, dysregulated expression of SNX27 can lead to seizures and epilepsy among a plethora of other neurodegenerative diseases [55].
This entry is adapted from the peer-reviewed paper 10.3390/cancers15010070
References
- Teasdale, R.D.; Collins, B.M. Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: Structures, functions, and roles in disease. Biochem. J. 2012, 441, 39–59.
- Ghai, R.; Mobli, M.; Norwood, S.J.; Bugarcic, A.; Teasdale, R.D.; King, G.F.; Collins, B.M. Phox homology band 4.1/ezrin/radixin/moesin-like proteins function as molecular scaffolds that interact with cargo receptors and Ras GTPases. Proc. Natl. Acad. Sci. USA 2011, 108, 7763–7768.
- Yong, X.; Zhao, L.; Hu, W.; Sun, Q.; Ham, H.; Liu, Z.; Ren, J.; Zhang, Z.; Zhou, Y.; Yang, Q.; et al. SNX27-FERM-SNX1 complex structure rationalizes divergent trafficking pathways by SNX17 and SNX27. Proc. Natl. Acad. Sci. USA 2021, 118, e2105510118.
- Joubert, L.; Hanson, B.; Barthet, G.; Sebben, M.; Claeysen, S.; Hong, W.; Marin, P.; Dumuis, A.; Bockaert, J. New sorting nexin (SNX27) and NHERF specifically interact with the 5-HT4a receptor splice variant: Roles in receptor targeting. J. Cell Sci. 2004, 117 Pt 22, 5367–5379.
- Lunn, M.L.; Nassirpour, R.; Arrabit, C.; Tan, J.; Mcleod, I.; Arias, C.M.; Sawchenko, P.E.; Yates, J.R.; Slesinger, P.A. A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction. Nat. Neurosci. 2007, 10, 1249–1259.
- Rincon, E.; Santos, T.; Avila-Flores, A.; Albar, J.P.; Lalioti, V.; Lei, C.; Hong, W.; Merida, I. Proteomics identification of sorting nexin 27 as a diacylglycerol kinase zeta-associated protein: New diacylglycerol kinase roles in endocytic recycling. Mol. Cell Proteom. 2007, 6, 1073–1087.
- Clairfeuille, T.; Mas, C.; Chan, A.S.; Yang, Z.; Tello-Lafoz, M.; Chandra, M.; Widagdo, J.; Kerr, M.C.; Paul, B.; Mérida, I.; et al. Molecular code for endosomal recycling of phosphorylated cargos by the SNX27-retromer complex. Nat. Struct Mol. Biol. 2016, 23, 921–932.
- Lauffer, B.E.; Melero, C.; Temkin, P.; Lei, C.; Hong, W.; Kortemme, T.; von Zastrow, M. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J. Cell Biol. 2010, 190, 565–574.
- Sheff, D.R.; Daro, E.A.; Hull, M.; Mellman, I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 1999, 145, 123–139.
- Zhang, H.; Huang, T.; Hong, Y.; Yang, W.; Zhang, X.; Luo, H.; Xu, H.; Wang, X. The retromer complex and sorting nexins in neurodegenerative diseases. Front. Aging Neurosci. 2018, 10, 79.
- van Weering, J.R.; Sessions, R.B.; Traer, C.J.; Kloer, D.P.; Bhatia, V.K.; Stamou, D.; Carlsson, S.R.; Hurley, J.H.; Cullen, P.J. Molecular basis for SNX-BAR-mediated assembly of distinct endosomal sorting tubules. EMBO J. 2012, 31, 4466–4480.
- Temkin, P.; Lauffer, B.; Jäger, S.; Cimermancic, P.; Krogan, N.J.; von Zastrow, M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signaling receptors. Nat. Cell Biol. 2011, 13, 715–721.
- Derivery, E.; Sousa, C.; Gautier, J.J.; Lombard, B.; Loew, D.; Gautreau, A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev. Cell. 2009, 17, 712–723.
- Harbour, M.E.; Breusegem, S.Y.; Seaman, M.N. Recruitment of the endosomal WASH complex is mediated by the extended ‘tail’ of Fam21 binding to the retromer protein Vps35. Biochem. J. 2012, 442, 209–220.
- Seaman, M.N.; Gautreau, A.; Billadeau, D.D. Retromer-mediated endosomal protein sorting: All WASHed up! Trends Cell Biol. 2013, 23, 522–528.
- Lee, S.; Chang, J.; Blackstone, C. FAM21 directs SNX27-retromer cargoes to the plasma membrane by preventing transport to the Golgi apparatus. Nat. Commun. 2016, 7, 10939.
- Temkin, P.; Morishita, W.; Goswami, D.; Arendt, K.; Chen, L.; Malenka, R. The Retromer Supports AMPA Receptor Trafficking During LTP. Neuron 2017, 94, 74–82.
- Stangl, A.; Elliott, P.R.; Pinto-Fernandez, A.; Bonham, S.; Harrison, L.; Schaub, A.; Kutzner, K.; Keusekotten, K.; Pfluger, P.T.; El Oualid, F.; et al. Regulation of the endosomal SNX27-retromer by OTULIN. Nat. Commun. 2019, 10, 4320.
- Singh, V.; Yang, J.; Cha, B.; Chen, T.E.; Sarker, R.; Yin, J.; Avula, L.R.; Tse, M.; Donowitz, M. Sorting nexin 27 regulates basal and stimulated brush border trafficking of NHE3. Mol. Biol. Cell. 2015, 26, 2030–2043.
- Bannert, K.; Berlin, P.; Reiner, J.; Lemcke, H.; David, R.; Engelmann, R.; Lamprecht, G. SNX27 regulates DRA activity and mediates its direct recycling by PDZ-interaction in early endosomes at the apical pole of Caco2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G854–G869.
- Balana, B.; Maslennikov, I.; Kwiatkowski, W.; Stern, K.M.; Bahima, L.; Choe, S.; Slesinger, P.A. Mechanism underlying selective regulation of G protein-gated inwardly rectifying potassium channels by the psychostimulant-sensitive sorting nexin 27. Proc. Natl. Acad. Sci. USA 2011, 108, 5831–5836.
- Munoz, M.B.; Slesinger, P.A. Sorting nexin 27 regulation of G protein-gated inwardly rectifying K(+) channels attenuates in vivo cocaine response. Neuron 2014, 82, 659–669.
- Chan, A.S.; Clairfeuille, T.; Landao-Bassonga, E.; Kinna, G.; Ng, P.Y.; Loo, L.S.; Cheng, T.S.; Zheng, M.; Hong, W.; Teasdale, R.D.; et al. Sorting nexin 27 couples PTHR trafficking to retromer for signal regulation in osteoblasts during bone growth. Mol. Biol. Cell. 2016, 27, 1367–1382.
- Lin, T.B.; Lai, C.Y.; Hsieh, M.C.; Wang, H.H.; Cheng, J.K.; Chau, Y.P.; Chen, G.D.; Peng, H.Y. VPS26A-SNX27 interaction-dependent mGluR5 recycling in dorsal horn neurons mediates neuropathic pain in rats. J. Neurosci. 2015, 35, 14943–14955.
- Sun, L.; Hu, X.; Chen, W.; He, W.; Zhang, Z.; Wang, T. Sorting nexin 27 interacts with Fzd7 and mediates Wnt signalling. Biosci. Rep. 2016, 36, e00296.
- Steinberg, F.; Gallon, M.; Winfield, M.; Thomas, E.C.; Bell, A.J.; Heesom, K.J.; Tavaré, J.M.; Cullen, P.J. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell Biol. 2013, 15, 461–471.
- Yang, Z.; Follett, J.; Kerr, M.C.; Clairfeuille, T.; Chandra, M.; Collins, B.M.; Teasdale, R.D. Sorting nexin 27 (SNX27) regulates the trafficking and activity of the glutamine transporter ASCT2. J. Biol. Chem. 2018, 293, 6802–6811.
- Sharma, P.; Parveen, S.; Shah, L.; Mukherjee, M.; Kalaidzidis, Y.; Kozielski, A.; Rosato, R.; Chang, J.; Datta, S. SNX27–retromer assembly recycles MT1-MMP to invadopodia and promotes breast cancer metastasis. J. Cell Biol. 2020, 219, e201812098.
- Zimmerman, S.P.; Hueschen, C.L.; Malide, D.; Milgram, S.L.; Playford, M.P. Sorting nexin 27 (SNX27) associates with zonula occludens-2 (ZO-2) and modulates the epithelial tight junction. Biochem. J. 2013, 455, 95–106.
- Hussain, N.K.; Diering, G.H.; Sole, J.; Anggono, V.; Huganir, R.L. Sorting nexin 27 regulates basal and activity-dependent trafficking of AMPARs. Proc. Natl. Acad. Sci. USA 2014, 111, 11840–11845.
- Nakagawa, T.; Asahi, M. β1-adrenergic receptor recycles via a membranous organelle, recycling endosome, by binding with sorting nexin27. J. Membr Biol. 2013, 246, 571–579.
- Bauch, C.; Koliwer, J.; Buck, F.; Hönck, H.H.; Kreienkamp, H.J. Subcellular sorting of the G-protein coupled mouse somatostatin receptor 5 by a network of PDZ-domain containing proteins. PLoS ONE 2014, 9, e88529.
- MacNeil, A.J.; Mansour, M.; Pohajdak, B. Sorting nexin 27 interacts with the cytohesin associated scaffolding protein (CASP) in lymphocytes. Biochem. Biophys. Res. Commun. 2007, 359, 848–853.
- Cai, L.; Loo, L.S.; Atlashkin, V.; Hanson, B.J.; Hong, W. Deficiency of sorting nexin 27 (SNX27) leads to growth retardation and elevated levels of N-methyl-D-aspartate (NMDA) receptor 2C (NR2C). Mol. Cell Biol. 2011, 31, 1734–1747.
- Choi, H.J.; Jang, H.J.; Park, E.; Tingskov, S.J.; Nørregaard, R.; Jung, H.J.; Kwon, T.H. Sorting Nexin 27 Regulates the Lysosomal Degradation of Aquaporin-2 Protein in the Kidney Collecting Duct. Cells 2020, 9, 1208.
- Valdes, J.L.; Tang, J.; McDermott, M.I.; Kuo, J.C.; Zimmerman, S.P.; Wincovitch, S.M.; Waterman, C.M.; Milgram, S.L.; Playford, M.P. Sorting nexin 27 protein regulates trafficking of a p21-activated kinase interacting exchange factor (β-Pix)-G protein-coupled receptor kinase interacting protein (GIT) complex via a PDZ domain interaction. J. Biol. Chem. 2011, 286, 39403–39416.
- Hayashi, H.; Naoi, S.; Nakagawa, T.; Nishikawa, T.; Fukuda, H.; Imajoh-Ohmi, S.; Kondo, A.; Kubo, K.; Yabuki, T.; Hattori, A.; et al. Sorting nexin 27 interacts with multidrug resistance-associated protein 4 (MRP4) and mediates internalization of MRP4. J Biol. Chem. 2012, 287, 15054–15065.
- Huang, T.Y.; Zhao, Y.; Li, X.; Wang, X.; Tseng, I.C.; Thompson, R.; Tu, S.; Willnow, T.E.; Zhang, Y.W.; Xu, H. SNX27 and SORLA Interact to Reduce Amyloidogenic Subcellular Distribution and Processing of Amyloid Precursor Protein. J. Neurosci. 2016, 36, 7996–8011.
- Meraviglia, V.; Ulivi, A.F.; Boccazzi, M.; Valenza, F.; Fratangeli, A.; Passafaro, M.; Lecca, D.; Stagni, F.; Giacomini, A.; Bartesaghi, R.; et al. SNX27, a protein involved in down syndrome, regulates GRP17 trafficking and oligodendrocyte differentiation. Glia 2016, 64, 1437–1460.
- Nourry, C.; Grant, S.G.N.; and Borg, J.P. PDZ domain proteins: Plug and play! Sci. STKE 2003, 179, re7.
- Damseh, N.; Danson, C.M.; Al-Ashhab, M.; Abu-Libdeh, B.; Gallon, M.; Sharma, K.; Yaacov, B.; Coulthard, E.; Caldwell, M.A.; Edvardson, S.; et al. A defect in the retromer accessory protein, SNX27, manifests by infantile myoclonic epilepsy and neurodegeneration. Neurogenetics 2015, 16, 215–221.
- Tello-Lafoz, M.; Ghai, R.; Collins, B.; Mérida, I. A role for novel lipid interactions in the dynamic recruitment of SNX27 to the T-cell immune synapse. Bioarchitecture 2014, 4, 215–220.
- Gunzer, M.; Weishaupt, C.; Hillmer, A.; Basoglu, Y.; Friedl, P.; Dittmar, K.E.; Kolanus, W.; Varga, G.; Grabbe, S. A spectrum of biophysical interaction modes between T cells and different antigen-presenting cells during priming in 3-D collagen and in vivo. Blood 2004, 104, 2801–2809.
- Onnis, A.; Finetti, F.; Baldari, C.T. Vesicular Trafficking to the Immune Synapse: How to Assemble Receptor-Tailored Pathways from a Basic Building Set. Front. Immunol. 2016, 7, 50.
- Monks, C.R.F.; Freiberg, B.A.; Kupfer, H.; Sciaky, N.; Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 1998, 395, 82–86.
- Monjas, A.; Alcover, A.; Alarcón, B. Engaged and bystander T cell receptors are down-modulated by different endocytotic pathways. J. Biol. Chem. 2004, 279, 55376–55384.
- Soares, H.; Lasserre, R.; Alcover, A. Orchestrating cytoskeleton and intracellular vesicle traffic to build functional immunological synapses. Immunol. Rev. 2013, 256, 118–132.
- Rincon, E.; de Guinoa, J.S.; Gharbi, S.I.; Sorzano, C.O.S.; Carrasco, Y.R.; Merida, I. Translocation dynamics of sorting nexin 27 in activated T cells. J. Cell Sci. 2011, 124, 776–788.
- Ghai, R.; Tello-Lafoz, M.; Norwood, S.J.; Yang, Z.; Clairfeuille, T.; Teasdale, R.D.; Merida, I.; Collins, B.M. Phosphoinositide binding by the SNX27 FERM domain regulates its localization at the immune synapse of activated T-cells. J. Cell Sci. 2015, 128, 553–565.
- Tello-Lafoz, M.; Martínez-Martínez, G.; Rodríguez-Rodríguez, C.; Albar, J.P.; Huse, M.; Gharbi, S.; Merida, I. Sorting nexin 27 interactome in T-lymphocytes identifies zona occludens-2 dynamic redistribution at the immune synapse. Traffic 2017, 18, 491–504.
- Almena, M.; Mérida, I. Shaping up the membrane: Diacylglycerol coordinates spatial orientation of signaling. Trends Biochem. Sci. 2011, 36, 593–603.
- Ávila-Flores, A.; Arranz-Nicolás, J.; Andrada, E.; Soutar, D.; Mérida, I. Predominant contribution of DGKζ over DGKα in the control of PKC/PDK-1-regulated functions in T cells. Immunol. Cell Biol. 2017, 95, 549–563.
- Tello-Lafoz, M.; Rodríguez-Rodríguez, C.; Kinna, G.; Loo, L.S.; Hong, W.; Collins, B.M.; Teasdale, R.D.; Mérida, I. SNX27 links DGKζ to the control of transcriptional and metabolic programs in T lymphocytes. Sci. Rep. 2017, 7, 16361.
- McMillan, K.J.; Korswagen, H.C.; Cullen, P.J. The emerging role of retromer in neuroprotection. Curr. Opin. Cell Biol. 2017, 47, 72–82.
- Wang, X.; Zhao, Y.; Zhang, X.; Badie, H.; Zhou, Y.; Mu, Y.; Loo, L.S.; Cai, L.; Thompson, R.C.; Yang, B.; et al. Loss of sorting nexin 27 contributes to excitatory synaptic dysfunction by modulating glutamate receptor recycling in Down’s syndrome. Nat. Med. 2013, 19, 473–480.
- Loo, L.S.; Tang, N.; Al-Haddawi, M.; Dawe, G.S.; Hong, W. A role for sorting nexin 27 in AMPA receptor trafficking. Nat. Commun. 2014, 5, 3176.
- Fujiyama, K.; Kajii, Y.; Hiraoka, S.; Nishikawa, T. Differential regulation by stimulants of neocortical expression of mrt1, arc, and homer1a mRNA in the rats treated with repeated methamphetamine. Synapse 2003, 49, 143–149.
- Willén, K.; Sroka, A.; Takahashi, R.H.; Gouras, G.K. Heterogeneous Association of Alzheimer’s Disease-Linked Amyloid-β and Amyloid-β Protein Precursor with Synapses. J. Alzheimers Dis. 2017, 60, 511–524.
- Cataldo, A.; Rebeck, G.W.; Ghetri, B.; Hulette, C.; Lippa, C.; Van Broeckhoven, C.; van Duijn, C.; Cras, P.; Bogdanovic, N.; Bird, T.; et al. Endocytic disturbances distinguish among subtypes of Alzheimer’s disease and related disorders. Ann. Neurol. 2001, 50, 661–665.
- Wang, X.; Huang, T.; Zhao, Y.; Zheng, Q.; Thompson, R.C.; Bu, G.; Zhang, Y.W.; Hong, W.; Xu, H. Sorting nexin 27 regulates Aβ production through modulating γ-secretase activity. Cell Rep. 2014, 9, 1023–1033.
- Nielsen, M.S.; Gustafsen, C.; Madsen, P.; Nyengaard, J.R.; Hermey, G.; Bakke, O.; Mari, M.; Schu, P.; Pohlmann, R.; Dennes, A.; et al. Sorting by the cytoplasmic domain of the amyloid precursor protein binding receptor SorLA. Mol. Cell Biol. 2007, 27, 6842–6851.
- Olmos-Serrano, J.L.; Kang, H.J.; Tyler, W.A.; Silbereis, J.C.; Cheng, F.; Zhu, Y.; Pletikos, M.; Jankovic-Rapan, L.; Cramer, N.P.; Galdzicki, Z.; et al. Down Syndrome Developmental Brain Transcriptome Reveals Defective Oligodendrocyte Differentiation and Myelination. Neuron 2016, 89, 1208–1222.
- Niciu, M.J.; Kelmendi, B.; Sanacora, G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol. Biochem. Behav. 2012, 100, 656–664.
This entry is offline, you can click here to edit this entry!
