Diet is understood to be the most important determinant in shaping the microbiota ecosystem as balanced diets of fruits and vegetables are shown to increase gut bacterial richness and diversity. As such, lifestyle changes specifically through dietary interventions represent an effective therapeutic modality for dyslipidemia by creating targeted and beneficial changes in gut microbial composition. Although many dietary choices influence the composition of the gut microbiota, two particular diets have been studied extensively and can lead to significant, yet opposite effects. In this section, the metabolic changes seen in Western Diet (WD)-induced dyslipidemia followed by the role of plant-based diets was first described, such as the Mediterranean diet (MD) play in optimizing gut microbial composition to restore defective metabolic states.
- mediterranean diet
- prebiotics
- probiotics
- synbiotics
- folate
- fecal microbiota transplantation
1. High Fat Diet, Gut Microbiota and Dyslipidemia
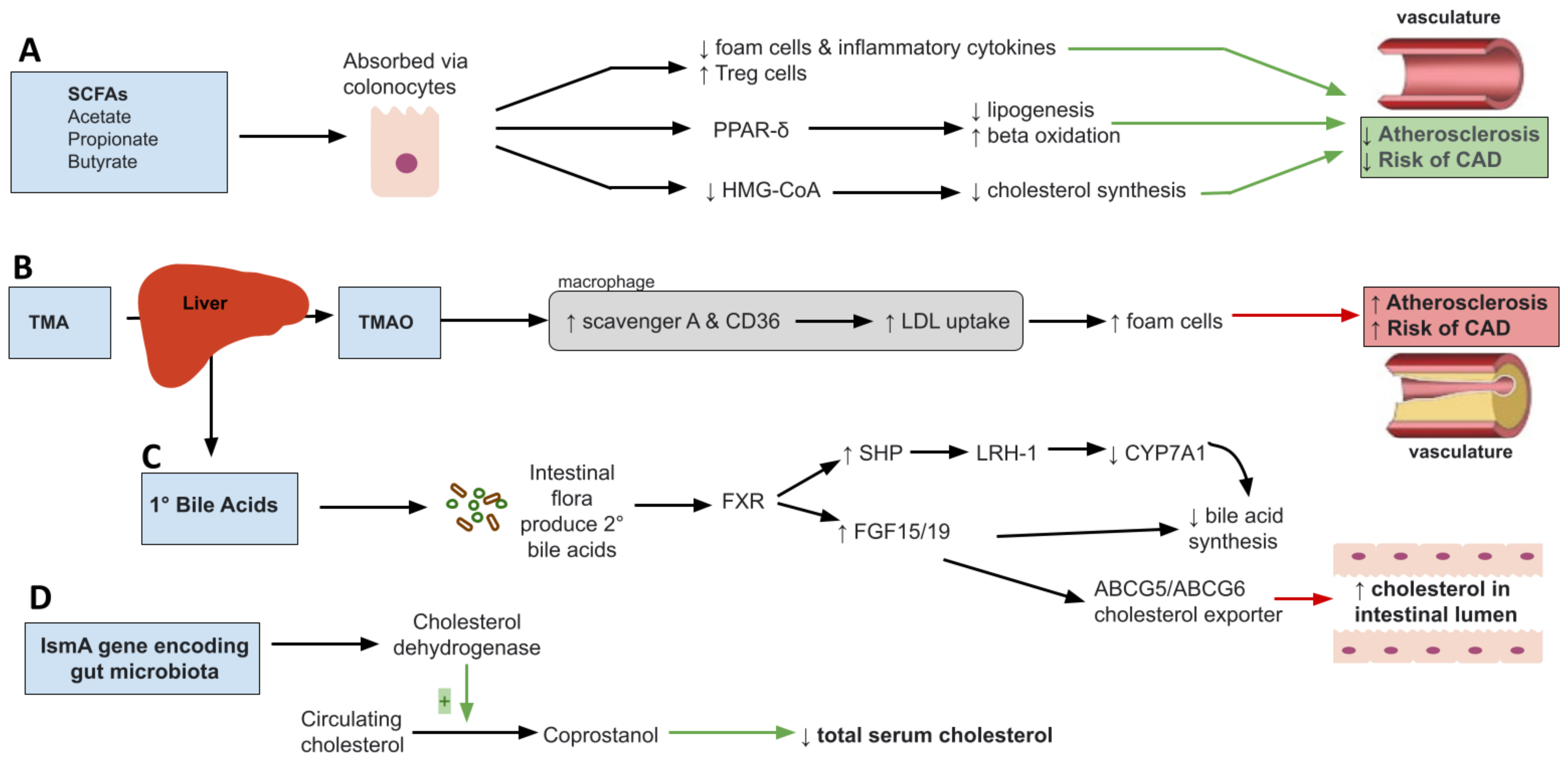
2. Mediterranean Diet, Gut Microbiota and Dyslipidemia
3. Current Pharmacologic Treatments for Dyslipidemia and Relations to Gut Microbiota
This entry is adapted from the peer-reviewed paper 10.3390/nu15010228
References
- Tomas, J.; Mulet, C.; Saffarian, A.; Cavin, J.B.; Ducroc, R.; Regnault, B.; Kun Tan, C.; Duszka, K.; Burcelin, R.; Wahli, W.; et al. High-fat diet modifies the PPAR-γ pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc. Natl. Acad. Sci. USA 2016, 113, E5934–E5943.
- Bailey, M.A.; Holscher, H.D. Microbiome-Mediated Effects of the Mediterranean Diet on Inflammation. Adv. Nutr. 2018, 9, 193–206.
- Määttä, A.M.; Salminen, A.; Pietiäinen, M.; Leskelä, J.; Palviainen, T.; Sattler, W.; Sinisalo, J.; Salomaa, V.; Kaprio, J.; Pussinen, P.J. Endotoxemia is associated with an adverse metabolic profile. Innate Immun. 2021, 27, 3–14.
- Gargiulo, S.; Gamba, P.; Testa, G.; Rossin, D.; Biasi, F.; Poli, G.; Leonarduzzi, G. Relation between TLR4/NF-κB signaling pathway activation by 27-hydroxycholesterol and 4-hydroxynonenal, and atherosclerotic plaque instability. Aging Cell 2015, 14, 569–581.
- Zhou, X.; Han, D.; Xu, R.; Li, S.; Wu, H.; Qu, C.; Wang, F.; Wang, X.; Zhao, Y. A model of metabolic syndrome and related diseases with intestinal endotoxemia in rats fed a high fat and high sucrose diet. PLoS ONE 2014, 9, e115148.
- Haneklaus, M.; O’Neill, L.A. NLRP3 at the interface of metabolism and inflammation. Immunol. Rev. 2015, 265, 53–62.
- Guo, C.; Chi, Z.; Jiang, D.; Xu, T.; Yu, W.; Wang, Z.; Chen, S.; Zhang, L.; Liu, Q.; Guo, X.; et al. Cholesterol Homeostatic Regulator SCAP-SREBP2 Integrates NLRP3 Inflammasome Activation and Cholesterol Biosynthetic Signaling in Macrophages. Immunity 2018, 49, 842–856.e847.
- Sun, R.; Peng, M.; Xu, P.; Huang, F.; Xie, Y.; Li, J.; Hong, Y.; Guo, H.; Liu, Q.; Zhu, W. Low-density lipoprotein receptor (LDLR) regulates NLRP3-mediated neuronal pyroptosis following cerebral ischemia/reperfusion injury. J. Neuroinflammation 2020, 17, 330.
- Chiu, S.; Williams, P.T.; Krauss, R.M. Effects of a very high saturated fat diet on LDL particles in adults with atherogenic dyslipidemia: A randomized controlled trial. PLoS ONE 2017, 12, e0170664.
- Hamamah, S.; Hajnal, A.; Covasa, M. Impact of Nutrition, Microbiota Transplant and Weight Loss Surgery on Dopaminergic Alterations in Parkinson’s Disease and Obesity. Int. J. Mol. Sci. 2022, 23, 7503.
- Rinott, E.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J.; et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: A randomized controlled trial. Genome Med. 2022, 14, 29.
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326.
- Lopez-Moreno, J.; Garcia-Carpintero, S.; Gomez-Delgado, F.; Jimenez-Lucena, R.; Vals-Delgado, C.; Alcala-Diaz, J.F.; Roncero-Ramos, I.; Rangel-Zuñiga, O.A.; Yubero-Serrano, E.M.; Malagon, M.M.; et al. Endotoxemia is modulated by quantity and quality of dietary fat in older adults. Exp. Gerontol. 2018, 109, 119–125.
- Kenđel Jovanović, G.; Mrakovcic-Sutic, I.; Pavičić Žeželj, S.; Šuša, B.; Rahelić, D.; Klobučar Majanović, S. The Efficacy of an Energy-Restricted Anti-Inflammatory Diet for the Management of Obesity in Younger Adults. Nutrients 2020, 12, 3583.
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients 2021, 13, 672.
- Juste, C.; Gérard, P. Cholesterol-to-Coprostanol Conversion by the Gut Microbiota: What We Know, Suspect, and Ignore. Microorganisms 2021, 9, 1881.
- Xue, Y.; Chen, H.; Zhang, S.; Bao, L.; Chen, B.; Gong, H.; Zhao, Y.; Qi, R. Resveratrol Confers Vascular Protection by Suppressing TLR4/Syk/NLRP3 Signaling in Oxidized Low-Density Lipoprotein-Activated Platelets. Oxid. Med. Cell. Longev. 2021, 2021, 8819231.
- Wang, P.; Ma, Y.; Wang, D.; Zhao, W.; Hu, X.; Chen, F.; Zhao, X. Protective Effects of Dietary Resveratrol against Chronic Low-Grade Inflammation Mediated through the Gut Microbiota in High-Fat Diet Mice. Nutrients 2022, 14, 1994.
- Ranaivo, H.; Thirion, F.; Béra-Maillet, C.; Guilly, S.; Simon, C.; Sothier, M.; Van Den Berghe, L.; Feugier-Favier, N.; Lambert-Porcheron, S.; Dussous, I.; et al. Increasing the diversity of dietary fibers in a daily-consumed bread modifies gut microbiota and metabolic profile in subjects at cardiometabolic risk. Gut Microbes 2022, 14, 2044722.
- Ma, Y.; Sun, Y.; Sun, L.; Liu, X.; Zeng, R.; Lin, X.; Li, Y. Effects of gut microbiota and fatty acid metabolism on dyslipidemia following weight-loss diets in women: Results from a randomized controlled trial. Clin. Nutr. 2021, 40, 5511–5520.
- Shibabaw, T. Omega-3 polyunsaturated fatty acids: Anti-inflammatory and anti-hypertriglyceridemia mechanisms in cardiovascular disease. Mol. Cell. Biochem. 2021, 476, 993–1003.
- Lim, R.R.X.; Park, M.A.; Wong, L.H.; Haldar, S.; Lim, K.J.; Nagarajan, N.; Henry, C.J.; Jiang, Y.R.; Moskvin, O.V. Gut microbiome responses to dietary intervention with hypocholesterolemic vegetable oils. NPJ Biofilms Microbiomes 2022, 8, 24.
- Raza, G.S.; Maukonen, J.; Makinen, M.; Niemi, P.; Niiranen, L.; Hibberd, A.A.; Poutanen, K.; Buchert, J.; Herzig, K.H. Hypocholesterolemic Effect of the Lignin-Rich Insoluble Residue of Brewer’s Spent Grain in Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2019, 67, 1104–1114.
- Xu, D.; Pan, D.; Liu, H.; Yang, C.; Yang, X.; Wang, X.; Liu, F.; Feng, M.; Wu, Q.; Shen, Y.; et al. Improvement in cardiometabolic risk markers following an oatmeal diet is associated with gut microbiota in mildly hypercholesterolemic individuals. Food Res. Int. 2022, 160, 111701.
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113.
- Lu, S.; Xu, S.; Chen, L.; Deng, Y.; Feng, J. Periplaneta americana Extract Pretreatment Alleviates Oxidative Stress and Inflammation and Increases the Abundance of Gut Akkermansia muciniphila in Diquat-Induced Mice. Antioxidants 2022, 11, 1806.
- Sun, N.X.; Tong, L.T.; Liang, T.T.; Wang, L.L.; Liu, L.Y.; Zhou, X.R.; Zhou, S.M. Effect of Oat and Tartary Buckwheat—Based Food on Cholesterol—Lowering and Gut Microbiota in Hypercholesterolemic Hamsters. J. Oleo Sci. 2019, 68, 251–259.
- Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J.; Shen, M. Flavonoids from Whole-Grain Oat Alleviated High-Fat Diet-Induced Hyperlipidemia via Regulating Bile Acid Metabolism and Gut Microbiota in Mice. J. Agric. Food Chem. 2021, 69, 7629–7640.
- Ito, M.; Nagasawa, M.; Omae, N.; Tsunoda, M.; Ishiyama, J.; Ide, T.; Akasaka, Y.; Murakami, K. A novel JNK2/SREBP-1c pathway involved in insulin-induced fatty acid synthesis in human adipocytes. J. Lipid Res. 2013, 54, 1531–1540.
- Hu, X.; Li, H.; Zhao, X.; Zhou, R.; Liu, H.; Sun, Y.; Fan, Y.; Shi, Y.; Qiao, S.; Liu, S.; et al. Multi-omics study reveals that statin therapy is associated with restoration of gut microbiota homeostasis and improvement in outcomes in patients with acute coronary syndrome. Theranostics 2021, 11, 5778–5793.
- Vieira-Silva, S.; Falony, G.; Belda, E.; Nielsen, T.; Aron-Wisnewsky, J.; Chakaroun, R.; Forslund, S.K.; Assmann, K.; Valles-Colomer, M.; Nguyen, T.T.D.; et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature 2020, 581, 310–315.
- Sun, B.; Li, L.; Zhou, X. Comparative analysis of the gut microbiota in distinct statin response patients in East China. J. Microbiol. 2018, 56, 886–892.
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128.
- Park, S.; Zhang, T.; Yue, Y.; Wu, X. Effects of Bile Acid Modulation by Dietary Fat, Cholecystectomy, and Bile Acid Sequestrant on Energy, Glucose, and Lipid Metabolism and Gut Microbiota in Mice. Int. J. Mol. Sci. 2022, 23, 5935.
- Li, B.; Zhang, J.; Chen, Y.; Wang, Q.; Yan, L.; Wang, R.; Wei, Y.; You, Z.; Li, Y.; Miao, Q.; et al. Alterations in microbiota and their metabolites are associated with beneficial effects of bile acid sequestrant on icteric primary biliary Cholangitis. Gut Microbes 2021, 13, 1946366.
- Jena, P.K.; Setayesh, T.; Sheng, L.; Di Lucente, J.; Jin, L.W.; Wan, Y.Y. Intestinal Microbiota Remodeling Protects Mice from Western Diet-Induced Brain Inflammation and Cognitive Decline. Cells 2022, 11, 504.
- Lin, S.H.; Cheng, P.C.; Tu, S.T.; Hsu, S.R.; Cheng, Y.C.; Liu, Y.H. Effect of metformin monotherapy on serum lipid profile in statin-naïve individuals with newly diagnosed type 2 diabetes mellitus: A cohort study. PeerJ 2018, 6, e4578.
- Tong, X.; Xu, J.; Lian, F.; Yu, X.; Zhao, Y.; Xu, L.; Zhang, M.; Zhao, X.; Shen, J.; Wu , S.; et al. Structural Alteration of Gut Microbiota during the Amelioration of Human Type 2 Diabetes with Hyperlipidemia by Metformin and a Traditional Chinese Herbal Formula: A Multicenter, Randomized, Open Label Clinical Trial. mBio 2018, 9, e02392-e17.
- Ermolenko, E.; Simanenkova, A.; Voropaeva, L.; Lavrenova, N.; Kotyleva, M.; Minasian, S.; Chernikova, A.; Timkina, N.; Gladyshev, N.; Dmitriev, A.; et al. Metformin Influence on the Intestinal Microbiota and Organism of Rats with Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 6837.
