Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Oxidized β-carotene (OxBC), a phytochemical that occurs naturally in plants, including fruits and vegetables, is formed by the spontaneous reaction of β-carotene with ambient oxygen. Synthetic OxBC, obtained by the full oxidation of β-carotene with air, shows considerable promise as a parts-per-million in-feed antimicrobial alternative additive that enhances health and performance in poultry, swine, and ruminant species. OxBC is predominantly composed of β-carotene–oxygen copolymers that have beneficial immune-modulating effects.
- antibiotic alternative
- oxidized β-carotene
- growth and health performance
1. Antimicrobial Alternatives for Enhancement of Health and Performance
Increased regulatory pressure banning or limiting the use of antibiotic growth promoters (AGPs) and growing consumer demand for sustainable agriculture practices, including products ‘Raised Without Antibiotics’ or ‘No Antibiotics Ever’, have been driving the search for alternative products [1][2][3][4]. Much effort has been made to discover and develop alternatives to AGPs to maintain or improve livestock health and performance. Alternatives include probiotics, prebiotics, synbiotics, organic acids, enzymes, phytogenics, antimicrobial peptides, hyperimmune egg antibodies, bacteriophages, clays, and metals. The mechanism of action, efficacy, and advantages and disadvantages of their uses have been reviewed [4]. Although the beneficial effects of many of the alternatives have been demonstrated, the consensus is that many currently available products lack consistency, and results can vary greatly. Additionally, their modes of action often are not well understood.
Antibiotic alternatives are intended to replace AGPs, whose primary function is to decrease microbial populations and promote growth via many different modes of action that may include alteration and/or inhibition of microbial growth, decreased inflammation, enhancement of innate immunity, reduced oxidative stress, and improved gut integrity [5].
There is no clear consensus on how AGPs act to promote antibiotic-mediated growth enhancement, although several ideas have been proposed [4]. An attractive hypothesis proposed by Niewold is that beneficial effects occur due to the interaction of antibiotics with host immune cells rather than from growth-inhibitory effects on microbiota [6]. Niewold proposed that antibiotics lower the inflammatory response and, thus, the production of proinflammatory cytokines that reduce the appetite and promote muscle catabolism. The anti-inflammatory role of AGPs reduces wasted energy and directs it toward production.
1.1. Classes of Alternatives
In reviewing alternatives, Gadde et al. commented that ideally, an alternative should have the same beneficial effects as AGPs to ensure optimum animal performance and to increase nutrient availability [4]. Two attractive proposed mechanisms of action of AGPs are modulation of the microbiome and immune activities. A practical alternative should possess both these properties in addition to improving feed conversion or growth or, ideally, both. Of the several classes of alternatives that have been considered, immunomodulators are an attractive opportunity.
1.2. Immune Modulators
An important feature of immune modulators, in contrast to vaccines, for example, is their ability to affect the immune system in a way that is less dependent on a specific pathogen threat, making them effective against a broad range of pathogens [7].
1.3. Phytochemicals
Phytochemicals are natural bioactive compounds that are derived from plants and incorporated into animal feeds to enhance productivity [5]. In recent years, phytochemicals have been used as natural growth promoters in the ruminant, swine, and poultry production industries. A wide variety of herbs and spices (e.g., thyme, oregano, rosemary, marjoram, yarrow, garlic, ginger, green tea, black cumin, coriander, and cinnamon) have been used in poultry for their potential application as AGP alternatives.
Lillehoj et al. [5] noted that a growing body of scientific evidence has demonstrated that many of the health-promoting activities of phytochemicals are mediated through their ability to enhance host defense against microbial infections. The immune-activating properties of medicinal plants such as dandelion, mustard, and safflower have been evaluated in vitro. All three extracts inhibit tumor cell growth, stimulate innate immunity, and exert antioxidant effects in poultry.
2. Carotenoids. Sources of Phytochemicals with a Unique Role in Health Promotion
The role of various carotenoids, including β-carotene, as natural pigments, and precursors of the retinoids with vitamin A activity, has long been known. However, over the last three decades, considerable empirical and scientific evidence has been gathered that suggests that carotenoids and their metabolites have functions beyond those that have been characterized as “normal” physiological roles [8][9][10][11]. These new roles are considered “functional”, as in functional foods, i.e., providing benefits beyond those attributable to the nutrient value of the compounds. This generally implies a health benefit that is in addition to the documented physiological function of the nutrient or related metabolite(s). Thus, carotenoids have been implicated in the reduced risk involved in the development of chronic diseases, for example, some types of cancers and certain cardiovascular and eye diseases [11][12][13].
Carotenoids represent the most abundant lipid-soluble phytochemicals. In vitro and in vivo studies have suggested they have antioxidant, antiapoptotic, and anti-inflammatory properties, many of which have been linked to the effect of carotenoids or their metabolic or breakdown products (apocarotenoids) on intracellular signaling cascades influencing gene expression and protein translation [10].
Although beneficial health effects attributed to carotenoids frequently have been attributed to a purported antioxidant capability [14], there are other plausible mechanisms of action of carotenoids and their apocarotenoids, including immunomodulatory and anti-inflammatory activities [8][11][15][16][17].
Carotenoids have been implicated in the modulation of the avian innate immune system [18][19][20][21][22]. Koutsos et al. [23] used lipopolysaccharide (LPS) or interleukin-1 (IL-1) to induce an acute phase response in broiler chicks and concluded that this response was implicated in reducing tissue carotenoid levels during infectious disease. In a subsequent study, this same group demonstrated that chicks fed 0 mg lutein had greater body weight losses and higher plasma haptoglobin and relative thymus, bursa, and spleen weights post-LPS challenge compared with chicks fed 40 mg lutein/kg diet [24]. The conclusion was that a lack of carotenoid exposure, either in ovo or post-hatch, increased systemic inflammation and that the innate immune response is critical as the first line of defense against invading pathogens. The components of this innate immune response possess the ability to regulate the transcription and translation of genes for additional mediators of the inflammatory response (i.e., cytokines and acute-phase proteins). For example, plasma haptoglobin and serum amyloid A protein are acute-phase proteins that respond positively to inflammatory stimulation in various species, including wild birds [25][26][27].
In livestock animals it was thought for some time that β-carotene is a source of activity beyond its provitamin A status. However, in 2012 in a review of the safety and efficacy of β-carotene as a feed additive for all animal species, an EFSA panel concluded that non-provitamin A effects, for example, on reproduction and immunity, had not yet been sufficiently demonstrated [28].
Subsequently, Burton et al. expanded upon the potential role of dietary carotenoids in the modulation of the innate immune system in various animal species by describing how naturally occurring carotenoids undergo spontaneous, autocatalytic oxidation (autoxidation) to produce many complex oxygen copolymers [29]. They suggested that these copolymers are the source of immune-enhancing activity in several species, including poultry, that formerly had been attributed to intact carotenoids [30].
3. Oxidized β-Carotene (OxBC). A Naturally Occurring Phytochemical Immunomodulator
3.1. What Is OxBC? Oxygen Copolymer and Apocarotenoid Compounds
The autoxidation of highly unsaturated hydrocarbon compounds preferentially proceeds by the addition of oxygen rather than the cleavage of the hydrocarbon into smaller breakdown compounds, as first reported by Miller and Mayo in 1956 [31]. Yet, despite β-carotene’s highly unsaturated conjugated polyene backbone and the many β-carotene oxidation product studies carried out over several decades [32][33][34], it was not until 2014 that a β-carotene-oxygen copolymer product (“copolymer”) was reported by researchers as the main product of the spontaneous oxidation of β-carotene [29]. Prior to that, β-carotene autoxidation was known to produce only a mixture of many apocarotenoids. There appear to be no known previous reports of a copolymer product.
It is now known that β-carotene oxygen copolymers occur naturally [29][35][36] when β-carotene undergoes oxidative degradation in a wide range of plant products during storage or drying [35][36].
In the laboratory, the full oxidation of β-carotene with air or pure oxygen in solution yields a complex, reproducible product, OxBC (Oxidized Beta-Carotene), containing 80–85% by weight of a copolymer compound and 15–20% of many small apocarotenoid breakdown products [29][37] (Figure 1).
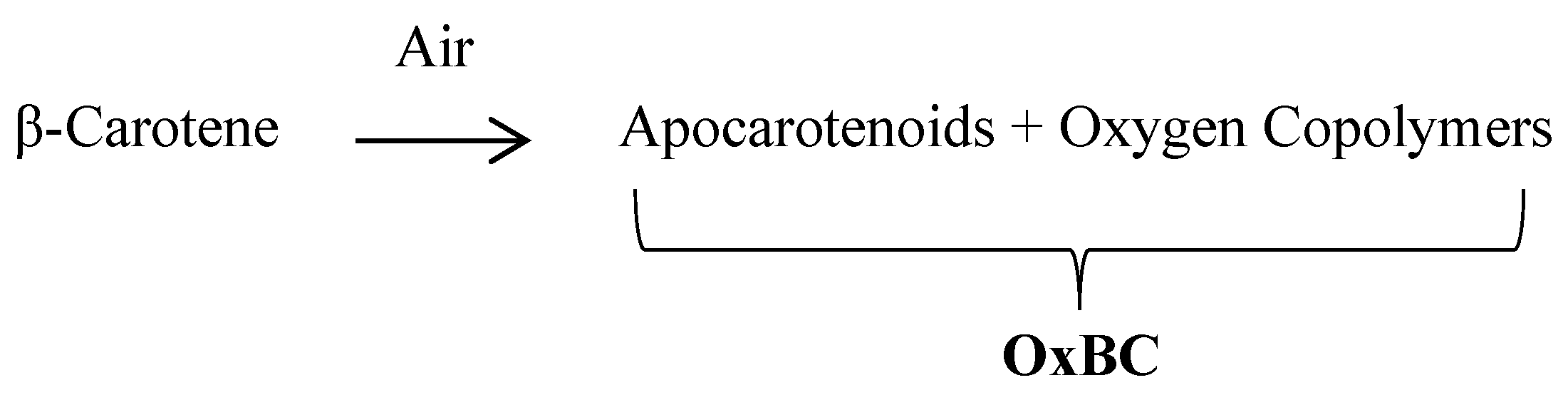
Figure 1. Formation of OxBC by spontaneous oxidation of β-carotene produces mainly a β-carotene oxygen copolymer compound and minor amounts of many apocarotenoid breakdown products.
The chemically complex OxBC is indirectly determined in foods and feeds using the apocarotenoid geronic acid (Figure 2) as a proxy marker compound for OxBC content [35].
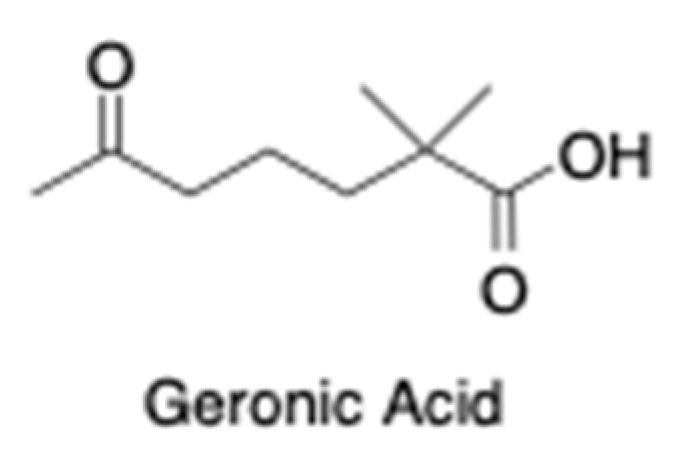
Figure 2. Geronic acid is an apocarotenoid product characteristic of OxBC that is used as a proxy marker compound for estimating OxBC.
Figure 3 illustrates the production of geronic acid as β-carotene is lost during the oxidation of dehydrated and powdered carrot over a period of days [38].
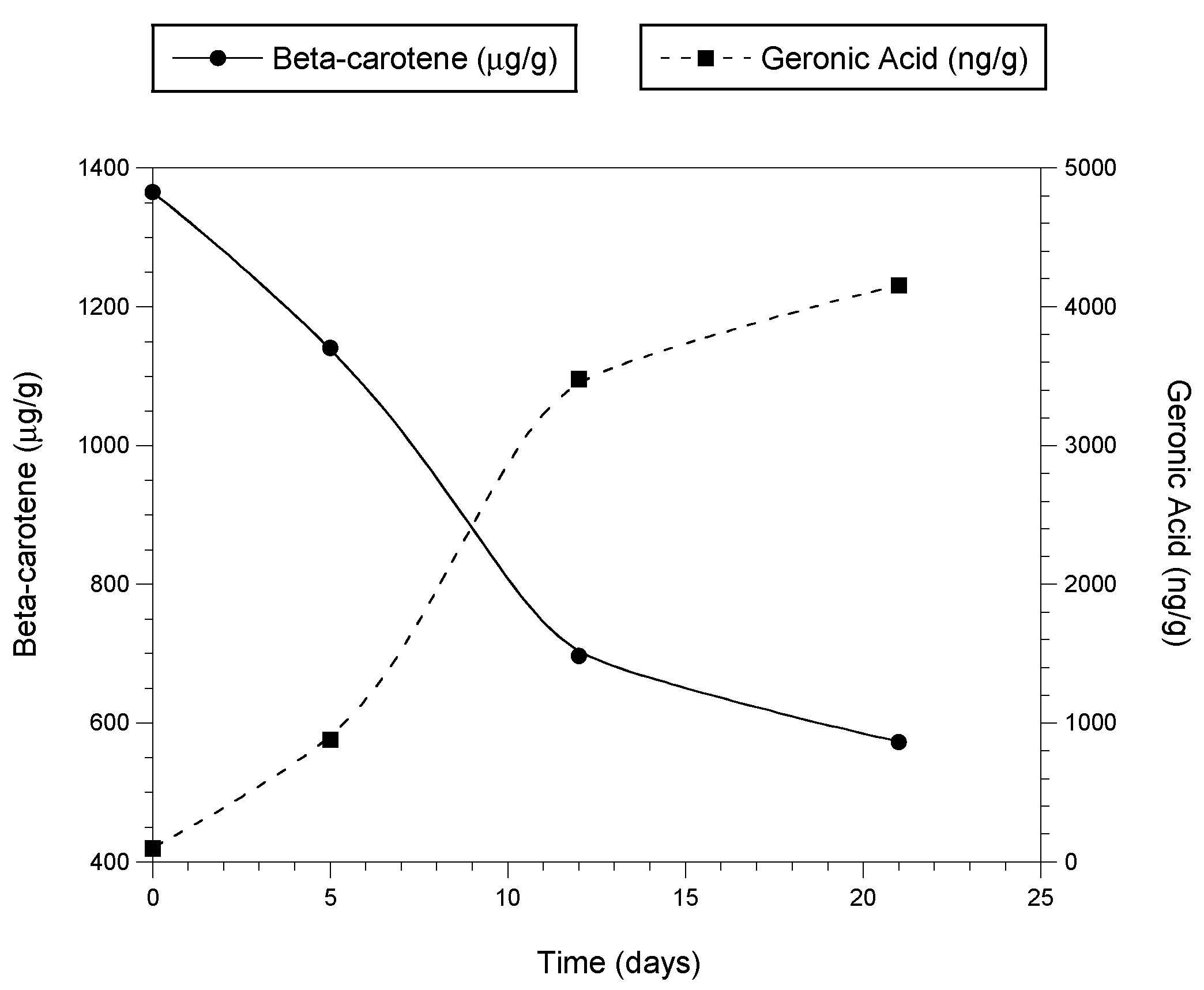
Figure 3. Formation of geronic acid as β-carotene is oxidized in dried, powdered carrot exposed to air. Measurements at time 0 used freshly dehydrated carrot purée. The dried purée was immediately ground to powder, spread thinly on a tray, and exposed to air and ambient light. Values at subsequent time points were determined using the powder.
Synthetic OxBC is produced commercially by the full, non-enzymatic air oxidation of pure β-carotene in an ethyl acetate solution.
The term apocarotenoid is applied somewhat loosely here, not only to include the double bond cleavage products but also compounds derived from some of these products by further chemical transformations during the oxidation reaction. Some of these compounds are also termed norisoprenoids [39][40].
The OxBC apocarotenoids are formed as by-products of the polymerization reaction. In the reaction with air, the final polymer:apocarotenoid ratio is approximately 4:1 (w/w). OxBC’s apocarotenoids contain no more than 18 carbon atoms, less than half of β-carotene’s 40 carbons [29], with a few present at 1% levels and the rest at much lower levels. Notably absent are those apocarotenoids containing more than 18 carbons, including vitamin A. These more reactive longer-chain apocarotenoid products (≥C20) formed early in the reaction are ultimately consumed and removed by further oxidation. The copolymer compound has been shown to be partially susceptible to further breakdown into apocarotenoids under acidic and basic conditions [37].
Several of OxBC’s apocarotenoids possess flavor and fragrance characteristics [39], being present, for example, in leaf products (e.g., tobacco, tea, mate), many essential oils, fruits (grapes, passionfruit, starfruit, quince, apple, nectarine, tomato, melon), spices (saffron, red pepper), wine, rum, coffee, oak wood, honey, and seaweeds. Thirteen of OxBC’s identified apocarotenoids are Generally Recognized As Safe (GRAS) human flavor agents [41].
3.2. OxBC’s Dual Immunological Function
OxBC exerts actions on the immune system through pathways that are distinct from either vitamin A or intact β-carotene, neither of which are present in OxBC. Uniquely, OxBC exhibits dual immunological activities relating to (1) enhanced innate immune detection or priming and response to pathogens [30] and (2) anti-inflammatory/pro-resolution activity that mitigates excessive immune responses and reduces the level of background inflammation [42][43].
Several in vitro and in vivo studies have demonstrated the immune-modulating activities of OxBC [30][42].
An evaluation of the ability of OxBC to influence the expression of genes relevant to core immune responses using quantitative real-time PCR arrays showed a pattern of activity consistent with two key activities [29]. First, OxBC upregulated the expression of genes encoding products that function in pathogen sensing and the detection of pathogen-associated molecule patterns (PAMPs), including Toll-like receptors (TLRs) and other proteins that act as cofactors for PAMP detection, such as CD14 (clusters of differentiation 14) and lymphocyte antigen 92 (LY96/MD2). Second, OxBC also appeared to down-regulate the expression of genes associated with the initiation and propagation of inflammatory responses, suggesting anti-inflammatory potential. This effect was observed for inflammatory cytokines such as tumour necrosis factor (TNF) and interleukin-1β, but also for cytokine receptors and other molecules that promote an inflammatory reaction. Of particular interest was the finding that OxBC inhibited key signaling molecules and regulators of the NF𝜅B pathway. This pathway plays pivotal roles in signaling events initiated by both the TLR system and inflammatory mediators such as TNF, suggesting a potential common mechanism behind both patterns of gene expression.
In vitro studies at the cellular level further clarified that it is the copolymers that are responsible for the innate immunological activities of OxBC, while the apocarotenoids appear to be inactive in this regard [30]. The results obtained for immune receptor levels, cytokine levels, and phagocytic activity indicated that OxBC could modulate innate immunity in a biologically beneficial way. These findings implied that the net result would be to prime the innate immune system to respond to subsequent challenges more rapidly. This is supported by the fact that OxBC had very little apparent effect on cytokine levels and phagocytic activity in the absence of a challenge. Unlike traditional immune stimulants, which directly trigger an inflammatory immune response, OxBC could potentially modulate but not stimulate and utilize an animal’s energy stores unless directly affected by stress.
As a result of the study of the biological activity of OxBC and other oxidized carotenoids, it has been proposed that carotenoid copolymer compounds are the actual agents responsible for many of the provitamin A-independent activities of β-carotene and other carotenoids [30].
It has not yet been established whether the source of anti-inflammatory activity of OxBC originates from the copolymer or the apocarotenoid fractions. In this regard it is of interest that β-ionone, a small molecule apocarotenoid present in OxBC, is reported to possess anti-inflammatory activity [44].
In addition to OxBC’s immune-modulating activity, several of OxBC’s apocarotenoids are recognized flavor agents [37][45], presenting the possibility that OxBC may also improve feed palatability.
3.3. Livestock Applications. Health and Performance and Metaphylaxis of Sub-Clinical Conditions
The utility of OxBC as a feed additive has been demonstrated in studies with piglets [46], sows [43], post-wean pigs [47], broiler chickens [48], and dairy cattle [42][49]. To illustrate, in piglets, dietary supplementation with OxBC improved growth performance and prevented the vaccine-induced growth lag associated with the PRRS (porcine reproductive and respiratory syndrome) vaccination [46]. In sows, supplementation with OxBC, beginning at late gestation and continuing through lactation, resulted in reduced proinflammatory cytokine levels in colostrum and milk concurrent with increased colostral and milk immunoglobulin levels [43]. In broilers, dietary supplementation with OxBC reduced the level of pathogen (Clostridium perfringens) recovered from the gut and protected against the reduction in growth performance associated with experimental induction of subclinical necrotic enteritis [48]. In dairy cattle, supplemental OxBC mitigated subclinical intramammary infections [49].
It has been proposed that the search for suitable alternatives to antibiotic growth promoters should yield substances that achieve effects similar to the antibiotics they are intended to replace, namely, a reduction in both bacterial load and inflammation [6][50][51][52]. The results from trials with pigs, poultry, and dairy cows highlight the utility of OxBC in achieving both outcomes. The benefits observed with piglets and gestating/lactating sows likely are consistent with the proposed anti-inflammatory actions of OxBC, whereas the reduction in C. perfringens in the poultry study and the elimination of the intramammary infections in lactating dairy cows support the benefits of innate immune priming. Note that OxBC has no direct anti-microbial effect and that the reduction or elimination of sub-clinical levels of bacterial pathogens in the poultry and dairy studies is consistent with the proposed immune priming actions, which positions host defenses to better detect and respond to the presence of various pathogens.
As of mid-2022, OxBC has been used commercially in the form of OxC-beta™ Livestock in various countries as a feed additive in approximately 16 million piglets, 94,000 sows, and 8.75 million poultry, and as a supplement for 12,000 dairy cows.
3.4. OxBC Safety
The question of the toxicity of β-carotene oxidation compounds arose indirectly as a result of the negative outcomes of several β-carotene human intervention clinical trials [53][54][55][56][57][58]. However, the physiological relevance of the cited supporting evidence, based entirely on in vitro model systems attempting to simulate oxidation conditions in vivo [59], was questioned in a review by an EFSA panel on the safety of β-carotene [60]. Additionally, synthetic OxBC does not contain any of the long-chain, retinoid-like apocarotenoids [29][60] that have been suggested as potentially toxic agents that may adversely interfere with vitamin A retinoid receptor activity.
A recent toxicology study of OxBC in rats has established a wide safety margin for OxBC with a Maximum Tolerated Dose (MTD) of 5000 mg/kg, an LD50 of 30,079 mg/kg, and a No Observed Adverse Effect Level (NOAEL) of 1875 mg/kg body weight [45]. Synthetic OxBC has been supplemented in livestock, pets, and humans at approximately 0.5 mg/kg body weight/day, a level 3750-fold below the NOAEL-based threshold.
An OxBC uptake study in mice established that both OxBC polymer and associated apocarotenoid compounds were naturally present in all tissues and fluids examined [45].
The safety of OxBC has been formally evaluated by several national regulatory authorities around the world. Notably, New Zealand, Australia, Brazil, Mexico, Thailand, and Philippine authorities have approved OxBC as safe for use in feeds for all animal species. In addition, OxBC is approved as an active ingredient in veterinary health products for companion animals in Canada. Further support for the safety of synthetic OxBC is provided by the history of commercial use in animal feeds and health supplements in the above countries, spanning almost a decade, with no serious adverse events reported. Furthermore, the natural occurrence of OxBC in many commonly used animal and human feeds and foodstuffs [35][36] indicates a long history of exposure to dietary OxBC for both animals and humans.
3.5. OxBC Stability
OxBC, both alone and in the form of the OxC-beta™ Livestock feed additive (Avivagen Inc., Ottawa, ON, Canada), shows multi-year stability as established by extensive chemical testing over a period of several years and in accelerated aging studies. Activity is not affected by pelleting, for example.
This entry is adapted from the peer-reviewed paper 10.3390/ani13020289
References
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Kwon, W.B.; Mathai, J.K.; Navarro, D.M.D.L.; Jaworski, N.W.; et al. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125.
- Tellez-Isaias, G.; Latorre, J.D. Editorial: Alternatives to Antimicrobial Growth Promoters and Their Impact in Gut Microbiota, Health and Disease: Volume II. Front. Vet. Sci. 2022, 9, 857583.
- Tellez, G.; Latorre, J.D. Editorial: Alternatives to Antimicrobial Growth Promoters and Their Impact in Gut Microbiota, Health and Disease. Front. Vet. Sci. 2017, 4, 196.
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health Res. Rev. 2017, 18, 26–45.
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76.
- Niewold, T.A. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult. Sci. 2007, 86, 605–609.
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z.Y. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014, 5, 217.
- Britton, G. Functions of carotenoid metabolites and breakdown products. In Carotenoids: Natural Functions; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2008; Volume 4, pp. 309–324.
- Wang, X.-D. Biological activities of carotenoid metabolites. In Carotenoids: Nutrition and Health; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2009; Volume 5, pp. 383–408.
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929.
- Sharoni, Y.; Linnewiel-Hermoni, K.; Khanin, M.; Salman, H.; Veprik, A.; Danilenko, M.; Levy, J. Carotenoids and apocarotenoids in cellular signaling related to cancer: A review. Mol. Nutr. Food Res. 2012, 56, 259–269.
- Kulczyński, B.; Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D. The role of carotenoids in the prevention and treatment of cardiovascular disease—Current state of knowledge. J. Funct. Foods 2017, 38, 45–65.
- Akhtar, S.; Ahmed, A.; Randhawa, M.A.; Atukorala, S.; Arlappa, N.; Ismail, T.; Ali, Z. Prevalence of vitamin A deficiency in South Asia: Causes, outcomes, and possible remedies. J. Health Popul. Nutr. 2013, 31, 413–423.
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Editors’ assessment. In Carotenoids. Nutrition and Health; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2009; Volume 5, pp. 409–422.
- Britton, G. Functions of intact carotenoids. In Carotenoids: Natural Functions; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2008; Volume 4, pp. 189–212.
- Chung, R.W.S.; Leanderson, P.; Lundberg, A.K.; Jonasson, L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 2017, 262, 87–93.
- Chew, B.; Park, J. The Immune System. In Carotenoids: Nutrition and Health; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2009; Volume 5, pp. 363–382.
- Blount, J.D.; Metcalfe, N.B.; Birkhead, T.R.; Surai, P.F. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 2003, 300, 125–127.
- McGraw, K.J.; Ardia, D.R. Carotenoids, Immunocompetence, and the Information Content of Sexual Colors: An Experimental Test. Am. Nat. 2003, 162, 704–712.
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S.
- Leclaire, S.; Bourret, V.; Blanchard, P.; de Franceschi, C.; Merkling, T.; Hatch, S.A.; Danchin, É. Carotenoids increase immunity and sex specifically affect color and redox homeostasis in a monochromatic seabird. Behav. Ecol. Sociobiol. 2015, 69, 1097–1111.
- Lopez-Rull, I.; Hornero-Mendez, D.; Frias, O.; Blanco, G. Age-Related Relationships between Innate Immunity and Plasma Carotenoids in an Obligate Avian Scavenger. PLoS ONE 2015, 10, e0141759.
- Koutsos, E.A.; Calvert, C.C.; Klasing, K.C. The effect of an acute phase response on tissue carotenoid levels of growing chickens (Gallus gallus domesticus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 135, 635–646.
- Koutsos, E.A.; Garcia Lopez, J.C.; Klasing, K.C. Carotenoids from in ovo or dietary sources blunt systemic indices of the inflammatory response in growing chicks (Gallus gallus domesticus). J. Nutr. 2006, 136, 1027–1031.
- Pepys, M.B.; Baltz, M.L.; Tennent, G.A.; Kent, J.; Ousey, J.; Rossdale, P.D. Serum amyloid A protein (SAA) in horses: Objective measurement of the acute phase response. Equine Vet. J. 1989, 21, 106–109.
- Ceron, J.J.; Eckersall, P.D.; Martynez-Subiela, S. Acute phase proteins in dogs and cats: Current knowledge and future perspectives. Vet. Clin. Pathol. 2005, 34, 85–99.
- Caliendo, V.; McKinney, P.; Bailey, T.; Kinne, J.; Wernery, U. Serum amyloid A as an indicator of health status in falcons. J. Avian Med. Surg. 2013, 27, 83–89.
- European Food Safety Authority. Scientific Opinion on the safety and efficacy of beta-carotene as a feed additive for all animal species and categories. EFSA J. 2012, 10, 2737.
- Burton, G.W.; Daroszewski, J.; Nickerson, J.G.; Johnston, J.B.; Mogg, T.J.; Nikiforov, G.B. ß-Carotene autoxidation: Oxygen copolymerization, non-vitamin A products and immunological activity. Can. J. Chem. 2014, 92, 305–316.
- Johnston, J.B.; Nickerson, J.G.; Daroszewski, J.; Mogg, T.J.; Burton, G.W. Biologically active polymers from spontaneous carotenoid oxidation. A new frontier in carotenoid activity. PLoS ONE 2014, 9, e111346.
- Miller, A.A.; Mayo, F.R. Oxidation of unsaturated compounds. I. The oxidation of styrene. J. Am. Chem. Soc. 1956, 78, 1017–1023.
- Handelman, G.J.; van Kuijk, F.J.G.M.; Chatterjee, A.; Krinsky, N.I. Characterization of products formed during the autoxidation of ß-carotene. Free Rad. Biol. Med. 1991, 10, 427–437.
- Mordi, R.C.; Walton, J.C.; Burton, G.W.; Hughes, L.; Ingold, K.U.; Lindsay, D.A.; Moffatt, D.J. Oxidative degradation of ß-carotene and ß-apo-8’-carotenal. Tetrahedron 1993, 49, 911–928.
- Crouzet, J.; Kanasawud, P.; Sakho, M. Thermal Generation of Carotenoid-Derived Compounds. In Carotenoid-Derived Aroma Compounds; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2001; Volume 802, pp. 115–129.
- Burton, G.W.; Daroszewski, J.; Mogg, T.J.; Nikiforov, G.B.; Nickerson, J.G. Discovery and characterization of carotenoid-oxygen copolymers in fruits and vegetables with potential health benefits. J. Agric. Food Chem. 2016, 64, 3767–3777.
- Schaub, P.; Wust, F.; Koschmieder, J.; Yu, Q.; Virk, P.; Tohme, J.; Beyer, P. Nonenzymatic beta-carotene degradation in provitamin A-biofortified crop plants. J. Agric. Food Chem. 2017, 65, 6588–6598.
- Mogg, T.J.; Burton, G.W. The β-carotene–oxygen copolymer: Its relationship to apocarotenoids and β-carotene function. Can. J. Chem. 2021, 99, 751–762.
- Burton, G.W.; Daroszewski, J.; Mogg, T.J.; Nikiforov, G.B.; Nickerson, J.G.; Groome, C.L. Plant or Microorganism-Derived Carotenoid-Oxygen Copolymer Compositions, Methods of Identifying, Quantifying and Producing Same and Uses Thereof. World Intellectual Property Organization. WO 2017/143460, 31 August 2017.
- Winterhalter, P.; Rouseff, R.L. (Eds.) Carotenoid-Derived Aroma Compounds; American Chemical Society: Washington, DC, USA, 2001; Volume 802.
- Melendez-Martinez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, e1801045.
- U.S. Food & Drug Administration. Substances Added to Food. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=FoodSubstances (accessed on 15 October 2022).
- Duquette, S.C.; Fischer, C.D.; Feener, T.D.; Muench, G.P.; Morck, D.W.; Barreda, D.R.; Nickerson, J.G.; Buret, A.G. Anti-inflammatory benefits of retinoids and carotenoid derivatives: Retinoic acid and fully oxidized β-carotene induce caspase-3-dependent apoptosis and promote efferocytosis of bovine neutrophils. Am. J. Vet. Res. 2014, 75, 1064–1075.
- Chen, J.; Chen, J.; Zhang, Y.; Lv, J.; Qiao, H.; Tian, M.; Cheng, L.; Chen, F.; Zhang, S.; Guan, W. Effects of maternal supplementation with fully oxidised β-carotene on the reproductive performance and immune response of sows, as well as the growth performance of nursing piglets. Brit. J. Nutr. 2021, 125, 62–70.
- Aloum, L.; Alefishat, E.; Adem, A.; Petroianu, G. Ionone Is More than a Violet’s Fragrance: A Review. Molecules 2020, 25, 5822.
- Burton, G.W.; Mogg, T.J.; Stupak, J.; Stark, F.C.; Twine, S.M.; Li, J. Safety and uptake of fully oxidized beta-carotene. Food Chem. Toxicol. 2022, 168, 113387.
- Hurnik, D.; Daroszewski, J.; Burton, G.W. Determination of the effect of a fully oxidized β-carotene dietary supplement on the immune system and growth performance of weaned pigs. In Proceedings of the American Association of Swine Veterinarians 42nd Annual Meeting, Phoenix, Arizona, 5–8 March 2011; pp. 277–279.
- Kinh, L.V.; Riley, W.W.; Nickerson, J.G.; Huyen, L.T.T.; Burton, G.W. Effect of oxidized β-carotene on swine growth performance under commercial production conditions in Vietnam. Animals 2022, 12, 3200.
- Kang, M.; Oh, J.Y.; Cha, S.Y.; Kim, W.I.; Cho, H.S.; Jang, H.K. Efficacy of polymers from spontaneous carotenoid oxidation in reducing necrotic enteritis in broilers. Poult. Sci. 2018, 97, 3058–3062.
- McDougall, S. Evaluation of fully oxidised β-carotene as a feed ingredient to reduce bacterial infection and somatic cell counts in pasture-fed cows with subclinical mastitis. N.Z. Vet. J. 2021, 69, 285–293.
- Khadem, A.; Soler, L.; Everaert, N.; Niewold, T.A. Growth promotion in broilers by both oxytetracycline and Macleaya cordata extract is based on their anti-inflammatory properties. Br. J. Nutr. 2014, 112, 1110–1118.
- Niewold, T.A. Organic more healthy? Green shoots in a scientific semi-desert. Br. J. Nutr. 2010, 103, 627–628.
- Soler, L.; Miller, I.; Hummel, K.; Razzazi-Fazeli, E.; Jessen, F.; Escribano, D.; Niewold, T. Growth promotion in pigs by oxytetracycline coincides with down regulation of serum inflammatory parameters and of hibernation-associated protein HP-27. Electrophoresis 2016, 37, 1277–1286.
- ATBC Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035.
- Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Meyskens, F.L., Jr.; Omenn, G.S.; Valanis, B.; Williams, J.H., Jr. The beta-carotene and retinol efficacy trial: Incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J. Natl. Cancer Inst. 2004, 96, 1743–1750.
- Omenn, G.S.; Goodman, G.; Thornquist, M.; Grizzle, J.; Rosenstock, L.; Barnhart, S.; Balmes, J.; Cherniack, M.G.; Cullen, M.R.; Glass, A.; et al. The beta-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high risk populations: Smokers and asbestos-exposed workers. Cancer Res. 1994, 54, 2038s–2043s.
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L., Jr.; Valanis, B.; Williams, J.H., Jr.; et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J. Natl. Cancer Inst. 1996, 88, 1550–1559.
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a Combination of Beta Carotene and Vitamin A on Lung Cancer and Cardiovascular Disease. New Engl. J. Med. 1996, 334, 1150–1159.
- Virtamo, J.; Taylor, P.R.; Kontto, J.; Mannisto, S.; Utriainen, M.; Weinstein, S.J.; Huttunen, J.; Albanes, D. Effects of alpha-tocopherol and beta-carotene supplementation on cancer incidence and mortality: 18-year postintervention follow-up of the Alpha-tocopherol, Beta-carotene Cancer Prevention Study. Int. J. Cancer 2014, 135, 178–185.
- European Food Safety Authority. Scientific Opinion on the re-evaluation of mixed carotenes (E 160a (i)) and beta-carotene (E 160a (ii)) as a food additive. EFSA Panel on Food Additives and Nutrient Sources added to Food. EFSA J. 2012, 10, 2593.
- Burton, G.W.; Mogg, T.J.; Riley, W.W.; Nickerson, J.G. beta-Carotene oxidation products—Function and safety. Food Chem. Toxicol. 2021, 152, 112207.
This entry is offline, you can click here to edit this entry!
