Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Biodegradable membranes with innovative antifouling properties are emerging as possible substitutes for conventional membranes. These types of membranes have the potential to be applied in a wide range of applications, from water treatment to food packaging and energy production.
- biopolymer
- biodegradable membrane
- green membrane
1. Introduction
Membrane technology has been extensively used in various applications, including but not limited to waste/wastewater treatment, gas separation, hemodialysis, energy production, drug delivery, and the food industry. Polymeric membranes are mainly employed in all those separation processes [1][2]. The production and disposal processes of polymeric membranes result in unsustainable accumulations of waste and several environmental concerns [3][4][5][6][7]. Even though these types of membranes are more prevalent and dominate the membrane market, they are still subject to waste disposal challenges, chemical, mechanical, and thermal stability concerns, as well as the membrane fouling issue [7]. As an environmentally friendly and sustainable approach to solving these problems, biodegradable membranes have been introduced, especially to reduce the amount of waste disposal. Under favorable conditions in the environment in terms of pH, humidity, and temperature, biodegradable membranes are broken down into non-toxic compounds by enzymes and microorganisms in nature [1]. Biodegradable membranes proved to be potential substitutes for conventional membranes in various applications such as oil/water separation, dye removal, tissue engineering, food packaging, fuel cells, etc. [1][8][9][10][11].
2. Green Membrane Materials
Synthetic polymer materials have been developed less than 100 years ago but because of their low ability to be utilized in nature are considered to have serious ecological impact on the environment. The interest in biodegradable plastics is constantly increasing, and their production is forecasted to reach more than 1.3 million tons in 2024 [12]. The main position on the biopolymers market belongs to the Asia-Pacific region, North America, and Europe [13]. All biodegradable polymeric materials considered in this chapter could be divided into two main classes: bio-sourced and synthetic. Synthetic materials, in turn, fall into two categories: petrochemical and made out of renewable sources, which is illustrated on Figure 1.
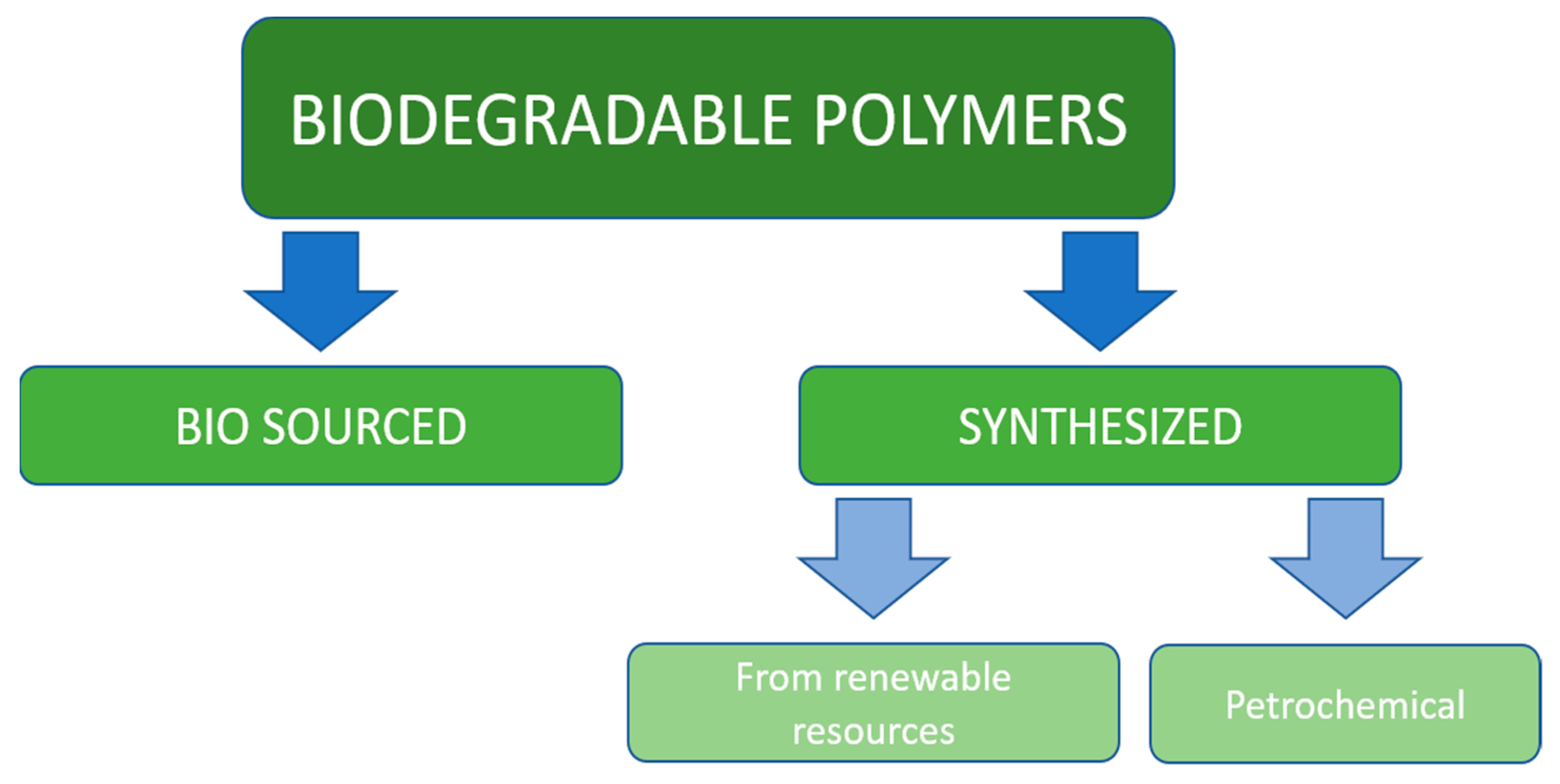
Figure 1. Classification of biodegradable polymers.
2.1. Bio-Sourced Polymers for Membrane Fabrication
Bio-sourced materials have been used by humanity since ancient times as materials for construction (wood), tools (wood, bones), cloth (plants, leather, wool, fur), jewelry (bones, wood, amber), and other. Nowadays bio-sourced crude is also used to create new materials, including membranes. The majority of bio-sourced polymers used for membrane fabrication are carbohydrates/polysaccharides and their derivatives. The origin of bio-sourced materials possesses a high degree of biodegradability that makes them attractive as green materials to be used for reducing pollution of the environment with plastic wastes.
2.1.1. Cellulose and Its Derivatives
Cellulose is one of the first materials used for membrane production. Thus, the first membrane for hemodialysis was produced from cellophane, which is the product of cellulose treatment. Cellulose is one of the most prevalent polysaccharides that occurs in natural sources like trees and plants. Saccharide monomer units in cellulose (see Figure 2A) macromolecules are arranged predominately in a linear manner, which results in its fiber structure and high mechanical properties.
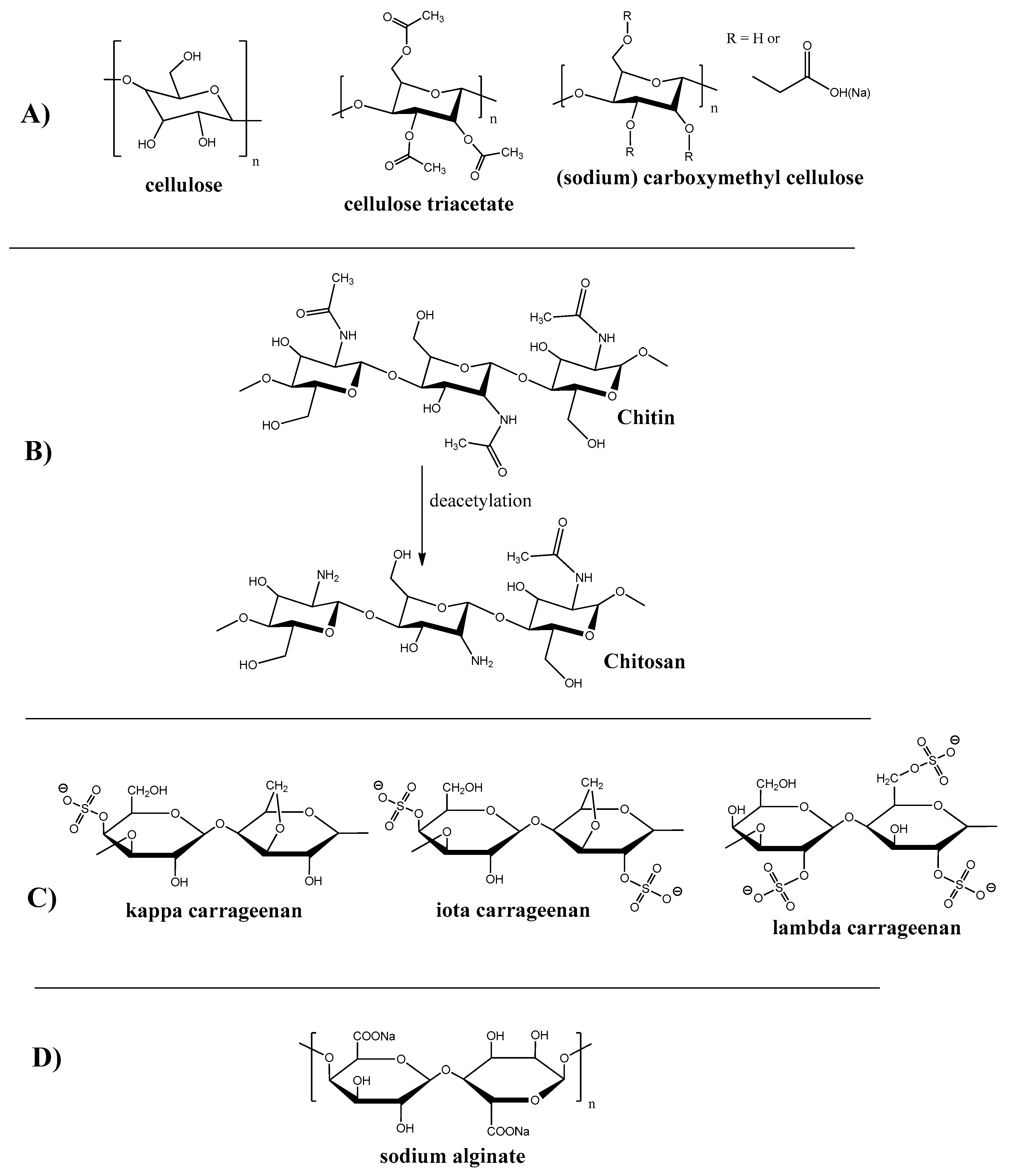
Figure 2. Chemical structure of polysaccharides polymers and their derivatives. (A) Cellulose and Its Derivatives; (B) Chitin and Chitosan; (C) Carrageenan; (D) Alginate.
Due to the presence of active hydroxyl groups in the saccharide unit, cellulose is widely used for its modification to obtain new artificial materials with improved properties. Some of the structures that resulted from cellulose modification are presented on Figure 2A.
Thus, the most common cellulose derivative is cellulose triacetate, which is, along with other cellulose derivatives, used as the fabrication material for membranes applied in many industries such as oil-water separation [14][15][16], antifouling improvement [17], virus removal [18], heavy metal adsorption [19][20], desalination [21][22][23], hemodialysis [24], and gas separation [25][26][27].
2.1.2. Chitin and Chitosan
Chitosan is the second (after cellulose) most abundant biopolymer in nature that can be found in insects, shells, mollusks, shrimps, crabs, fungi etc. It also can be derived from chitin (the exoskeleton of many living creatures, insects in particular) by its deacetylation (see Figure 2B).
Though this biopolymer is widely spread in nature, the research of chitosan began only in the 1980s because of the structure’s features and insolubility in water [28].
Examples of chitin and chitosan membranes applications are ion exchange membranes [29][30][31], wastewater treatment [32][33], oil-water separation [34], dye removal [35], etc.
2.1.3. Carrageenan
Carrageenan is a naturally occurred polysaccharide containing sulphate groups. This polymer can be extracted from red algae, particularly from Rhodophyceae [36]. Depending on the degree of sulfanation, there are three classes of carrageenan: kappa, iota, and lambda. The structure of carrageenan is shown on Figure 2C. One carrageenan application example is the super-oleophobic membrane for the removal of dyes and heavy metals [37].
2.1.4. Other Polysaccharides and Biopolymers
Starch is another example of a polysaccharide. It is one of the most abundant biopolymers in nature and can be found in such common products as potato, corn, and rice. Due to its branched structure, resulting in poor mechanical properties, starch can be hardly used as the main material for membrane production [38]. Thus, starch was reported to be used as an additive to produce membranes for wastewater treatment [39][40][41].
Cyclodextrin (CD) can also be used as an additive for the improvement of hydrophilicity and permeability of membranes used for water treatment [42]. The functionalization of hydroxyl groups to amino, sulphonyl, and other groups resulted in the further improvement of membrane selectivity by enhancing porosity and hydrophilicity [43].
Alginate is a naturally occurring anionic water-soluble linear polysaccharide composed of α-L-guluronic acid and β-D-mannuronic acid (see Figure 2D), typically obtained from brown seaweed (Phaeophyceae), including Laminaria hyperborea, Laminaria digitata, Laminaria japonica, Ascophyllum nodosum, and Macrocystis pyrifera [44]. Alginate-containing membranes were reported to be used for dyes and heavy metal removal [45][46][47][48][49].
Silk fibroin is another natural biopolymer, extracted from insects’ cocoons when treating them with hot alkali solution [50]. This protein is added to membranes to improve their adsorption of heavy metal ions [51][52].
Similar to fibroin, collagen can be extracted from animal connective tissues [53]. Despite being sensitive to pH, temperature, and bacteria, collagen can be used for membrane fabrication, though its application is limited. Due to its protein nature, collagen is perfectly suitable to create fiber-type membranes for oil-water separation [54] and pervaporation [55][56].
Polyhydroxybutyrate (PHB) is bio-derived polyester (see Figure 3) that is produced by some microorganisms such as Cupriavidus necator, Methylobacterium rhodesianum, or Bacillus megaterium during assimilation of glucose or starch [57].
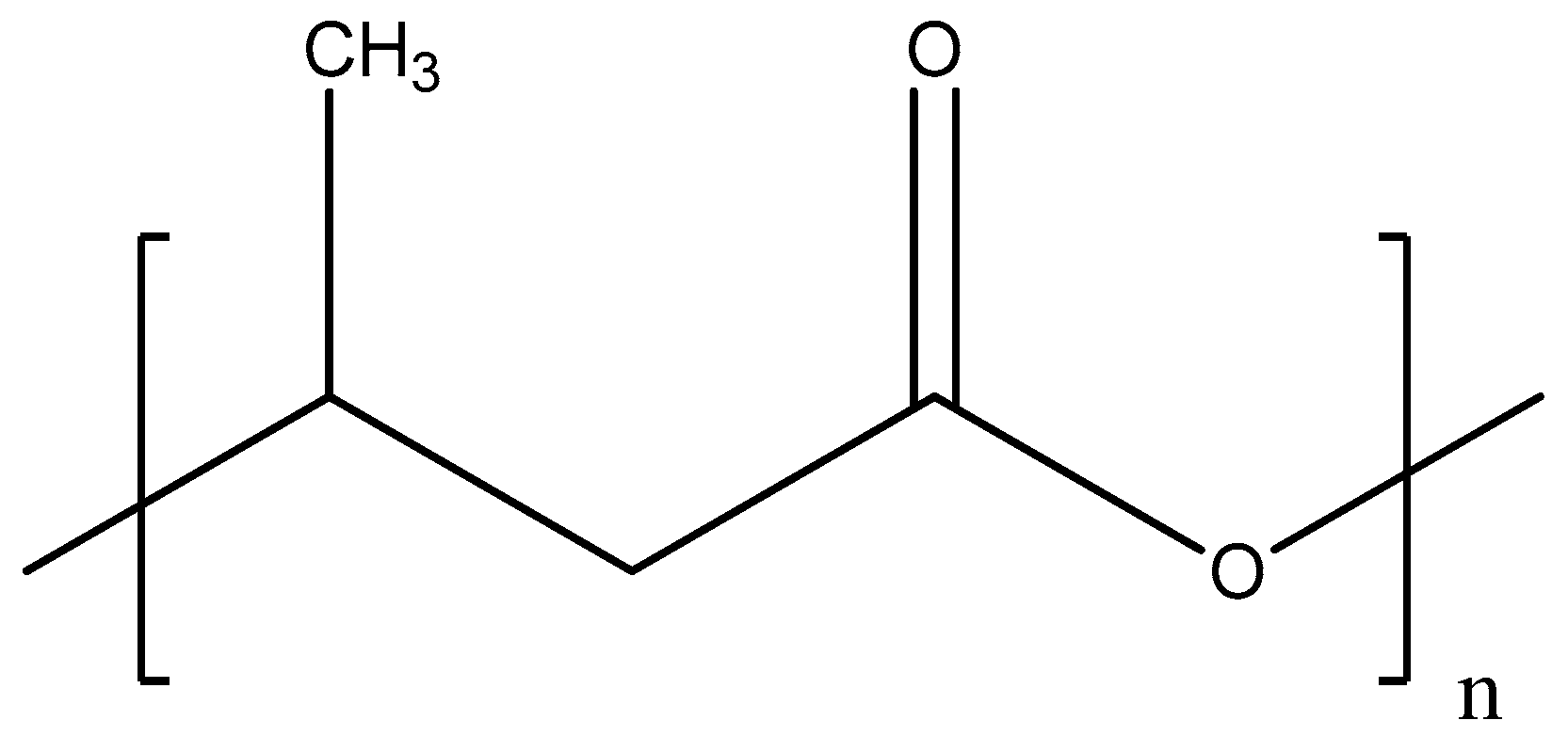
Figure 3. Chemical structure of polyhydroxybutyrate.
This polymer has a tensile strength close to polypropylene, making it suitable to manufacture fibers. As a result, PHB has been used to prepare electrospun fibers-based membranes with improved antibacterial properties for medical applications, dye removal, and microfiltration [58][59][60][61].
2.2. Synthetic Biodegradable Polymers
2.2.1. Polymers Synthesized from Renewable Sources
Polylactic Acid
Polylactic acid (PLA) is a thermoplastic polyester that is produced by polycondensation of lactic acid or ring-opening polymerization of cyclic diester-lactide (see Figure 4). Lactic acid, in turn, is produced from a renewable bio-source, such as sugarcane or starch, [62] by aerobic fermentation [63]. PLA is one of the most researched and mass-produced biodegradable polymers.
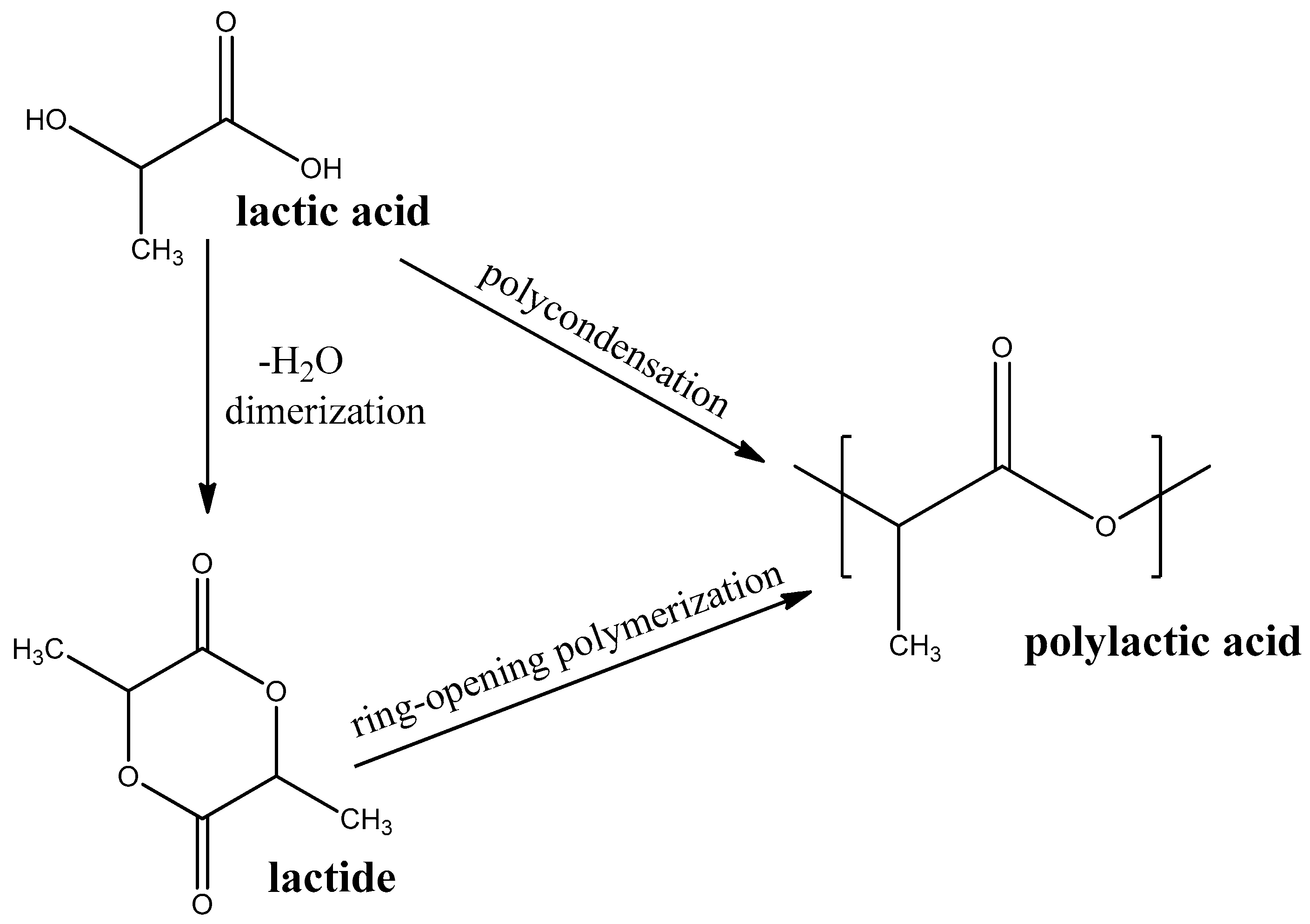
Figure 4. Synthesis routes PLA.
PLA belongs to semicrystalline polymers. PLA-application temperatures are limited by its low glass-transition temperature (Tg = 55–60 °C) and melting temperature (Tm = 170–180 °C) [64]. PLA is suitable to prepare membranes based on electro-spun fibers for oil-water separation [65] and tissue regeneration [66]. PLA biodegradation proceeds quite fast to CO2 and water [67], which meets all the requirements of all standards for biodegradable plastics [68]. PLA is also suitable for decomposition as a part of compost [69].
Polybutylene Succinate
Polybutylene succinate (PBS) is another (like PLA) example of a biodegradable polyester that is produced by polycondensation of succinic acid and 1,4-butanediol. Succinic acid can be produced through biomass fermentation [70] by further hydrogenation to 1,4-butanediol [71]. The further polycondensation process of succinic acid and 1,4-butanediol proceeds in two steps and requires catalysts to produce a polymeric PBS from its oligomers, obtained in the first step (see Figure 5). The first mass production of this polymer began in 1990s in Japan by the Showa Highpolymer company [72]. PBS are reported to be used for the fabrication of membranes for pervaporation and other applications [73][74][75]. PBS biodegradation in soil was reported to proceed with 65% carbon mineralization (conversion to CO2) for 425 days [76].
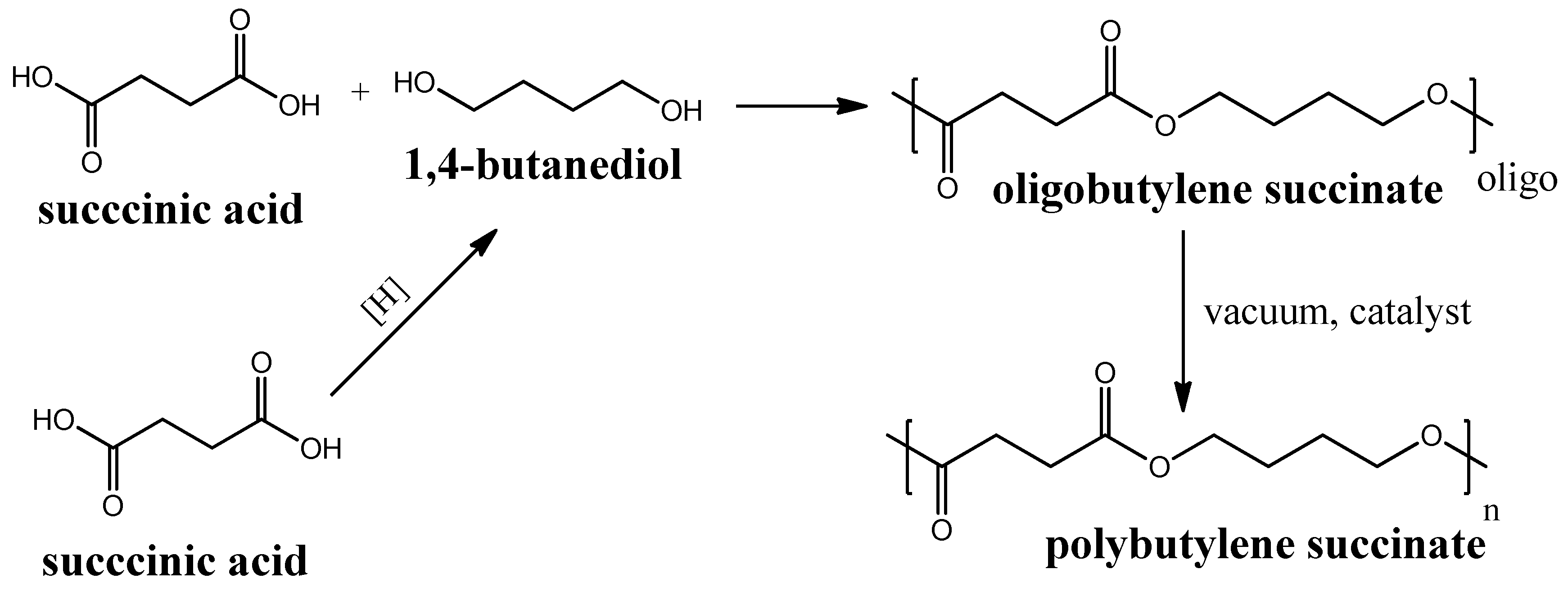
Figure 5. Circuit diagram of polybutylene succinate synthesis.
2.2.2. Petrochemical Polymers
Poly-ε-caprolactone
Poly-ε-caprolactone is an aliphatic polyester, produced by ring-opening polymerization of ε-caprolactone (see Figure 6A) [77]. This polymer has a low Tg = −55–60 °C and low melting point of Tm = 60–65 °C and good resistance to oil and water. Examples of using poly-ε-caprolactone for membranes include dye removal [78], wound dressing [79], antifouling [80], treatment blood infections [81], and guided tissue regeneration [82] applications. Furthermore, poly-ε-caprolactone is highly degradable polymer. Thus, Pseudozyma japonica-Y7-09 enzyme, which is present in different microorganisms, results in Poly-ε-caprolactone degradation by 93.3% for 15 days for polymer film and 43.2% for 30 days when the polymer was made as foam plastic [83].
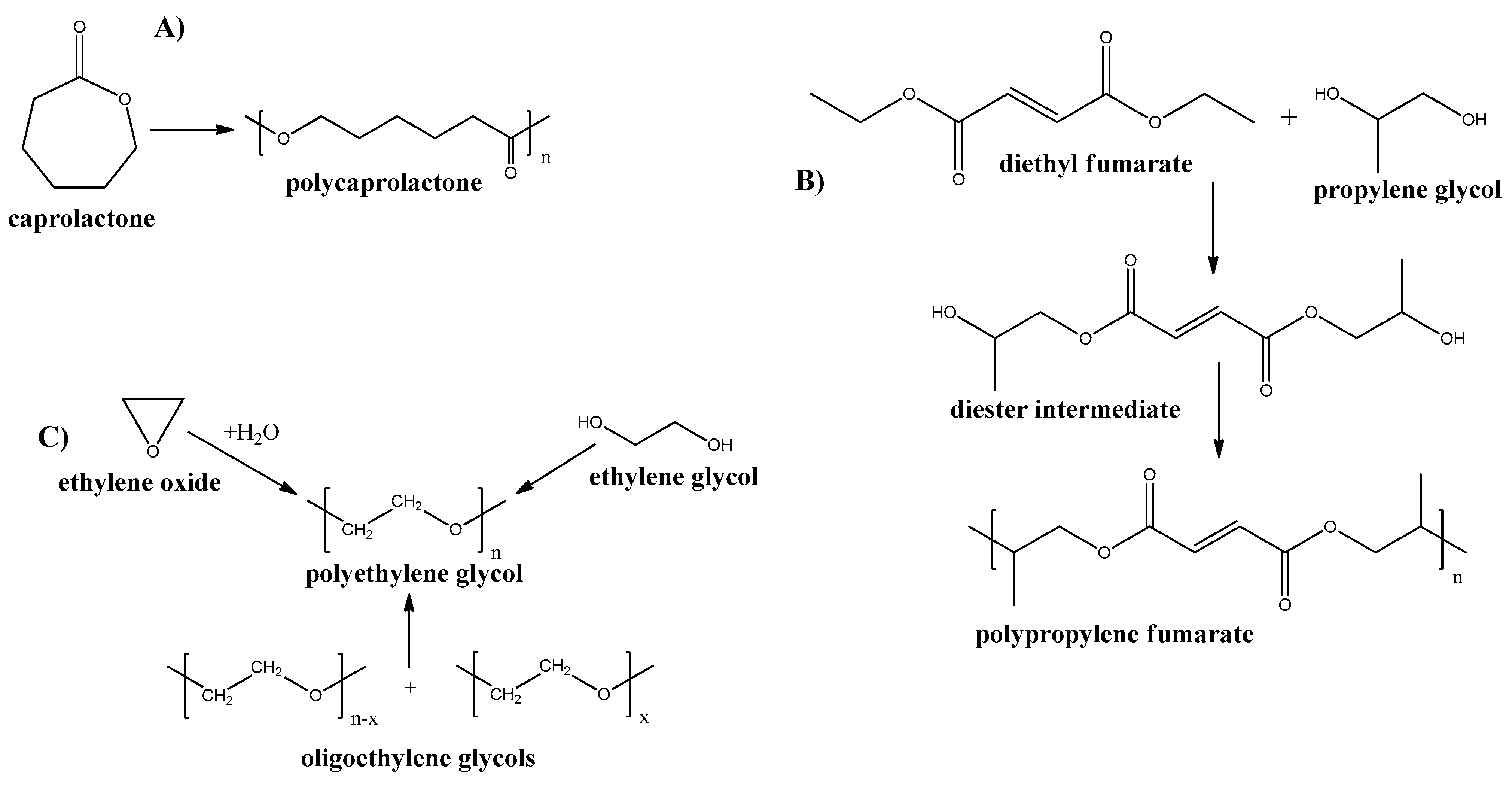
Figure 6. Synthesis routes of some petrochemical derived biodegradable plastics. (A) Poly-ε-caprolactone; (B) Poly-propylene Fumarate; and (C) Poly-ethylene Glycol.
Poly-propylene Fumarate
Polypropylene fumarate (PPF) is an aliphatic polyester, containing a double bond in the main chain. This polymer can be produced by multistep synthesis (see Figure 6B) [84]. Because of the presence of double bonds, this polymer can be cross-linked by various methods which, results in the broad mechanical properties of the resultant structures [85]. Although, the mechanical properties are still low and limit the use of PPF for the membrane and other applications. However, some new approaches have been applied by the introduction of nanofillers into PPF to improve its properties [86][87][88]. The in vitro biodegradation of PPF scaffolds revealed nearly 17% mass loss at 6 weeks [89].
Poly-ethylene Glycol
Polyethylene glycol (PEG) is a synthetic polyether that can be produced by various ways: from ethylene oxide, ethylene glycol, or ethylene glycol oligomers (see Figure 6C).
PEG production has been reported in 1859 by both A. V. Lourenço and Charles Adolphe Wurtz, independently. PEG membranes are predominately used for drug release systems [90][91][92][93][94]. In addition, PEG is well known to possess sensitivity to oxidative degradation [95]; thus, this material is substantial to oxidative biological degradation by different microorganisms [96][97][98].
Poly-vinyl Alcohol
Polyvinyl alcohol (PVA), unlike most of other vinyl polymers, cannot be produced by polymerization of its monomer units because vinyl alcohol is thermodynamically unstable. PVA is predominately produced by hydrolysis of polyvinyl acetate (see Figure 7). Hydrolysis reaction is usually proceeded in the presence of ethanol but can be easily done without it.
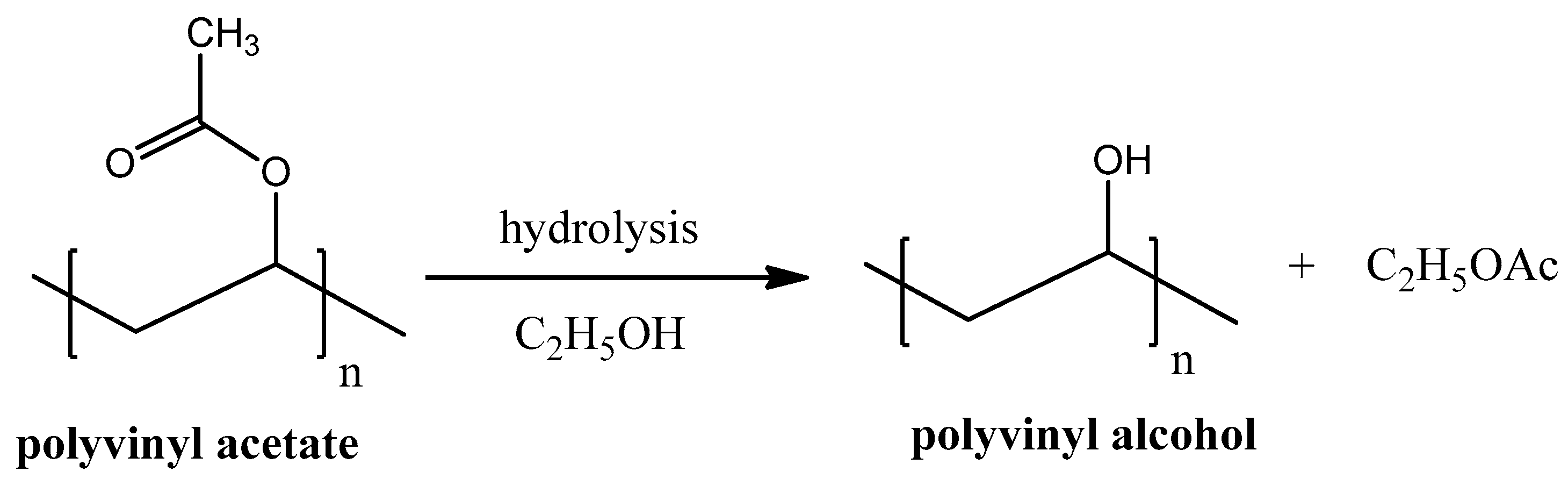
Figure 7. Polyvinyl alcohol synthesis from polyvinyl acetate hydrolysis.
PVA has been reported to use for membrane preparation for wound dressing applications [99][100]. In addition, PVA is biodegradable in the presence of different microorganisms under two-step metabolic processes, including oxidation and hydrolysis [101].
Polyurethane and Polyurethane Urea
Polyurethanes (PU) are one of the most produced polymers in the world. Around 25 million tons of this class of polymer was produced in 2019, which is about of 6% of all manufactured polymers [102]. The interest to polyurethanes is determined by the broad chemical variety of monomer units, resulting in a wide range of physical properties–from rigid and strong plastics to flexible elastomers [103]. Linear PU are basically produced during polycondensation reaction between diisocyanates and diols (see Figure 8A, step 2A) with the ability to produce polyurethane urea when a diamine chain extender is used on the second synthesis step (see Figure 8A, step 2B).
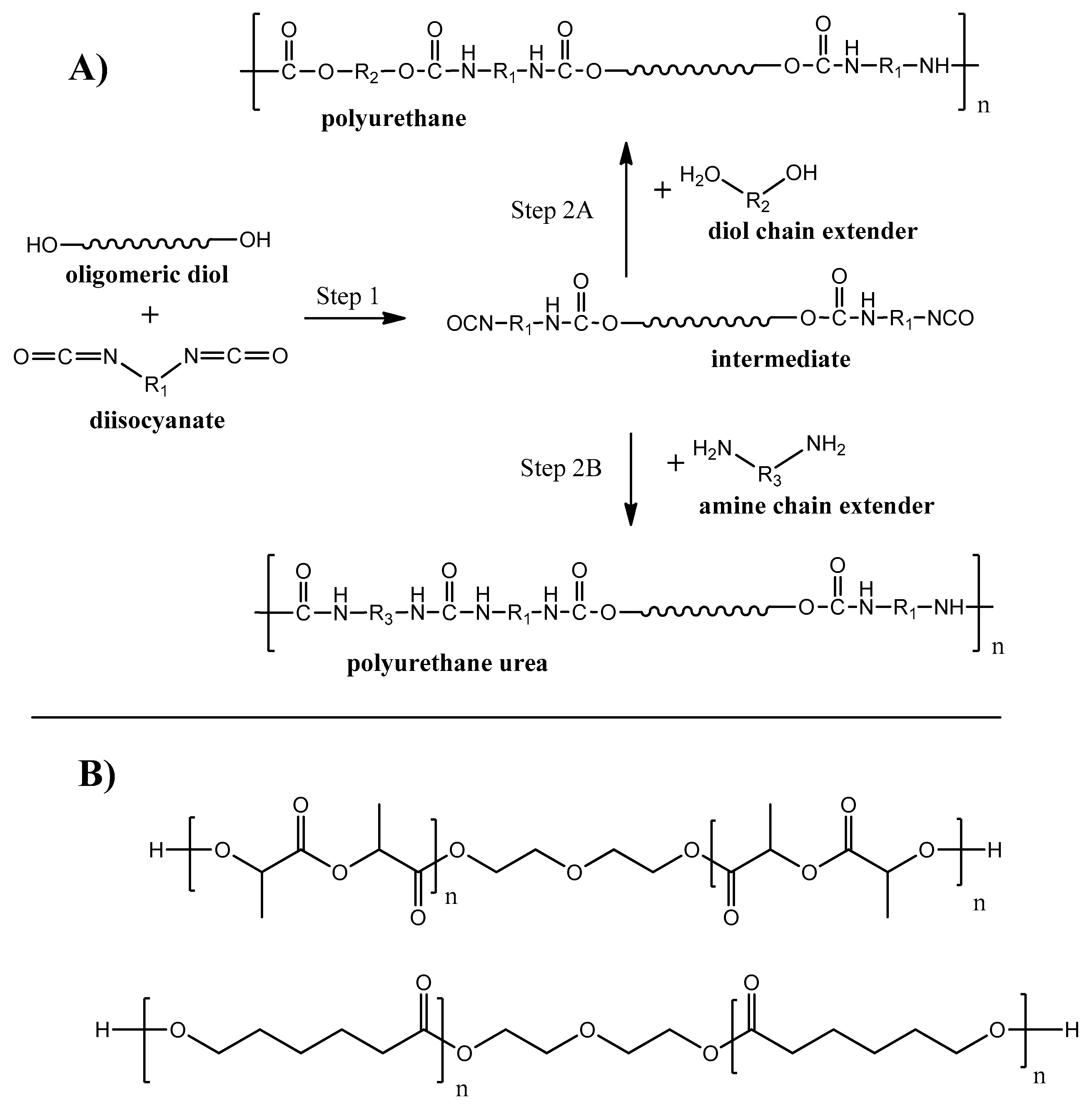
Figure 8. Synthesis routes of biodegradable PU and PU urea.
Polyurethanes were known for a long time as non-degradable polymers. For the last decades, biodegradable PU were developed by using monomer units with hydrolysable bonds (e.g., ester, amide) groups [104][105]. Examples of oligomeric diols [106][107] used for biodegradable PU synthesis are given on Figure 8B. A broad opportunity to adjust and control PU chemistry and structure makes PU degradable by enzymes, fungi, and bacteria [108].
PU applications include sprayable membranes [109], tissue repair [110], and many other biomedical applications [110].
The biodegradable membranes synthesis routes and main applications are summarized in Table 1.
Table 1. Green chemistry biodegradable membranes chemistry and applications.
| No. | Membrane Material | Synthesis Method | Main Applications |
|---|---|---|---|
| Bio-sourced | |||
| 1 | Cellulose derivatives (acetates, carboxymethyl etc.) | Processing of cellulose containing biomaterials (plants, trees) | Water treatment and separation, gas separation, biomedical applications |
| 2 | Chitin and chitosan | Processing of chitin containing biomaterials (insects, shrimps, crabs etc.). Deacetylation results in chitosan | Ion exchange, water treatment and separation, dye removal |
| 3 | Carrageenan | Processing of biomaterials (algae) | Dyes and heavy metals removal |
| 4 | Starch | Processing of starch containing biomaterials (potato, corn, etc.) | Used as additive and source of other materials |
| 5 | Cyclodextrin | Enzymatic conversion of starch | Water treatment |
| 6 | Alginate | Processing of biomaterials (algae) | Dyes and heavy metals removal |
| 7 | Silk fibroin | Processing of biomaterials (insects cocoons) | Heavy metals removal |
| 8 | Collagen | Processing of biomaterials (animal connecting tissues) | Water separation, pervaporation |
| 9 | Polyhydroxybutyrate | Glucose and starch conversion by microorganisms | Biomedical application, dye removal, microfiltration |
| Synthesized from bio-sourced chemicals | |||
| 1 | Polylactic acid | Polycondensation or ring-opening polymerization of lactic acid, produced by aerobic fermentation of glucose or starch. | Water separation, tissue regeneration |
| 2 | Polybutylene succinate | Polycondensation of succinic acid and 1,4-butanediol. Succinic acid is produced by biomass fermentation. 1,4-butanediol is produced by succinic acid hydrogenation | Pervaporation |
| Petrochemical | |||
| 1 | Poly-ε-caprolactone | Ring-opening polymerization of ε-caprolactone | Biomedical applications |
| 2 | Polypropylene fumarate | Polycondensation of fumarate with propylene glycol | Biomedical applications |
| 3 | Poly-ethylene glycol | Polycondensation of ethylene glycol or ring-opening polymerization of ethylene oxide | Drug release systems |
| 4 | Poly-vinyl alcohol | Hydrolysis of polyvinyl acetate | Wound dressing |
| 5 | Polyurethane and polyurethane urea | Polycondensation of diisocyanates with diols and diamines | Biomedical application, agriculture |
This entry is adapted from the peer-reviewed paper 10.3390/app122412837
References
- Ghorbani, M.; Vakili, M.H.; Ameri, E. Fabrication and evaluation of a biopolymer-based nanocomposite membrane for oily wastewater treatment. Mater. Today Commun. 2021, 28, 102560.
- Bandehali, S.; Sanaeepur, H.; Amooghin, A.E.; Shirazian, S.; Ramakrishna, S. Biodegradable polymers for membrane separation. Sep. Purif. Technol. 2021, 269, 118731.
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344.
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798.
- Al Sharabati, M.; Abokwiek, R.; Al-Othman, A.; Tawalbeh, M.; Karaman, C.; Orooji, Y.; Karimi, F. Biodegradable polymers and their nano-composites for the removal of endocrine-disrupting chemicals (EDCs) from wastewater: A review. Environ. Res. 2021, 202, 111694.
- Agarwal, S. Biodegradable Polymers: Present Opportunities and Challenges in Providing a Microplastic-Free Environment. Macromol. Chem. Phys. 2020, 221, 2000017.
- Ouda, M.; Ibrahim, Y.; Kallem, P.; Govindan, B.; Banat, F.; Hasan, S.W. Highly permeable, environmentally-friendly, antifouling polylactic ultrafiltration membranes. J. Clean. Prod. 2022, 330, 129871.
- Habiba, U.; Siddique, T.A.; Talebian, S.; Lee, J.J.L.; Salleh, A.; Ang, B.C.; Afifi, A.M. Effect of deacetylation on property of electrospun chitosan/PVA nanofibrous membrane and removal of methyl orange, Fe(III) and Cr(VI) ions. Carbohydr. Polym. 2017, 177, 32–39.
- Suh, J.-K.F.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598.
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643.
- Choudhury, R.R.; Gohil, J.M.; Dutta, K. Poly(vinyl alcohol)-based membranes for fuel cell and water treatment applications: A review on recent advancements. Polym. Adv. Technol. 2021, 32, 4175–4203.
- European Bioplastics, Bioplastics Market Data. Available online: https://www.european-bioplastics.org/market-update-2020-bioplastics-continue-to-become-mainstream-as-the-global-bioplastics-market-is-set-to-grow-by-36-percent-over-the-next-5-years/ (accessed on 23 July 2021).
- Bioplastics & Biopolymers Market by Type (Non-Biodegradable/Bio-Based, Biodegradable), End-Use Industry (Packaging, Consumer Goods, Automotive & Transportation, Textiles, Agriculture & Horticulture), Region—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/market-reports/biopolymersbioplastics-market-88795240.html (accessed on 23 July 2021).
- Rohrbach, K.; Li, Y.; Zhu, H.; Liu, Z.; Dai, J.; Andreasen, J.; Hu, L. A cellulose based hydrophilic, oleophobic hydrated filter for water/oil separation. Chem. Commun. 2014, 50, 13296–13299.
- Li, X.; Shan, H.; Zhang, W.; Li, B. 3D printed robust superhydrophilic and underwater superoleophobic composite membrane for high efficient oil/water separation. Sep. Purif. Technol. 2020, 237, 116324.
- Xu, X.; Long, Y.; Li, Q.; Li, D.; Mao, D.; Chen, X.; Chen, Y. Modified cellulose membrane with good durability for effective oil-in-water emulsion treatment. J. Clean. Prod. 2019, 211, 1463–1470.
- Yao, A.; Yan, Y.; Tan, L.; Shi, Y.; Zhou, M.; Zhang, Y.; Zhu, P.; Huang, S. Improvement of filtration and antifouling performance of cellulose acetate membrane reinforced by dopamine modified cellulose nanocrystals. J. Membr. Sci. 2021, 637, 119621.
- Szekeres, G.P.; Németh, Z.; Schrantz, K.; Németh, K.; Schabikowski, M.; Traber, J.; Pronk, W.; Hernádi, K.; Graule, T. Copper-Coated Cellulose-Based Water Filters for Virus Retention. ACS Omega 2018, 3, 446–454.
- Pei, X.; Gan, L.; Tong, Z.; Gao, H.; Meng, S.; Zhang, W.; Wang, P.; Chen, Y. Robust cellulose-based composite adsorption membrane for heavy metal removal. J. Hazard. Mater. 2021, 406, 124746.
- Saber-Samandari, S.; Saber-Samandari, S.; Heydaripour, S.; Abdouss, M. Novel carboxymethyl cellulose based nanocomposite membrane: Synthesis, characterization and application in water treatment. J. Environ. Manag. 2016, 166, 457–465.
- Li, S.; Wang, D.; Xiao, H.; Zhang, H.; Cao, S.; Chen, L.; Ni, Y.; Huang, L. Ultra-low pressure cellulose-based nanofiltration membrane fabricated on layer-by-layer assembly for efficient sodium chloride removal. Carbohydr. Polym. 2021, 255, 117352.
- Zheng, K.; Zhou, S.; Cheng, Z.; Huang, G. Thin-film composite forward osmosis membrane prepared from polyvinyl chloride/cellulose carbamate substrate and its potential application in brackish water desalination. J. Appl. Polym. Sci. 2021, 138, 49939.
- Elkony, Y.; Mansour, E.; Elhusseiny, A.; Ebrahim, S. Effect of cellulose acetate/cellulose triacetate ratio on reverse osmosis blend membrane performance. Polym. Eng. Sci. 2020, 60, 2852–2863.
- Hoseinpour, V.; Ghaee, A.; Vatanpour, V.; Ghaemi, N. Surface modification of PES membrane via aminolysis and immobilization of carboxymethylcellulose and sulphated carboxymethylcellulose for hemodialysis. Carbohydr. Polym. 2018, 188, 37–47.
- Mubashir, M.; Dumee, L.F.; Fong, Y.Y.; Jusoh, N.; Lukose, J.; Chai, W.S.; Show, P.L. Cellulose acetate-based membranes by interfacial engineering and integration of ZIF-62 glass nanoparticles for CO2 separation. J. Hazard. Mater. 2021, 415, 125639.
- Nikolaeva, D.; Azcune, I.; Tanczyk, M.; Warmuzinski, K.; Jaschik, M.; Sandru, M.; Vankelecom, I.F.J. The performance of affordable and stable cellulosebased poly-ionic membranes in CO2/N2 and CO2/CH4 gas separation. J. Membr. Sci. 2018, 564, 552–561.
- Liu, L.; Doherty, C.M.; Ricci, E.; Chen, G.Q.; De Angelis, M.G.; Kentish, S.E. The influence of propane and n-butane on the structure and separation performance of cellulose acetate membranes. J. Membr. Sci. 2021, 638, 119677.
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186.
- Ali, Q.; Taweepreda, W.; Techato, K. Preparation and characterization of polymer electrolyte membrane from chloroacetate chitosan/chitosan blended with epoxidized natural rubber. Polym. Test. 2020, 82, 106294.
- Xiang, Y.; Yang, M.; Guo, Z.; Cui, Z. Alternatively chitosan sulfate blending membrane as methanol-blocking polymer electrolyte membrane for direct methanol fuel cell. J. Membr. Sci. 2009, 337, 318–323.
- Zhang, H.-P.; Gandhi, N.S.; Gu, Y.; Zhang, Y.; Tang, Y. Chitosan/graphene complex membrane for polymer electrolyte membrane fuel cell: A molecular dynamics simulation study. Int. J. Hydrogen Energy 2020, 45, 25960–25969.
- Tang, C.C.H.; Zhang, L. Efficient adsorption of Hg2+ ions on chitin/cellulose composite membranes prepared via environmentally friendly pathway. Chem. Eng. J. 2011, 173, 689–697.
- Shen, S.S.; Yang, J.J.; Liu, C.X.; Bai, R.B. Immobilization of copper ions on chitosan/cellulose acetate blend hollow fiber membrane for protein adsorption. RSC Adv. 2017, 7, 10424–10431.
- Wu, J.X.; Zhang, J.; Kang, Y.L.; Wu, G.; Chen, S.C.; Wang, Y.Z. Reusable and recyclable superhydrophilic electrospun nanofibrous membranes with in situ cocross-linked polymer—Chitin nanowhisker network for obust oil-in-water emulsion separation. ACS Sustain. Chem. Eng. 2017, 6, 1753–1762.
- Zhijiang, C.; Ping, X.; Cong, Z.; Tingting, Z.; Jie, G.; Kongyin, Z. Preparation and characterization of a bi-layered nano-filtration membrane from a chitosan hydrogel and bacterial cellulose nanofiber for dye removal. Cellulose 2018, 25, 5123–5137.
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Solanki, H.K. RETRACTED: Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014, 105, 97–112.
- Prasannan, A.; Udomsin, J.; Tsai, H.-C.; Wang, C.-F.; Lai, J.-Y. Robust underwater superoleophobic membranes with bio-inspired carrageenan/laponite multilayers for the effective removal of emulsions, metal ions, and organic dyes from wastewater. Chem. Eng. J. 2020, 391, 123585.
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70.
- Zarei, V.G.A. Preparation and characterization of biodegradable cellulose acetate-starch membrane. Polym.-Plast. Technol. Eng. 2013, 52, 387–392.
- Woranuch, S.; Pangon, A.; Puagsuntia, K.; Subjalearndee, N.; Intasanta, V. Starch-based and multi-purpose nanofibrous membrane for high efficiency nanofiltration. RSC Adv. 2017, 7, 35368–35375.
- Nabavi, M.M.K.; Ghaffarian, V.; Zabihi, E. Polyacrylonitrile/starch semibiodegradable blend membrane: Preparation, morphology and performance. Desalin. Water Treat. 2016, 57, 495–504.
- Wu, B.T.H.; Wu, P. Preparation and characterization of anti-fouling β-cyclodextrin/polyester thin film nanofiltration composite membrane. J. Membr. Sci. 2013, 428, 301–308.
- Adams, E.N.F.; Krause, R.; Hoek, E.; Mamba, B. Application of polysulfone/cyclodextrin mixed-matrix membranes in the removal of natural organic matter from wate. Phys. Chem. Earth Parts A/B/C 2014, 67, 71–78.
- Smidsrød, G.S.O. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78.
- Jeon, J.L.Y.S.; Kim, J.-H. Dye adsorption characteristics of alginate/polyaspartate hydrogels. J. Ind. Eng. Chem. 2008, 14, 726–731.
- Abdel-Halim, S.S.A.-D.E. Removal of heavy metals from their aqueous solutions through adsorption onto natural polymers. Carbohydr. Polym. 2011, 84, 454–458.
- Lim, J.P.C.S. Synthesis of an innovative calcium-alginate magnetic sorbent for removal of multiple contaminants. Appl. Surf. Sci. 2007, 253, 5772–5775.
- Chen, J.H.; Xing, H.T.; Guo, H.X.; Li, G.P.; Weng, W.; Hu, S.R. Preparation, characterization and adsorption properties of a novel 3-aminopropyltriethoxysilane functionalized sodium alginate porous membrane adsorbent for Cr(III) ions. J. Hazard. Mater. 2013, 248–249, 285–294.
- Wang, Q.; Ju, J.; Tan, Y.; Hao, L.; Ma, Y.; Wu, Y.; Zhang, H.; Xia, Y.; Sui, K. Controlled synthesis of sodium alginate electrospun nanofiber membranes for multi-occasion adsorption and separation of methylene blue. Carbohydr. Polym. 2019, 205, 125–134.
- Costa, R.S.F.; Boccaccini, A. Fibrous Protein-Based Biomaterials (Silk, Keratin, Elastin, and Resilin Proteins) for Tissue Regeneration and Repair Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Elsevier: Amsterdam, The Netherlands, 2018; pp. 175–204.
- Khosa, S.S.S.M.A.; Feng, X. Metal sericin complexation and ultrafiltration of heavy metals from aqueous solution. Chem. Eng. J. 2014, 244, 446–456.
- Gao, A.; Xie, K.; Song, X.; Zhang, K.; Hou, A. Removal of the heavy metal ions from aqueous solution using modified natural biomaterial membrane based on silk fibroin. Ecol. Eng. 2017, 99, 343–348.
- Shoulders, R.T.R.M.D. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958.
- Xiao, H.; Wang, Y.; Hao, B.; Cao, Y.; Cui, Y.; Bi Shi, X. Collagen Fiber-Based Advanced Separation Materials: Recent Developments and Future Perspectives. Adv. Mater. 2022, 34, 2107891.
- Suzuki, H.K.F.; Shibue, T. Formation having a tanning gradient structure of collagen membrane by the pervaporation technique. J. Membr. Sci. 2000, 165, 169–175.
- Maser, F.; Ströher-Glowienka, C.; Kimmerle, K.; Gudernatsch, W. Collagen film as a new pervaporation membrane. J. Membr. Sci. 1991, 61, 269–278.
- Ackermann, J.U.; Müller, S.; Lösche, A.; Bley, T. Wolfgang Babel Methylobacterium rhodesianum cells tend to double the DNA content under growth limitations and accumulate PHB. J. Biotechnol. 1995, 39, 9–20.
- Lin, X.; Li, S.; Wang, Y.; Yang, X.; Jung, J.; Li, Z.; Ren, X.; Sun, Y. Fabrication of pH-responsive hydrophobic/hydrophilic antibacterial polyhydroxybutyrate/poly-ε-caprolactone fibrous membranes for biomedical application. Mater. Chem. Phys. 2021, 260, 124087.
- Lin, X.; Yin, M.; Liu, Y.; Li, L.; Ren, X.; Sun, Y.; Huang, T.-S. Biodegradable polyhydroxybutyrate/poly-ε-caprolactone fibrous membranes modified by silica composite hydrol for super hydrophobic and outstanding antibacterial application. J. Ind. Eng. Chem. 2018, 63, 303–311.
- Guo, J.; Zhang, Q.; Cai, Z.; Zhao, K. Preparation and dye filtration property of electrospun polyhydroxybutyrate–calcium alginate/carbon nanotubes composite nanofibrous filtration membrane. Sep. Purif. Technol. 2016, 161, 69–79.
- Tomietto, P.; Carré, M.; Loulergue, P.; Paugam, L.; Audic, J.-L. Polyhydroxyalkanoate (PHA) based microfiltration membranes: Tailoring the structure by the non-solvent induced phase separation (NIPS) process. Polymer 2020, 204, 122813.
- Leja, G.L.K. Polymer biodegradation and biodegradable polymers—A review. Pol. J. Environ. Stud. 2010, 19, 255–266.
- Ummartyotin, C.P.S. Strategies for development and implementation of bio-based materials as effective renewable resources of energy: A comprehensive review on adsorbent technology. Renew. Sustain. Energy Rev. 2016, 62, 654–664.
- Singhvi, D.G.M. Biomass to biodegradable polymer (PLA). RSC Adv. 2013, 3, 13558–13568.
- Jing, Y.; Zhang, L.; Huang, R.; Bai, D.; Bai, H.; Zhang, Q.; Fu, Q. Ultrahigh-performance electrospun polylactide membranes with excellent oil/water separation ability via interfacial stereocomplex crystallization. J. Mater. Chem. A 2017, 5, 19729–19737.
- Liu, X.; He, X.; Jin, D.; Wu, S.; Wang, H.; Yin, M.; Aldalbahi, A.; El-Newehy, M.; Mo, X.; Wu, J. A biodegradable multifunctional nanofibrous membrane for periodontal tissue regeneration. Acta Biomater. 2020, 108, 207–222.
- Leejarkpai, T.; Suwanmanee, U.; Rudeekit, Y.; Mungcharoen, T. Biodegradable kinetics of plastics under controlled composting conditions. Waste Manag. 2011, 31, 1153–1161.
- Mochizuki, M. Properties and application of aliphatic polyester products. Biopolymers 2005, 20.
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation Assessment of Poly (Lactic Acid) Filled with Functionalized Titania Nanoparticles (PLA/TiO2) under Compost Conditions. Nanoscale Res. Lett. 2019, 14, 56.
- Lee, J.-S.; Lin, C.-J.; Lee, W.-C.; Teng, H.-Y.; Chuang, M.-H. Production of succinic acid through the fermentation of Actinobacillus succinogenes on the hydrolysate of Napier grass. Biotechnol. Biofuels Bioprod. 2022, 15, 9.
- Le, S.D.; Nishimura, S. Highly Selective Synthesis of 1,4-Butanediol via Hydrogenation of Succinic Acid with Supported Cu–Pd Alloy Nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 18483–18492.
- Guo, J.X.A.B.-H. Plastics from Bacteria: Natural Functions and Applications. Microbiol. Monogr. 2010, 14, 347–385.
- Mizuno, S.; Maeda, T.; Kanemura, C.; Hotta, A. Biodegradability, reprocessability, and mechanical properties of polybutylene succinate (PBS) photografted by hydrophilic or hydrophobic membranes. Polym. Degrad. Stab. 2015, 117, 58–65.
- Almwli, H.H.A.; Mousavi, S.M.; Kiani, S. Preparation of poly(butylene succinate)/polyvinylpyrrolidone blend membrane for pervaporation dehydration of acetone. Chem. Eng. Res. Des. 2021, 165, 361–373.
- Ghaffarian, V.; Mousavi, S.M.; Bahreini, M.; Jalaei, H. Polyethersulfone/poly (butylene succinate) membrane: Effect of preparation conditions on properties and performance. J. Ind. Eng. Chem. 2014, 20, 1359–1366.
- Nelson, T.F.; Baumgartner, R.; Jaggi, M.; Bernasconi, S.M.; Battagliarin, G.; Sinkel, C.; Künkel, A.; Kohler, H.-P.E.; McNeill, K.; Sander, M. Biodegradation of poly(butylene succinate) in soil laboratory incubations assessed by stable carbon isotope labelling. Nat. Commun. 2022, 13, 5691.
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504.
- Geravand, M.H.A.; Saljoughi, E.; Mousavi, S.M.; Kiani, S. Biodegradable polycaprolactone/MXene nanocomposite nanofiltration membranes for the treatment of dye solutions. J. Taiwan Inst. Chem. Eng. 2021, 128, 124–139.
- Ho, T.T.-P.; Doan, V.K.; Tran, N.M.-P.; Nguyen, L.K.-K.; Le, A.N.-M.; Ho, M.H.; Trinh, N.-T.; Van Vo, T.; Tran, L.D.; Nguyen, T.-H. Fabrication of chitosan oligomer-coated electrospun polycaprolactone membrane for wound dressing application. Mater. Sci. Eng. C 2021, 120, 111724.
- Li, W.; Zong, Y.; Liu, Q.; Sun, Y.; Li, Z.; Wang, H.; Li, Z. A highly stretchable and biodegradable superamphiphobic fluorinated polycaprolactone nanofibrous membrane for antifouling. Prog. Org. Coat. 2020, 147, 105776.
- Zafari, M.; Aghajani, S.; Boroujeni, M.M.; Nosrati, H. Vancomycin-loaded electrospun polycaprolactone/nano-hydroxyapatite membrane for the treatment of blood infections. Med. Hypotheses 2020, 144, 109992.
- Nowwarote, N.; Chanjavanakul, P.; Kongdecha, P.; Clayhan, P.; Chumprasert, S.; Manokawinchoke, J.; Egusa, H.; Pavasant, P.; Osathanon, T. Characterization of a bioactive Jagged1-coated polycaprolactone-based membrane for guided tissue regeneration. Arch. Oral Biol. 2018, 88, 24–33.
- Abdel-Motaal, F.F.; El-Sayed, M.A.; El-Zayat, S.A.; Ito, S.I. Biodegradation of poly (epsilon-caprolactone) (PCL) film and foam plastic by Pseudozyma japonica sp. nov., a novel cutinolytic ustilaginomycetous yeast species. 3 Biotech 2014, 4, 507–512.
- MShung, A.K.; Timmer, M.D.; Jo, S.; Engel, P.S.; Mikos, A.G. Kinetics of poly(propylene fumarate) synthesis by step polymerization of diethyl fumarate and propylene glycol using zinc chloride as a catalyst. J. Biomater. Sci. Polym. Ed. 2002, 13, 95–108.
- Frazier, V.K.L.D.D.; Gerhart, T.N.; Altobelli, D.E.; Hayes, W.C. In-Vivo Degradation Of A Poly(Propylene-Fumarate) Biodegradable, Particulate Composite Bone Cement. MRS Online Proc. Library 1995, 394, 15–19.
- Diez-Pascual, M.A. Mechanical properties of poly(propylene fumarate)/nanotube composites: Experimental results and theoretical predictions. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bangkok, Thailand, 24–26 February 2018; Volume 383.
- Díez-Pascual, A.L.D.-V.A.M. Multifunctional poly (glycolic acid-copropylene fumarate) electrospun fibers reinforced with graphene oxide and hydroxyapatite nanorods. J. Mater. Chem. B 2017, 5, 4084–4096.
- Zhang, P.; Tian, R.; Lv, R.; Na, B.; Liu, Q. Water-permeable polylactide blend membranes for hydrophilicity-based separation. Chem. Eng. J. 2015, 269, 180–185.
- Wolfe, M.S.; Dean, D.; Chen, J.E.; Fisher, J.P.; Han, S.; Rimnac, C.M.; Mikos, A.G. In vitro degradation and fracture toughness of multilayered porous poly(propylene fumarate)/beta-tricalcium phosphate scaffolds. J. Biomed. Mater. Res. 2002, 61, 159–164.
- Mitsuhashi, K.; Ohta, S.; Ito, T. Analysis of model drug permeation through highly crosslinked and biodegradable polyethylene glycol membranes. J. Membr. Sci. 2022, 645, 120218.
- Cavallo, A.; Madaghiele, M.; Masullo, U.; Lionetto, M.G.; Sannino, A. Photo-crosslinked poly(ethylene glycol) diacrylate (PEGDA) hydrogels from low molecular weight prepolymer: Swelling and permeation studies. J. Appl. Polym. Sci. 2017, 134, 2.
- He, X.C.H.; Lee, L.J. Design of a novel hydrogel-based intelligent system for controlled drug release. J. Control. Release 2004, 95, 391–402.
- Tokuyama, Y.N.H.; Ban, T. Diffusion coefficient of solute in heterogeneous and macroporous hydrogels and its correlation with the effective crosslinking density. J. Membr. Sci. 2020, 595, 117533.
- Asai, H.; bin Miswan, M.H.; Shimada, N.; Nakane, K.; Sakai, T.; Ogata, N. Preparation and characterization of a nanofiber mat consisting of Tetra-PEG prepolymers. J. Appl. Polym. Sci. 2014, 132, 41353.
- Ulbricht, J.; Jordan, R.; Luxenhofer, R. On the biodegradability of polyethylene glycol, polypeptoids and poly(2-oxazoline)s. Biomaterials 2014, 35, 4848–4861.
- Kawai, F. Microbial degradation of polyethers. Appl. Microbiol. Biotechnol. 2002, 58, 30–38.
- Yamanaka, F.K.a.H. Biodegradation of polyethylene glycol by symbiotic mixed culture (obligate mutualism). Arch. Microbiol. 1986, 146, 125–129.
- Ulrike Kohlweyer, B.T.; Schrader, T.; Andreesen, J.R. Tetrahydrofuran degradation by a newly isolated culture of Pseudonocardia sp. strain K1. FEMS Microbiol. Lett. 2000, 186, 301–306.
- Fahmy, A.; Kamoun, E.A.; El-Eisawy, R.; El-Fakharany, E.M.; Taha, T.H.; El-Damhougy, B.K.; Abdelhai, F. Poly(vinyl alcohol)-hyaluronic Acid Membranes for Wound Dressing Applications: Synthesis and in vitro Bio-Evaluations. J. Braz. Chem. Soc. 2015, 26, 1466–1474.
- Yang, C.C.H.J.M. Preparation and characterizaton of poly(dimethyl amino ethyl methacrylate) modified poly(vinyl alcohol) membrane by UV radiation for the permeation of 5-fluorouracil. J. Appl. Polym. Sci. 2012, 123, 3182–3188.
- Ben Halima, N. Poly(vinyl alcohol): Review of its promising applications and insights into biodegradation. RSC Adv. 2016, 6, 39823–39832.
- Market Volume of Polyurethane Worldwide from 2015 to 2025, with a Forecast for 2022 to 2029, Polyurethane Global Market Volume 2015–2029. Statista. Available online: https://www.statista.com/statistics/720341/global-polyurethane-market-size-forecast/ (accessed on 23 July 2021).
- Cooper, J.G.S.L. Advances in Polyurethane Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016.
- Zhang, C.; Wen, X.; Vyavahare, N.R.; Boland, T. Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet technique. Biomaterials 2008, 29, 3781–3791.
- Pavlova, M.; Draganova, M. Biocompatible and biodegradable polyurethane polymers. Biomaterials 1993, 14, 1024–1029.
- Wong, C.S.; Liu, X.; Xu, Z.; Lin, T.; Wang, X. Elastin and collagen enhances electrospun aligned polyurethane as scaffolds for vascular graft. J. Mater. Sci. Mater. Med. 2013, 24, 1865–1874.
- Xu, C.; Huang, Y.; Wu, J.; Tang, L.; Hong, Y. Triggerable Degradation of Polyurethanes for Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2015, 7, 20377–20388.
- Howard, G. Biodegradation of polyurethane: A review. Int. Biodeterior. Biodegrad. 2002, 49, 245–252.
- Braunack, M.V.; Zaja, A.; Tam, K.; Filipović, L.; Filipović, V.; Wang, Y.; Bristow, K.L. A Sprayable Biodegradable Polymer Membrane (SBPM) technology: Effect of band width and application rate on water conservation and seedling emergence. Agric. Water Manag. 2020, 230, 105900.
- Xu, C.; Hong, Y. Rational design of biodegradable thermoplastic polyurethanes for tissue repair. Bioact. Mater. 2021, 15, 250–271.
This entry is offline, you can click here to edit this entry!
