Allogeneic stem cell transplantation (allo-SCT) represented the first immunotherapy to treat hematologic malignancies: it has been considered as a cure for the disease and never as an approach to extend the life of patients. The success of allo-SCT derives both from the ability to treat patients with intensive chemoradiotherapy and from the potent graft-versus-leukemia effects mediated by donor immunity. The treatment of hematologic malignancies, particularly acute lymphoblastic leukemia and certain forms of lymphomas, has been revolutionized by the commercial introduction of genetically modified autologous T-lymphocyte therapy (CAR-T).
- allogeneic stem cell transplantation
- autologous stem cell transplantation
- CAR-T treatment
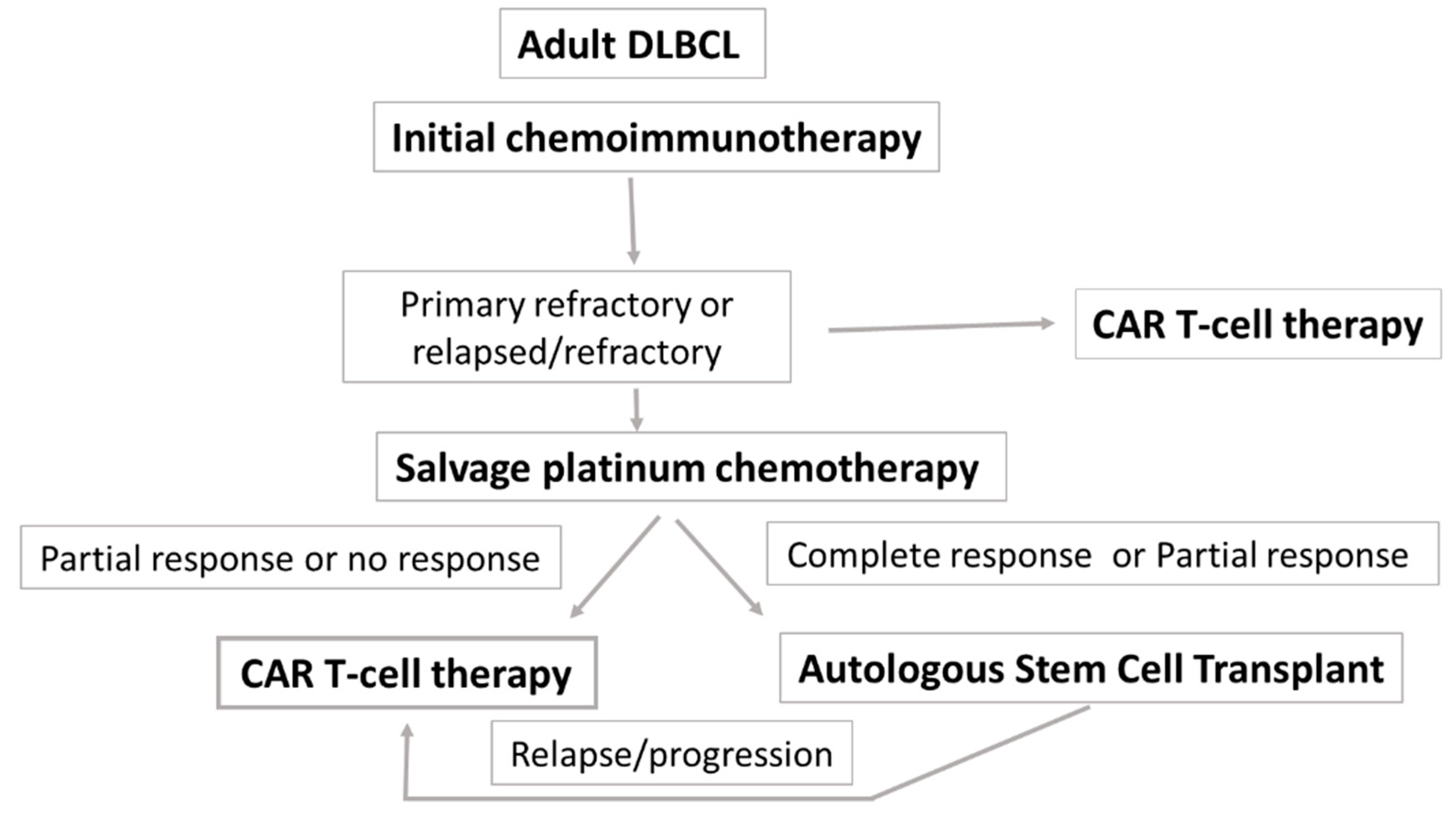
1. R/R DLBCL
| JULIET | ZUMA-1 | TRANSCEND NHL 001 | |
|---|---|---|---|
| CAR T-cell agent | Tisa-cel | Axi-cel | Liso-cel |
| Patient population | Adults with R/R DLBCL post/ineligible for Auto-SCT | Adults with R/R DLBCL | Adults with R/R DLBCL |
| Patients apheresed/treated, n | 165/111 | 111/101 | 344/269 |
| ORR, % CR, % |
52% 40% |
82% 54% |
73% 53% |
| Survival | 12-mo PFS 65% 12-mo OS 49% |
PFS 5.8 mo 18-mo OS 52% |
12-mo RFS 44% 12-mo OS 58% |
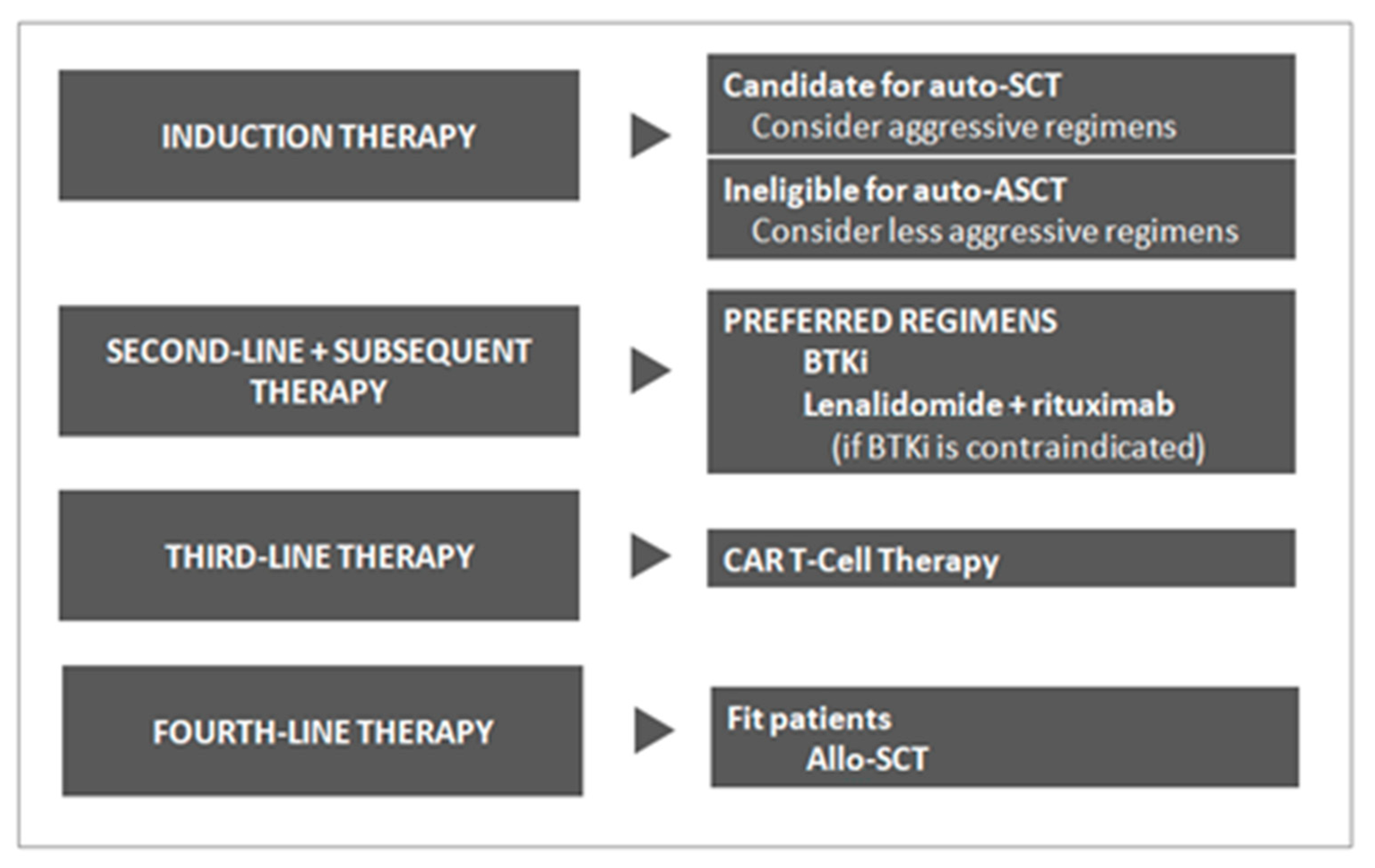
2. MCL
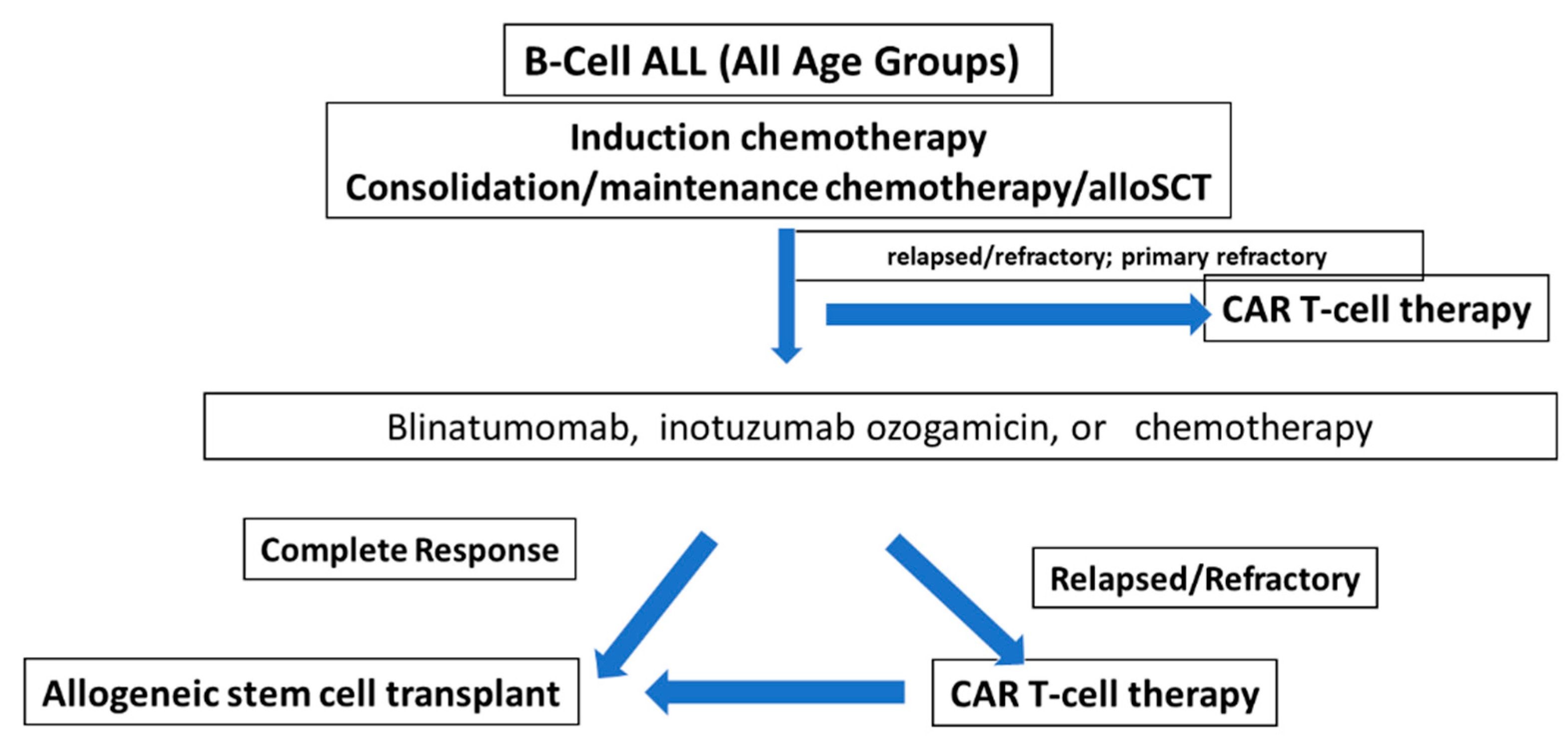
3. The Rationale for Clinical Development of CAR T-Cell Therapy in ALL
This entry is adapted from the peer-reviewed paper 10.3390/ijms24021045
References
- Snowden, J.A.; Sánchez-Ortega, I.; Corbacioglu, S.; Basak, G.W.; Chabannon, C.; de la Camara, R.; Dolstra, H.; Duarte, R.F.; Glass, B.; Greco, R.; et al. European Society for Blood and Marrow Transplantation (EBMT). Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2022. Bone Marrow Transplant. 2022, 57, 1217–1239.
- van Kampen, R.J.; Canals, C.; Schouten, H.C.; Nagler, A.; Thomson, K.J.; Vernant, J.P.; Buzyn, A.; Boogaerts, M.A.; Luan, J.J.; Maury, S.; et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin’s lymphoma relapsing after an autologous stem-cell transplantation: An analysis of the European Group for Blood and Marrow Transplantation Registry. J. Clin. Oncol. 2011, 29, 1342–1348.
- Glass, B.; Hasenkamp, J.; Wulf, G.; Dreger, P.; Pfreundschuh, M.; Gramatzki, M.; Silling, G.; Wilhelm, C.; Zeis, M.; Görlitz, A.; et al. German High-Grade Lymphoma Study Group. Rituximab after lymphoma-directed conditioning and allogeneic stem-cell transplantation for relapsed and refractory aggressive non-Hodgkin lymphoma (DSHNHL R3): An open-label, randomised, phase 2 trial. Lancet Oncol. 2014, 15, 757–766.
- Dodero, A.; Patriarca, F.; Milone, G.; Sarina, B.; Miceli, R.; Iori, A.; Barretta, F.; Terruzzi, E.; Mussetti, A.; Pini, M.; et al. Allogeneic Stem Cell Transplantation for Relapsed/Refractory B Cell Lymphomas: Results of a Multicenter Phase II Prospective Trial including Rituximab in the Reduced-Intensity Conditioning Regimen. Biol. Blood Marrow Transplant 2017, 23, 1102–1109.
- Fenske, T.S.; Ahn, K.W.; Graff, T.M.; DiGilio, A.; Bashir, Q.; Kamble, R.T.; Ayala, E.; Bacher, U.; Brammer, J.E.; Cairo, M.; et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br. J. Haematol. 2016, 174, 235–248.
- Passweg, J.R.; Baldomero, H.; Chabannon, C.; Corbacioglu, S.; de la Cámara, R.; Dolstra, H.; Glass, B.; Greco, R.; Mohty, M.; Neven, B.; et al. Impact of the SARS-CoV-2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: A report from the EBMT activity survey. Bone Marrow Transplant. 2022, 57, 742–752.
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019, 20, 31–42.
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 2019, 380, 45–56.
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852.
- Nastoupil, L.J.; Jain, M.D.; Feng, L.; Spiegel, J.Y.; Ghobadi, A.; Lin, Y.; Dahiya, S.; Lunning, M.; Lekakis, L.; Reagan, P.; et al. Standard-of- care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: Results from the US Lymphoma CAR T Consortium. J. Clin. Oncol. 2020, 38, 3119–3128.
- Locke, F.L.; Jacobson, C.; Perales, M.A.; Kersten, M.J.; Westin, J.R. Primary analysis of ZUMA-7: A phase 3 randomized trial of axicabtagene ciloleucel (Axi-Cel) versus standard-of-care therapy in patients with relapsed/refractory large B-cell lym- phoma. Blood 2021, 138, 2.
- Kamdar, M.S.; Scott, R.; Arnason Jon, E.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.G.; Hernandez-Ilizaliturri, F.J.; et al. Lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T cell therapy, versus standard of care (SOC) with salvage chemotherapy (CT) followed by autologous stem cell transplantation (ASCT) as second-line (2L) treatment in patients (Pts) with relapsed or refractory (R/R) large B-cell lymphoma (LBCL): Results from the randomized phase 3 transform study. Blood 2021, 138 (Suppl. 1), 91.
- Frontzek, F.; Karsten, I.; Schmitz, N.; Lenz, G. Current options and future perspectives in the treatment of patients with relapsed/refractory diffuse large B-cell lymphoma. Ther. Adv. Hematol. 2022, 13, 20406207221103321.
- Hamadani, M.; Gopal, A.K.; Pasquini, M.; Kim, S.; Qiu, X.; Ahmed, S.; Lazaryan, A.; Bhatt, V.R.; Daly, A.; Lulla, P.; et al. Allogeneic transplant and CAR-T therapy after autologous transplant failure in DLBCL: A noncomparative cohort analysis. Blood Adv. 2022, 6, 486–494.
- Zurko, J.; Ramdial, J.; Shadman, M.; Ahmed, S.; Szabo, A.; Iovino, L.; Tomas, A.A.; Sauter, C.; Perales, M.A.; Shah, N.N.; et al. Allogeneic transplant following CAR T-cell therapy for large B-cell lymphoma. Haematologica 2022, 18, 1.
- Logue, J.M.; Chavez, J.C. How to Sequence Therapies in Diffuse Large B-Cell Lymphoma Post-CAR-T Cell Failure. Curr. Treat. Options Oncol. 2021, 22, 112.
- Sermer, D.; Batlevi, C.; Palomba, M.L.; Shah, G.; Lin, R.J.; Perales, M.A.; Scordo, M.; Dahi, P.; Pennisi, M.; Afuye, A.; et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020, 4, 4669–4678.
- Harmanen, M.; Hujo, M.; Sund, R.; Sorigue, M.; Khan, M.; Prusila, R.; Klaavuniemi, T.; Kari, E.; Jantunen, E.; Sunela, K.; et al. Survival of patients with mantle cell lymphoma in the rituximab era: Retrospective binational analysis between 2000 and 2020. Br. J. Haematol. 2022.
- Dreyling, M.; Jurczak, W.; Jerkeman, M.; Silva, R.S.; Rusconi, C.; Trneny, M.; Offner, F.; Caballero, D.; Joao, C.; Witzens-Harig, M.; et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lym- phoma: An international, randomised, open-label, phase 3 study. Lancet 2016, 387, 770–778.
- Kumar, A. What is the role of up-front autologous stem cell transplantation in mantle cell lymphoma? Hematol. Am. Soc. Hematol. Educ. Progrom 2022, 2022, 155–162.
- Martin, P.; Maddocks, K.; Leonard, J.P.; Ruan, J.; Goy, A.; Wagner-Johnston, N.; Rule, S.; Advani, R.; Iberri, D.; Phillips, T.; et al. Post-ibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016, 127, 1559–1563.
- Fenske, T.S.; Zhang, M.J.; Carreras, J.; Ayala, E.; Burns, L.J.; Cashen, A.; Costa, L.J.; Freytes, C.O.; Gale, R.P.; Hamadani, M.; et al. Autologous or reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chemotherapy-sensitive mantle-cell lymphoma: Analysis of transplantation timing and modality. J. Clin. Oncol. 2013, 32, 273–281.
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342.
- Fielding, A.K.; Richards, S.M.; Chopra, R.; Lazarus, H.M.; Litzow, M.R.; Buck, G.; Durrant, I.J.; Luger, S.M.; Marks, D.I.; Franklin, I.M.; et al. Medical Research Council of the United Kingdom Adult ALL Working Party; Eastern Cooperative Oncology Group. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007, 109, 944–950.
- Kantarjian, H.; Thomas, D.; Jorgensen, J.; Jabbour, E.; Kebriaei, P.; Rytting, M.; York, S.; Ravandi, F.; Kwari, M.; Faderl, S.; et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: A phase 2 study. Lancet Oncol. 2012, 13, 403–411.
- Topp, M.S.; Gökbuget, N.; Stein, A.S.; Zugmaier, G.; O’Brien, S.; Bargou, R.C.; Dombret, H.; Fielding, A.K.; Heffner, L.; Larson, R.A.; et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2015, 16, 57–66.
- Badar, T.; Szabo, A.; Advani, A.; Wadleigh, M.; Arslan, S.; Khan, M.A.; Aldoss, I.; Siebenaller, C.; Schultz, E.; Hefazi, M.; et al. Real-world outcomes of adult B-cell acute lymphocytic leukemia patients treated with blinatumomab. Blood Adv. 2020, 4, 2308–2316.
- Short, N.J.; Macaron, W.; Konopleva, M.; Ravandi, F.; Jain, N.; Issa, G.C.; Kadia, T.; Sasaki, K.; Kebriaei, P.; Yilmaz, M.; et al. Dismal outcomes of patients with relapsed/refractory Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia after failure of both inotuzumab ozogamicin and blinatumomab. Am. J. Hematol. 2022, 97, E201–E204.
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 439–448.
- Shah, B.D.; Ghobadi, A.; Oluwole, O.O.; Logan, A.C.; Boissel, N.; Cassaday, R.D.; Leguay, T.; Bishop, M.R.; Topp, M.S.; Tzachanis, D.; et al. KTE- X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: Phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 2021, 398, 491–502.
- Mohty, M.; Gautier, J.; Malard, F.; Aljurf, M.; Bazarbachi, A.; Chabannon, C.; Kharfan-Dabaja, M.A.; Savani, B.N.; Huang, H.; Kenderian, S.; et al. CD19 chimeric antigen receptor-T cells in B-cell leukemia and lymphoma: Current status and perspectives. Leukemia 2019, 33, 2767–2778.
- Hay, K.A.; Gauthier, J.; Hirayama, A.V.; Voutsinas, J.M.; Wu, Q.; Li, D.; Gooley, T.A.; Cherian, S.; Chen, X.; Pender, B.S.; et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood 2019, 133, 1652–1663.
- Zhang, X.; Lu, X.A.; Yang, J.; Zhang, G.; Li, J.; Song, L.; Su, Y.; Shi, Y.; Zhang, M.; He, J.; et al. Efficacy and safety of anti- CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leu- kemia with high-risk features. Blood Adv. 2020, 4, 2325–2338.
- Shalabi, H.; Delbrook, C.; Stetler-Stevenson, M. Chimeric antigen receptor T-cell therapy can render patients with ALL into PCR-negative remission and can be an effective bridge to transplant. Biol. Blood Marrow Transplant. 2018, 24, S25–S26.
- Park, J.H.; Riviere, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 449–459.
