Hypoxia imposes conflicting demands on cardio-respiratory function. Being systemically O2 supply-dependent on cardiac output (CO) and arterial O2 concentration, fish can respond to and cope with hypoxia through cardio-respiratory adjustments to preserve systemic O2 delivery, thus maintaining aerobic metabolism, or by reducing O2 demands via anaerobic metabolism or metabolic depression. In 1986, Hochachka firstly proposed metabolic arrest, i.e., a simultaneous reduction in metabolic rate and metabolic demands, as a key adaptation to O2 deprivation in organisms capable of long-term anoxic survival.
- fish heart
- cyprinids
- contractility
- metabolism
1. Introduction
2. Hypoxia-Related Metabolic Responses of the Fish Heart
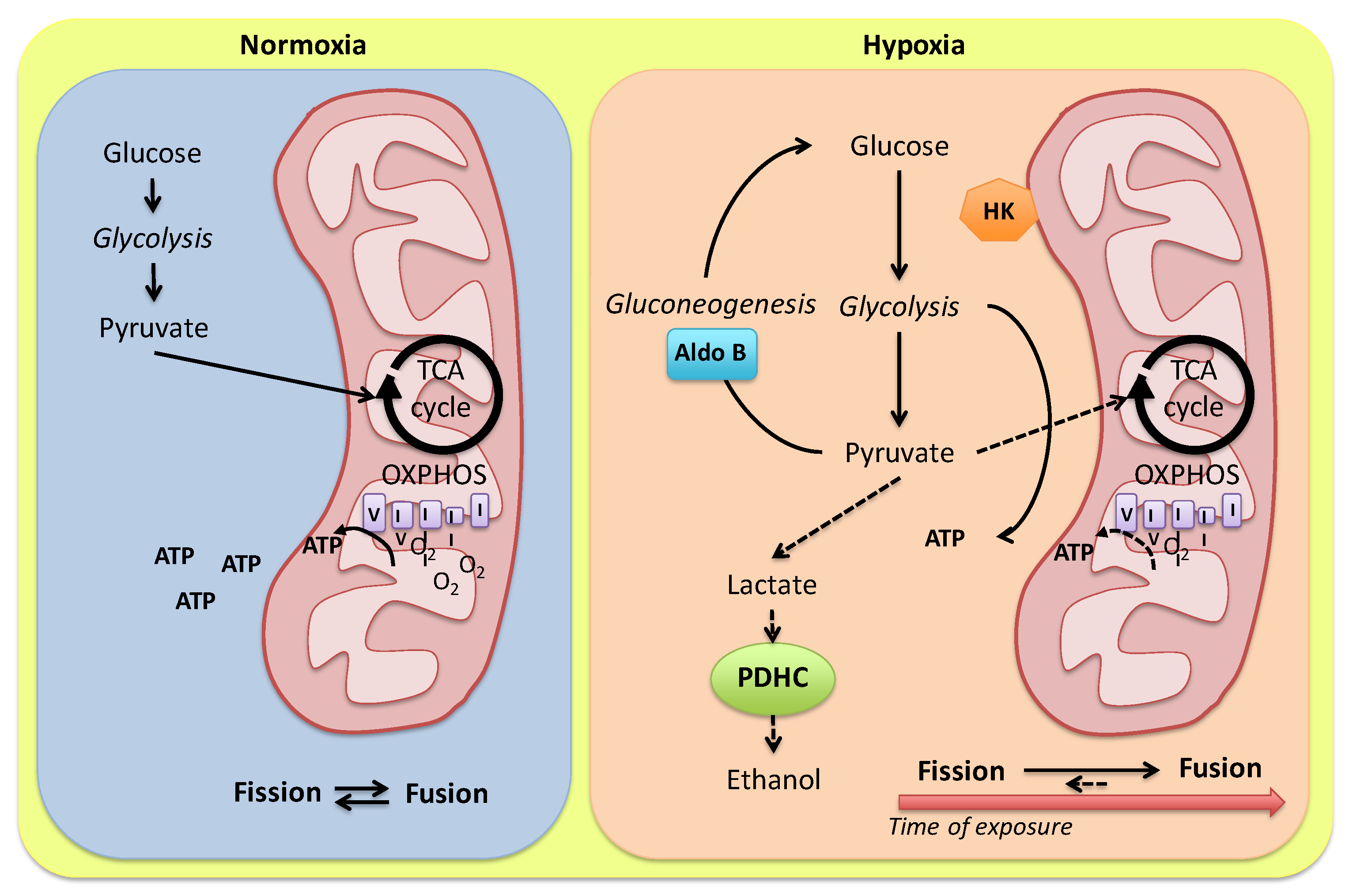
This entry is adapted from the peer-reviewed paper 10.3390/ijms24021460
References
- Chapman, L.J.; Mckenzie, D.J. Behavioral responses and ecological consequences. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 27, pp. 25–77.
- Urbina, M.A.; Forster, M.E.; Glover, C.N. Leap of faith: Voluntary emersion behaviour and physiological adaptations to aerial exposure in a non-aestivating freshwater fish in response to aquatic hypoxia. Physiol. Behav. 2011, 103, 240–247.
- Richards, J.G. Metabolic and molecular responses of fish to hypoxia. In Fish Physiology; Jeffrey, G., Richards, A.P.F., Colin, J.B., Eds.; Academic Press: Cambridge, MA, USA, 2009; Volume 27, pp. 443–485.
- Rogers, N.J.; Urbina, M.A.; Reardon, E.E.; McKenzie, D.J.; Wilson, R.W. A new analysis of hypoxia tolerance in fishes using a database of critical oxygen level (P crit). Conserv. Physiol. 2016, 4, cow012.
- Ultsch, G.R.; Regan, M.D. The utility and determination of P(crit) in fishes. J. Exp. Biol. 2019, 222, jeb203646.
- Hochachka, P.W. Defense strategies against hypoxia and hypothermia. Science 1986, 231, 234–241.
- Bickler, P.E.; Buck, L.T. Hypoxia tolerance in reptiles, amphibians, and fishes: Life with variable oxygen availability. Annu. Rev. Physiol. 2007, 69, 145–170.
- Shoubridge, E.A.; Hochachka, P.W. Ethanol: Novel end product of vertebrate anaerobic metabolism. Science 1980, 209, 308–309.
- Fagernes, C.E.; Stenslokken, K.O.; Rohr, A.K.; Berenbrink, M.; Ellefsen, S.; Nilsson, G.E. Extreme anoxia tolerance in crucian carp and goldfish through neofunctionalization of duplicated genes creating a new ethanol-producing pyruvate decarboxylase pathway. Sci. Rep. 2017, 7, 7884.
- Imbrogno, S.; Capria, C.; Tota, B.; Jensen, F.B. Nitric oxide improves the hemodynamic performance of the hypoxic goldfish (Carassius auratus) heart. Nitric Oxide 2014, 42, 24–31.
- Stecyk, J.A.; Stenslokken, K.O.; Farrell, A.P.; Nilsson, G.E. Maintained cardiac pumping in anoxic crucian carp. Science 2004, 306, 77.
- Gattuso, A.; Garofalo, F.; Cerra, M.C.; Imbrogno, S. Hypoxia Tolerance in Teleosts: Implications of Cardiac Nitrosative Signals. Front. Physiol. 2018, 9, 366.
- Farrell, A.P.; Stecyk, J.A. The heart as a working model to explore themes and strategies for anoxic survival in ectothermic vertebrates. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 147, 300–312.
- Imbrogno, S.; Aiello, D.; Filice, M.; Leo, S.; Mazza, R.; Cerra, M.C.; Napoli, A. MS-based proteomic analysis of cardiac response to hypoxia in the goldfish (Carassius auratus). Sci. Rep. 2019, 9, 18953.
- Penhoet, E.E.; Kochman, M.; Rutter, W.J. Molecular and catalytic properties of aldolase C. Biochemistry 1969, 8, 4396–4402.
- Penhoet, E.E.; Rutter, W.J. Catalytic and immunochemical properties of homomeric and heteromeric combinations of aldolase subunits. J. Biol. Chem. 1971, 246, 318–323.
- Brooks, S.P.J.; Storey, K.B. Is glycolytic rate controlledby the reversible binding of enzymes to subcellular structures? In Biochemistry and Molecular Biology of Fishes; Hochachka, P.W., Mommsen, T.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 4, pp. 291–307.
- Treberg, J.R.; MacCormack, T.J.; Lewis, J.M.; Almeida-Val, V.M.; Val, A.L.; Driedzic, W.R. Intracellular glucose and binding of hexokinase and phosphofructokinase to particulate fractions increase under hypoxia in heart of the amazonian armored catfish (Liposarcus pardalis). Physiol. Biochem. Zool. 2007, 80, 542–550.
- Duncan, J.A.; Storey, K.B. Role of enzyme binding in muscle metabolism of the goldfish. Can. J. Zool. 1991, 69, 1571–1576.
- Sidell, B.D.; Stowe, D.B.; Hansen, C.A. Carbohydrate Is the Preferred Metabolic Fuel of the Hagfish (Myxine glutinosa) Heart. Physiol. Zool. 1984, 57, 266–273.
- Imbrogno, S. The eel heart: Multilevel insights into functional organ plasticity. J. Exp. Biol. 2013, 216, 3575–3586.
- Bailey, J.R.; MacDougall, R.; Clowe, S.; Driedzic, W.R. Anoxic performance of the american eel (Anguilla rostrata L.) heart requires extracellular glucose. J. Exp. Zool. 2000, 286, 699–706.
- Vornanen, M.; Haverinen, J. Glycogen dynamics of crucian carp (Carassius carassius) in prolonged anoxia. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2016, 186, 999–1007.
- Shoubridge, E.A.; Hochachka, P.W. The integrationand control of metabolism in the anoxic goldfish. Mol. Physiol. 1983, 4, 165–195.
- Farhat, E.; Turenne, E.D.; Choi, K.; Weber, J.M. Hypoxia-induced remodelling of goldfish membranes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 237, 110326.
- Farhat, E.; Cheng, H.; Romestaing, C.; Pamenter, M.; Weber, J.M. Goldfish Response to Chronic Hypoxia: Mitochondrial Respiration, Fuel Preference and Energy Metabolism. Metabolites 2021, 11, 187.
- Pedersen, C.L.; Faggiano, S.; Helbo, S.; Gesser, H.; Fago, A. Roles of nitric oxide, nitrite and myoglobin on myocardial efficiency in trout (Oncorhynchus mykiss) and goldfish (Carassius auratus): Implications for hypoxia tolerance. J. Exp. Biol. 2010, 213, 2755–2762.
- Pamenter, M.E. Mitochondria: A multimodal hub of hypoxia tolerance. Can. J. Zool. 2014, 92, 569–589.
- Du, S.N.N.; Mahalingam, S.; Borowiec, B.G.; Scott, G.R. Mitochondrial physiology and reactive oxygen species production are altered by hypoxia acclimation in killifish (Fundulus heteroclitus). J. Exp. Biol. 2016, 219, 1130–1138.
- Onukwufor, J.O.; Stevens, D.; Kamunde, C. Combined effects of cadmium, temperature and hypoxia-reoxygenation on mitochondrial function in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2017, 182, 129–141.
- Sappal, R.; MacDougald, M.; Fast, M.; Stevens, D.; Kibenge, F.; Siah, A.; Kamunde, C. Alterations in mitochondrial electron transport system activity in response to warm acclimation, hypoxia-reoxygenation and copper in rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 2015, 165, 51–63.
- Cook, D.G.; Iftikar, F.I.; Baker, D.W.; Hickey, A.J.R.; Herbert, N.A. Low-O2 acclimation shifts the hypoxia avoidance behaviour of snapper (Pagrus auratus) with only subtle changes in aerobic and anaerobic function. J. Exp. Biol. 2013, 216, 369–378.
- Hickey, A.J.R.; Renshaw, G.M.C.; Speers-Roesch, B.; Richards, J.G.; Wang, Y.; Farrell, A.P.; Brauner, C.J. A radical approach to beating hypoxia: Depressed free radical release from heart fibres of the hypoxia-tolerant epaulette shark (Hemiscyllum ocellatum). J. Comp. Physiol. B 2012, 182, 91–100.
- Gerber, L.; Clow, K.A.; Katan, T.; Emam, M.; Leeuwis, R.H.J.; Parrish, C.C.; Gamperl, A.K. Cardiac mitochondrial function, nitric oxide sensitivity and lipid composition following hypoxia acclimation in sablefish. J. Exp. Biol. 2019, 222, jeb208074.
- St-Pierre, J.; Boutilier, R.G. Aerobic capacity of frog skeletal muscle during hibernation. Physiol. Biochem. Zool. 2001, 74, 390–397.
- O’Brien, K.M. Mitochondrial biogenesis in cold-bodied fishes. J. Exp. Biol. 2011, 214, 275–285.
- Filice, M.; Barca, A.; Amelio, D.; Leo, S.; Mazzei, A.; Del Vecchio, G.; Verri, T.; Cerra, M.C.; Imbrogno, S. Morpho-functional remodelling of the adult zebrafish (Danio rerio) heart in response to waterborne angiotensin II exposure. Gen. Comp. Endocrinol. 2021, 301, 113663.
- Cerra, M.C.; Imbrogno, S.; Amelio, D.; Garofalo, F.; Colvee, E.; Tota, B.; Icardo, J.M. Cardiac morphodynamic remodelling in the growing eel (Anguilla anguilla L.). J. Exp. Biol. 2004, 207, 2867–2875.
- Farhat, E.; Talarico, G.G.M.; Grégoire, M.; Weber, J.M.; Mennigen, J.A. Epigenetic and post-transcriptional repression support metabolic suppression in chronically hypoxic goldfish. Sci. Rep. 2022, 12, 5576.
