Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cell spheroids (CSs) are three-dimensional models in vitro that have a microenvironment similar to tissues. Such three-dimensional cellular structures are of great interest in the field of nano biomedical research, as they can simulate information about the characteristics of nanoparticles (NPs) by avoiding the use of laboratory animals.
- cell spheroids
- carbon nanotubes
- CNTs
- nanomedicines
- penetration
1. Introduction
The field of nanomedicine offers great opportunities for the development of new materials that can improve the therapy of various diseases [1][2]. Carbon nanotubes (CNTs) are fairly new nanomaterials that have unique properties and potential in various fields [3][4]. In particular, CNTs can broaden the horizon of biomedical research due to their important chemical, thermal, electrical, mechanical, and structural properties, which are currently of great interest. CNTs have a high modulus of elasticity and possess the properties of metallic, semiconducting, and superconducting materials [5]. Also, CNTs have a nanoarchitecture that allows both encapsulation of molecules inside and conjugation to the surface [6]. It has been shown that CNTs can be used in many applications, including biosensors [7][8], nanofluidic systems [9], biopharmaceutical applications [10], and diagnostic tools and devices in radiation oncology [11]. Unfortunately, CNTs still have no direct application in clinical settings due to the poor understanding of their biological properties and behavior in living objects [12]. In addition, in large-scale production, CNTs must also have well-characterized biological, environmental, and safety profiles. CNTs can vary significantly in size, morphology, structure, and purity depending on the method of preparation, purification, and functionalization used for their synthesis. Therefore, the interaction of CNTs with the biological environment is very complex and sometimes unpredictable, which requires an additional study on complex living systems [13].
2. Properties, Modifications, and Application of CNTs
The nanoparticles made completely of carbon are known as carbon nanomaterials (CNMs). CNMs can be divided into 0D-CNMs (i.e., fullerenes, particulate diamonds, and carbon dots), 1D-CNMs (i.e., CNTs, carbon nanofibers (CNFs), and diamond nanorods), 2D-CNMs (i.e., graphene, graphite sheets, and diamond nanoplatelets), and 3D-CNMs. All decreased dimensionalities, including fullerenes, contain CNMs made completely of sp2-bonded graphitic carbon. All of the materials presented above can also be used for nano-biomedical applications, as evidenced by already existing scientific work [14][15][16][17].
Carbon nanotubes are an allotropic form of carbon. CNTs are well-ordered, high-aspect-ratio hollow graphite rods that were identified by Iijima in 1991 [18]. Since then, CNTs have been widely used in many areas, including electrode materials [19], nanoelectronics components [20], biosensors [21], strengthening of materials [22], and as components of biomaterials for drug delivery or other types of therapy [23][24][25][26]. The synthesis of CNTs is a broad topic and will not be described here in detail; however, it should be noted that the most used methods are electric-arc discharge [27], laser ablation [28], and the wide family of catalytic chemical vapor deposition (CCVD) methods [29]. A CNTs can be described as a rolled layer of graphene that can be opened and closed at the ends with fullerene caps [30].
One of the most important parameters of the CNTs is the number of concentric walls (Figure 1). The number of walls primarily determines the diameter of the CNTs. For example, single-walled carbon nanotubes (SWCNTs) have a small diameter (usually 1–2 nm), while multi-walled carbon nanotubes (MWCNTs) reach a diameter of up to 100 nm. Ref. [31] However, an increase in the number of walls also increases the number of defects, thus facilitating their modification and functionalization. Double-walled carbon nanotubes (DWCNTs) are located in the middle and are also quite promising since the diameter is still quite small, mechanical properties and electrical conductivity remain high due to the inner layer, but their surface modification is also possible due to the second wall [32].
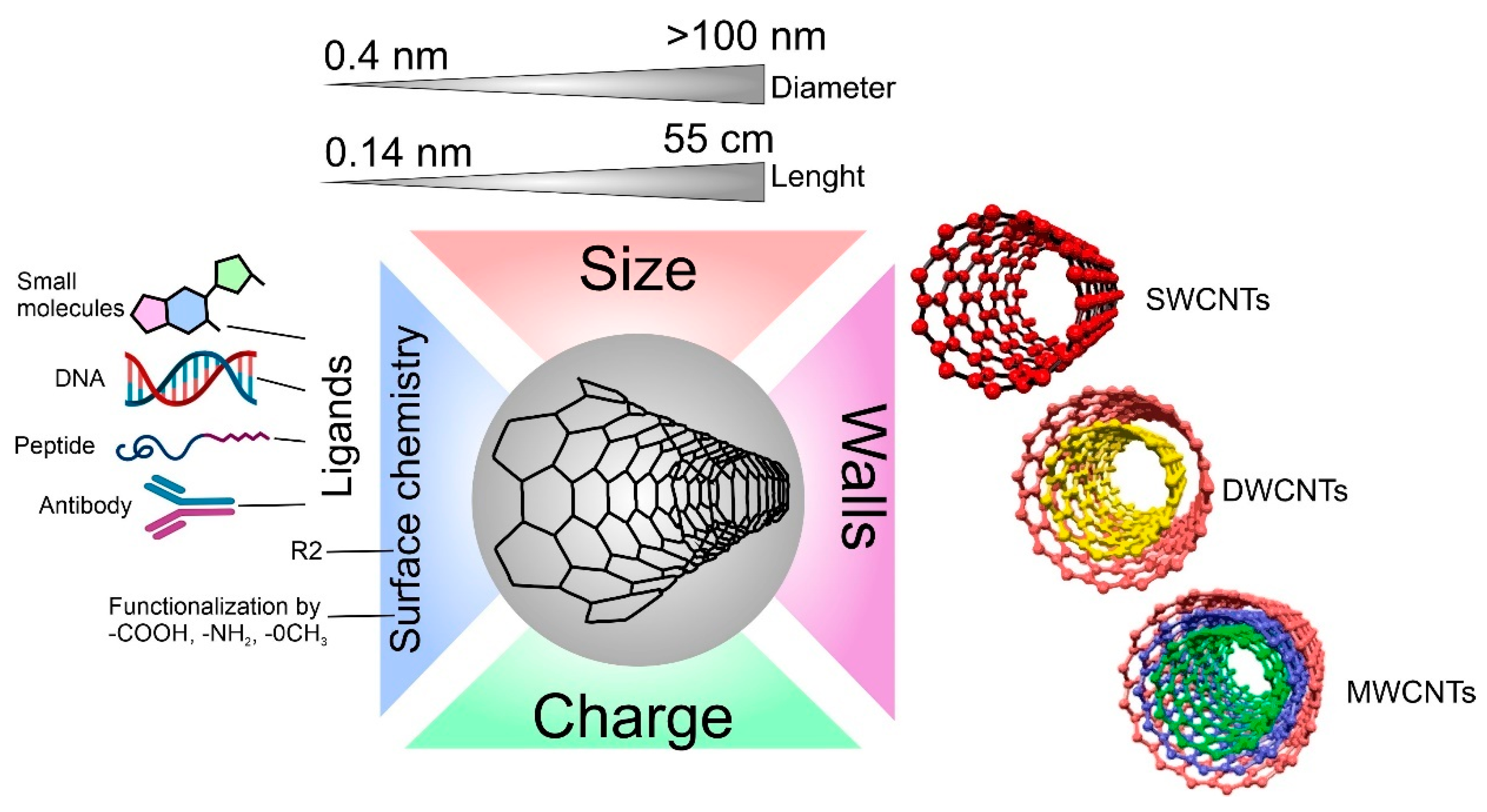
Figure 1. Carbon nanotube properties are important for interaction with cells.
The difference in the number of walls in CNTs can also affect cell viability in different ways [33]. For example, it has already been described that the difference in the cytotoxic effect on cells between single-walled and multi-walled CNTs is quite large [34]. It is hypothesized that MWCNTs lead to the production of reactive oxygen species (ROS), causing inflammation, while SWCNTs increase oxidative stress through damage to mitochondria [35]. Additionally, the difference in toxicity between multi-wall and single-wall CNTs is associated with their hydrophobic–hydrophobic interaction of MWCNTs with the cell membrane and following hole formation and loss of the plasma membrane integrity [36].
When synthesizing CNTs, parameters such as the diameter and length of the CNTs can be tuned; for example, it can be tuned by different flow rates and flow duration of the carbon precursor gas (C2H2) on the growth of CNTs by a thermal CVD method [37]. By changing the length of the carbon tubes, researchers change the specific area, which can be a very important parameter when using CNTs to load and deliver molecules [38]. However, the length and diameter of the CNTs also influence the degree of toxicity of the CNTs in vivo and in vitro [39]. It is proven that with an increase in the length of CNTs, the toxic effect also increases. This is because macrophages can more easily envelop CNTs with a shorter length [40][41].
Another key parameter, both for the physicochemical properties of CNTs and biocompatibility, is the surface chemistry of CNTs. Surface chemistry determines properties such as charge, hydrophobicity [42], photocatalytic activity [43], and the ability to bind to various biological molecules (one of the most important factors for the formation of a protein crown and connection with cells) [44]. Each of these factors can affect both in vitro co-localization and in vivo biodistribution [45][46]. One of the methods for functionalizing the surface of CNTs with groups is plasma treatment [47]. The advantage of plasma treatment is that it does not pollute the environment and provides a wide range of functional groups depending on the plasma parameters. Fine-tuning of the surface is achieved by changing the plasma processing parameters such as power, gases used, processing time, and gas pressure [48]. Surface functionalization of CNTs can provide good targeting to the desired cell type, such as surface functionalization with antibodies that selectively bind to the desired receptors (e.g., EGFR) on cancer cells. Such functionalization technologies are widely used in radioactivity and drug-delivery systems [49].
Depending on the properties of CNTs, they find various applications for biomedical purposes. Some of the most obvious applications of CNTs are molecule delivery [50], photothermal therapy [51], use as biosensors [52], and as a component for the synthesis of hybrid materials for tissue engineering [53]. The choice of a molecule for delivery and its loading/conjugation primarily depends on the purpose of delivery. It can be peptides, nucleic acids, therapeutic molecules, etc.
Peptide delivery has already been demonstrated using the foot-and-mouth disease virus (FMDV) B-cell epitope, which was covalently bound to amino groups on the surface of CNTs. After conjugation, the peptides adopt a suitable secondary structure and can be recognized by specific monoclonal and polyclonal antibodies. Immunization of mice with FMDV peptide-nanotube conjugates induced a high humoral response compared to the free peptide. Similar results indicate the possibility of using carbon nanotubes as components for vaccines [54]. Delivery of nucleic acids using CNTs is also possible. This direction is extremely promising. For example, by functionalizing the surface with ammonium, nucleic acids bind to the surface of the CNT via electrostatic interaction [55]. The search for new and effective delivery systems for therapeutic agents also suggests the possibility of using CNTs as a carrier. Anti-cancer drugs such as doxorubicin (DOX) successfully bind to the surface of CNTs via π-π stacking, making the CNTs–DOX conjugation the basis of CNT-based drug delivery systems for the delivery of DOX to cancer cells [56].
CNTs can also be used for photothermal therapy, as they have excellent optical absorption in the visible and near-infrared sectors. When irradiated with near-infrared light, the local temperature of the tissues in which the CNTs are located rises to 40–45 °C and kills the cells that are within the heating radius [51]. Induction of high temperature for sufficient time causes physical damage such as protein denaturation and membrane lysis and can increase oxidative stress, eventually causing coagulative necrosis or apoptosis. The wide electromagnetic absorption spectrum of CNTs creates exceptional properties compared to other plasmon-heated nanomaterials (e.g., gold nanoshells and nanorods), which depend on the size and shape of CNTs [57]. Studies show that CNTs can achieve thermal destruction using tenfold-lower doses in solution and using threefold-lower laser power than that required for gold nanorods, and these also indicate that MWCNTs are more potent than bulk single-walled nanotubes in transferring the NIR light into heat.
Currently, the scientific community has identified three possible mechanisms of CNT cell toxicity. The first is based on irreparable mechanical damage to the membrane (cellular or nuclear) [58]. It is very likely that endocytosis, phagocytosis, or nanopermeasurement, which are the main ways in which the nanomaterial interacts with the lipid membrane, are strictly dependent on the geometry of the CNTs, especially their length [59]. The next putative mechanism of toxicity is oxidative stress, resulting from an increase in reactive oxygen species (ROS) and leading to numerous side effects in the cell, such as apoptosis, necrosis, cytochrome c release, oxidative DNA damage, reduced proliferation, inhibition of cell growth, etc. [58]. The last mechanism, the mechanism of genotoxicity, is in one way or another associated with DNA damage, characterized by a wide spectrum: CNTs interaction with proteins involved in chromosome aberration; CNTs effect on the mitotic spindle, micronuclei formation, indirect DNA oxidation, DNA breakage, etc. Although the toxic mechanisms of CNTs have been studied from several perspectives, there is still a strong correlation between triggered or inhibited molecular pathways and cell types [60]. Despite the described complexity of the processes occurring inside the cells targeted by CNTs, some scientific works suggest ways to overcome the toxic effects of CNTs by modifying the material surface with functionalizing groups, coating with metal oxides, or protein attachment. For example, coating with recombinant C1q, which is a protein that activates the classical pathway of the complement system involved in the innate immune system, is a promising approach to regulating inflammation. In addition, several theoretical studies on modeling a possible cellular response to CNTs demonstrate the mechanical interaction of nanotubes with the lipid layer or with proteins, suggesting a safer geometry of CNTs, which furthers the understanding of the action of CNTs on cells [61].
3. Properties, Fabrication, and Application of CSs
The use of cell cultures is the first step in biomaterial development, research, and clinical activities. There are a huge number of methods that are used to determine the cellular condition and behavior, for example, when exposed to cytostatics or on biomaterials surface [62]. However, in most scientific and research works for such tests, 2D cultures are used, i.e., cells located on the surface of the culture plastic and forming a monolayer [63]. However, we are in a three-dimensional world and consist of tissues, which in turn are also three-dimensional. Due to this three-dimensionality, tissues in the body have a large number of gradients, which can be mechanical [64], chemical [65], electrical [66], etc. [67]. Such gradients are practically impossible to obtain in 2D cultures, which makes them suitable but extremely distant from real biological objects. In this regard, the direction of studying 3D CSs has been actively developed in recent years [68]. Such 3D CSs can have properties much closer to real tissues and successfully fill the gap between cell culture and laboratory animals, which makes their use significant for biological research [69].
One important purpose of using an in vitro cell testing system is to replicate the cell microenvironment (Figure 2) [70]. For example, cells within a tissue are surrounded by neighboring cells and an extracellular matrix, which constantly provide the cells with biochemical and mechanical signals [71]. This 3D network of cell–cell and cell–ECM interactions maintains the specificity and homeostasis of a particular tissue [72]. As a result, testing and detection of interactions between NPs and 2D cultures cannot be called reliable since the tissue-specific properties characteristic of these cells in a 3D environment are lost. The complex 3D network of the tissue microenvironment influences not only the penetration and distribution of CNTs but also the function of many physiological factors [73]. In addition, conventional animal testing often fails to predict the actual efficacy of a therapeutic agent in humans because the cells, microenvironment, and physiology of animals differ from those of humans. This species gap can be bridged by culturing human cells in 3D. It is also necessary to understand that tissues have their specific properties, such as the size of the intercellular space [74], tissue stiffness [75], cell density [76], phagocytic function [77], and cell morphology [68]. As in solid tumors, cells in spheroids form layers; the outer layer consists of proliferating cells, followed by a layer of senescent cells. In the very center is the necrotic core. This gradient in cell survival and proliferation depends on the availability of nutrients and oxygen [78].
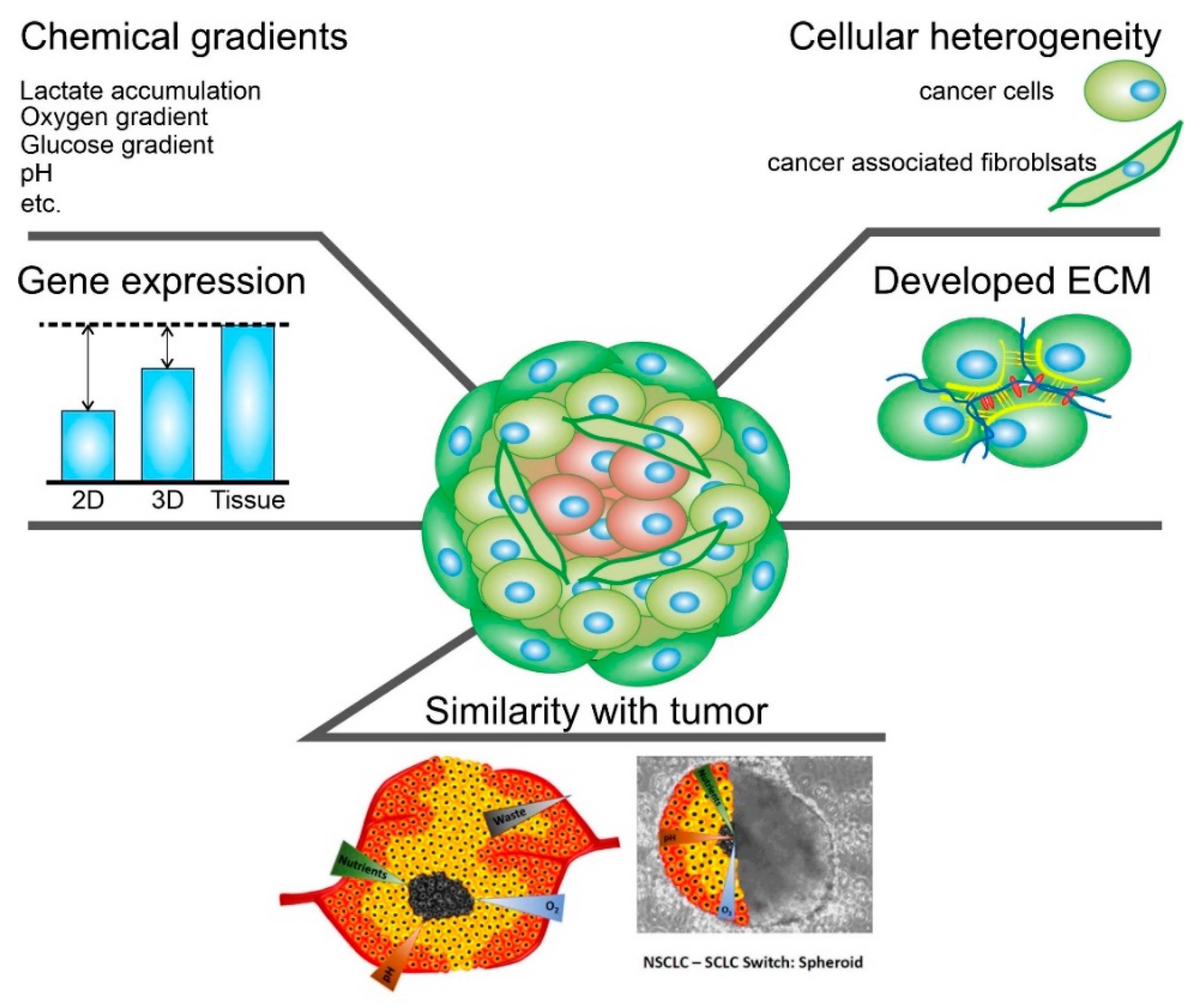
Figure 2. Schematic representation of the main characteristics of 3D spheroids that are crucial.
Intercellular contacts inside spheroids are much more complicated than in 2D cultures. Cells deposit ECM components such as collagen, laminin, fibronectin, proteoglycans, tenascin, etc. There are also a large number of intercellular compounds; for example, α5- and β1-integrin, E-cadherins are a barrier to cytostatic molecules [79].
The spheroids can be formed from one or more cell types, such as breast cancer cells and fibroblasts, endothelial cells, and immune cells. In this way, cellular heterogeneity, which is present in normal and oncological tissues, can be achieved [80][81].
Optimizing spheroids for nanoparticle testing, in particular CNTs, is one of the important aspects of working with 3D cultures [82]. The cells used in the experiments should be in culture from 1 to 20 passages. The cells should be kept in a humidified incubator at 37 °C and 5% CO2. Standard culture medium should be used for cultivation. Cells should have a 70–80% fill rate. Cultures should be transplanted with trypsin/EDTA solution (0.05% (wt/vol) trypsin and 0.02% (wt/vol) EDTA). There are several methods for creating cell spheroids, the hanging drop method [83], ultra non-adhesive well plates [83], magnetic nanoparticles [84], incubation in hydrogels [85], and the use of bioreactors. For CNTs testing, the first three methods are the most optimal since they are the easiest to use in all laboratories and have the smallest variation in the size of the spheroids. To create a spheroid 400 µm in diameter on the fourth day of formation, the desired concentration and cell proliferation rate must be determined. For this purpose, spheroids are formed from different numbers of cells (from 250 to 3000 cells/spheroid in the case of ultra non-adhesive plates, magnetic nanoparticles, and suspended droplet method). When creating spheroids, it is recommended to use a multichannel pipette, which will reduce the standard deviation among the spheroid diameters to 5% in one plate and 10% in different experiments. Cells form a spheroid within 96 h in a CO2 incubator at 37 °C. A phase-contrast microscope with 5x and 10x lenses is used to determine the size of the spheroid. The microscope is used to assess the integrity, diameter, volume and roundness of the spheroid. Once the optimal number of cells has been determined, it is possible to proceed with CNTs testing. This requires titration of CNTs and making 2x solutions of the substances tested. After that, 50 µL of the medium must be removed from the plate where the spheroids are formed, and 50 µL of the test solution must be added, thus making a concentration of CNTs of the desired concentration. The spheroids can then be incubated with the CNTs for the desired time.
4. Mechanism of CNTs Uptake by Cells and Spheroids
The ECM that surrounds the cells serves as a good barrier to the penetration of therapeutic agents and NPs, including CNTs [86]. There are two types of transport of molecules and NPs into the spheroid: transcellular and diffusion through the extracellular matrix [87]. In the first case, the cells must absorb the carriers and pass them on to each other until the carrier reaches the cells of the necrotic nucleus. In the second case, CNTs must pass into the extracellular space, which usually has a size of 25–500 nm (Figure 3) [88]. In both cases, it depends on two parameters: the type of tissue and the properties of CNTs. It is worth paying attention not only to the properties of CNTs but also to the properties of other models NPs that have already been studied for penetration into tumor spheroids. The main properties of nano- and micro-sized objects that can be absorbed by cells are their size, shape, charge, surface chemistry, and rigidity [89].
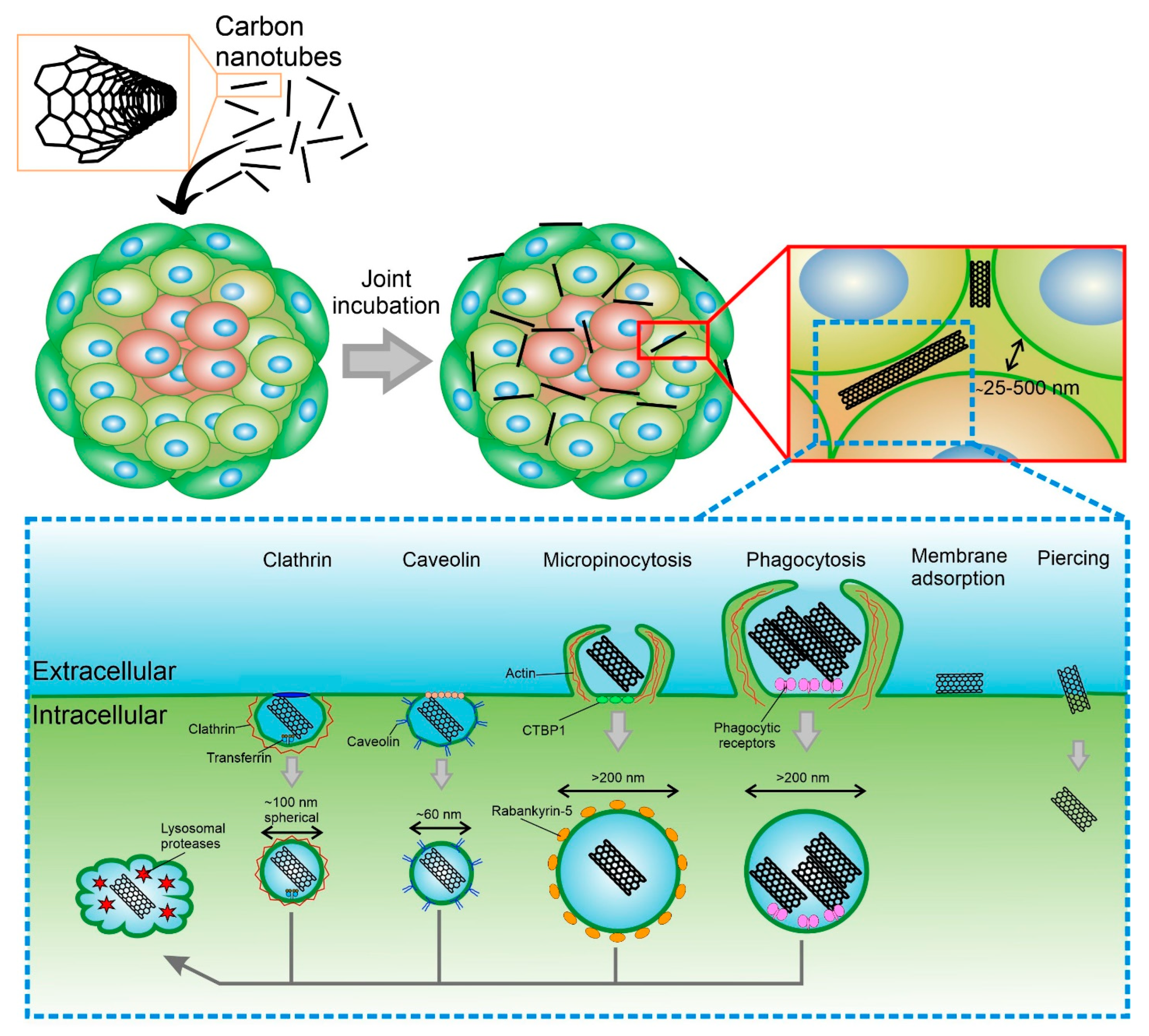
Figure 3. Overview of the primary mechanisms of uptake CNTs into cellular spheroid and cell.
There is now agreement in the literature that smaller particles penetrate spheroids faster. This has been tested with particles and spheroids of various types. CNTs are highly anisotropic objects with diameters ranging from ∼0.4 to ∼100 nm and lengths from ∼0.14 nm to ∼55 cm, so it is difficult to compare them with existing models. However, it is known that when 50 and 100 nm gold NPs penetrate for 24 h, 50 nm NPs penetrate deeper [90]. Similar results depending on the size could be obtained when the spheroids were immobilized in the “tumor-on-chip” system. This system made it possible to analyze the penetration of NPs in combination with real-time observation of the accumulation of NPs. Small spherical PEG-coated NPs (40 and 70 nm) rapidly accumulated in MDA-MB-435 spheroids and accumulated in the interstitial space, while larger NPs (110 and 150 nm) were more and more rejected from accumulation in the tumor [91]. Although the rule is clear that smaller particles have better penetration, no clear upper limit has been reported so far that would lead to the complete exclusion of particles from spheroid models, although there are indications that penetration becomes low after sizes larger than 1000 nm.
The next important feature is the shape of the particles. As mentioned earlier, CNTs are highly anisotropic particles. However, it has been previously repeatedly demonstrated that elongated small particles enter 2D cell culture much better than spheres. The results of the study show that the rate of internalization increases as the aspect ratio increases. If an equal number of particles are added per cell, then the total volume of internalized particles increases with the volume of individual particles [92]. However, as said above, a 2D system is very different from a 3D. Jiacheng Zhao et al. in their work describe particles from poly(1-O-methacryloyl-β-d-fructopyranose)-b-poly(methyl methacrylate) having the shape of spheres (diameter 30 nm), rods (diameter 30, length 120), and carriers (hollow sphere 160 nm in diameter). The study showed that there is no difference between the passage of spheres and rods into the spheroids, and both types of particles enter the spheroid at the same speed, unlike carriers [93].
CNTs can be internalized both by the outer layers of cells and by cells that are closer to the center of the spheroid. When NPs are ingested by cells, including CNTs, there are several types of internalization: active (energy-dependent), passive (energy-independent), and diffusion. The active pathway of CNTs’ internalization through the cell membrane occurs by endocytosis. In the case of endocytosis, CNTs enter cells inside vesicles (endosomes), and then they are gradually transported to the perinucleolar space, becoming lysosomes [94]. Studies related to the selective inhibition of endocytosis pathways showed that CNTs internalization includes several pathways, such as macropinocytosis, caveolae-mediated endocytosis, and clathrin-dependent endocytosis [95]. The results show that macropinocytosis is the main mechanism of internalization of SWCNTs, while clathrin-mediated endocytosis is length-dependent and relatively important for the shortest CNTs. Phagocytosis allows the uptake of CNTs longer than 1 μm and conglomerates, as well as microsized composite particles with CNTs embedded in their structure [96][97]. When cells were incubated with CNTs at 4 °C, the internalization of particles was strongly reduced because low-temperature blocks all types of endocytosis. It is also known that the contact of CNTs with the cell membrane occurs from the tip [98]. For nanotubes with end caps or a carbon sheath at the ends, the uptake process involves tip recognition via receptor binding, rotation induced by asymmetric elastic deformation at the tube-bilayer interface, and finally, penetration into the cell in a nearly vertical direction. For nanotubes without caps and sheaths on their ends, the needle entry mode is not realized.
Passive diffusion of CNTs is not dependent on temperature or endocytosis, as the particles simply penetrate through the lipid bilayer [99]. It is already known that CNTs functionalized with amino acids can easily penetrate the cell without entering the lysosome. Removal of CNTs involves processes of exocytosis and enzymatic degradation. It has been reported that CNTs are displaced from cells by exocytosis several hours or days after internalization [99].
This entry is adapted from the peer-reviewed paper 10.3390/c8040056
References
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer-and hybrid-based biomaterials for interstitial, connective, vascular, nerve, visceral and musculoskeletal tissue engineering. Polymers 2020, 12, 620.
- Saveleva, M.S.; Eftekhari, K.; Abalymov, A.; Douglas, T.E.L.; Volodkin, D.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of Hybrid Materials—The Place of Inorganics-in-Organics in it, Their Composition and Applications. Front. Chem. 2019, 7, 179.
- Zhang, Q.; Huang, J.-Q.; Zhao, M.-Q.; Qian, W.-Z.; Wei, F. Carbon Nanotube Mass Production: Principles and Processes. ChemSusChem 2011, 4, 864–889.
- Prajapati, S.K.; Malaiya, A.; Kesharwani, P.; Soni, D.; Jain, A. Biomedical applications and toxicities of carbon nanotubes. Drug Chem. Toxicol. 2022, 45, 435–450.
- Rahman, G.; Najaf, Z.; Mehmood, A.; Bilal, S.; Shah, A.; Mian, S.; Ali, G. An Overview of the Recent Progress in the Synthesis and Applications of Carbon Nanotubes. C 2019, 5, 3.
- Bors, L.; Erdő, F. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. Sci. Pharm. 2019, 87, 6.
- Hwang, H.S.; Jeong, J.W.; Kim, Y.A.; Chang, M. Carbon Nanomaterials as Versatile Platforms for Biosensing Applications. Micromachines 2020, 11, 814.
- Menaa, F.; Fatemeh, Y.; Vashist, S.K.; Iqbal, H.; Sharts, O.N.; Menaa, B. Graphene, an Interesting Nanocarbon Allotrope for Biosensing Applications: Advances, Insights, and Prospects. Biomed. Eng. Comput. Biol. 2021, 12, 117959722098382.
- Zheng, G.; Zhang, Y.; Zhang, L.; Qian, L.; Cai, Y.; Lv, H.; Kang, X.; Guo, D.; Wang, X.; Huang, J.; et al. Evaluation of a micro/nanofluidic chip platform for diagnosis of central nervous system infections: A multi-center prospective study. Sci. Rep. 2020, 10, 1568.
- Rode, A.; Sharma, S.; Mishra, D.K. Carbon Nanotubes: Classification, Method of Preparation and Pharmaceutical Application. Curr. Drug Deliv. 2018, 15, 620–629.
- Sheikhpour, M.; Naghinejad, M.; Kasaeian, A.; Lohrasbi, A.; Shahraeini, S.S.; Zomorodbakhsh, S. The Applications of Carbon Nanotubes in the Diagnosis and Treatment of Lung Cancer: A Critical Review. Int. J. Nanomed. 2020, 15, 7063–7078.
- Heller, D.A.; Jena, P.V.; Pasquali, M.; Kostarelos, K.; Delogu, L.G.; Meidl, R.E.; Rotkin, S.V.; Scheinberg, D.A.; Schwartz, R.E.; Terrones, M.; et al. Banning carbon nanotubes would be scientifically unjustified and damaging to innovation. Nat. Nanotechnol. 2020, 15, 164–166.
- Baibarac, M.; Gómez-Romero, P. Nanocomposites Based on Conducting Polymers and Carbon Nanotubes: From Fancy Materials to Functional Applications. J. Nanosci. Nanotechnol. 2006, 6, 289–302.
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978.
- Roy, R.K.; Lee, K.-R. Biomedical applications of diamond-like carbon coatings: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83, 72–84.
- Ðorđević, L.; Arcudi, F.; Cacioppo, M.; Prato, M. A multifunctional chemical toolbox to engineer carbon dots for biomedical and energy applications. Nat. Nanotechnol. 2022, 17, 112–130.
- Xue, Y.; Feng, X.; Roberts, S.C.; Chen, X. Diamond and carbon nanostructures for biomedical applications. Funct. Diam. 2021, 1, 221–242.
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58.
- De, B.; Banerjee, S.; Verma, K.D.; Pal, T.; Manna, P.K.; Kar, K.K. Carbon Nanotube as Electrode Materials for Supercapacitors. In Handbook of Nanocomposite Supercapacitor Materials II; Springer: Berlin/Heidelberg, Germany, 2020; pp. 229–243.
- Dmitriev, V.; Gomes, F.; Nascimento, C. Nanoelectronic Devices Based on Carbon Nanotubes. J. Aerosp. Technol. Manag. 2015, 7, 53–62.
- Tîlmaciu, C.-M.; Morris, M.C. Carbon nanotube biosensors. Front. Chem. 2015, 3.
- Lin, D.; Saei, M.; Suslov, S.; Jin, S.; Cheng, G.J. Super-strengthening and stabilizing with carbon nanotube harnessed high density nanotwins in metals by shock loading. Sci. Rep. 2015, 5, 15405.
- Abalymov, A.; Van der Meeren, L.; Volodkin, D.; Parakhonskiy, B.; Skirtach, A.G. Carbon Nanotubes Transform Soft Gellan Gum Hydrogels into Hybrid Organic–Inorganic Coatings with Excellent Cell Growth Capability. C 2021, 7, 18.
- Kaur, J.; Gill, G.S.; Jeet, K. Applications of Carbon Nanotubes in Drug Delivery. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–135.
- Lin, Z.; Jiang, B.P.; Liang, J.; Wen, C.; Shen, X.C. Phycocyanin functionalized single-walled carbon nanohorns hybrid for near-infrared light-mediated cancer phototheranostics. Carbon N. Y. 2019, 143, 814–827.
- Gao, C.; Jian, J.; Luo, L.; Liang, J.; Li, Z.; Pang, M.; Cai, H.; Shen, X.-C. Single-walled carbon nanohorns-based smart nanotheranostic: From phototherapy to enzyme-activated fluorescence imaging-guided photodynamic therapy. J. Colloid Interface Sci. 2022, 628, 273–286.
- Sharma, R.; Sharma, A.K.; Sharma, V. Synthesis of carbon nanotubes by arc-discharge and chemical vapor deposition method with analysis of its morphology, dispersion and functionalization characteristics. Cogent Eng. 2015, 2, 1094017.
- Chrzanowska, J.; Hoffman, J.; Małolepszy, A.; Mazurkiewicz, M.; Kowalewski, T.A.; Szymanski, Z.; Stobinski, L. Synthesis of carbon nanotubes by the laser ablation method: Effect of laser wavelength. Phys. Status Solidi 2015, 252, 1860–1867.
- DUPUIS, A. The catalyst in the CCVD of carbon nanotubes—A review. Prog. Mater. Sci. 2005, 50, 929–961.
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis—Review. J. Mater. Chem. 2011, 21, 15872.
- Laurent, C.; Flahaut, E.; Peigney, A. The weight and density of carbon nanotubes versus the number of walls and diameter. Carbon N. Y. 2010, 48, 2994–2996.
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A review on carbon nanotube: An overview of synthesis, properties, functionalization, characterization, and the application. Mater. Sci. Eng. B 2021, 268, 115095.
- Chetyrkina, M.R.; Fedorov, F.S.; Nasibulin, A.G. In vitro toxicity of carbon nanotubes: A systematic review. RSC Adv. 2022, 12, 16235–16256.
- Pacurari, M.; Castranova, V.; Vallyathan, V. Single- and Multi-Wall Carbon Nanotubes Versus Asbestos: Are the Carbon Nanotubes a New Health Risk to Humans? J. Toxicol. Environ. Heal. Part A 2010, 73, 378–395.
- Nahle, S.; Foliguet, B.; Lovera, M.; Zahra, L.; Rihn, B.; Ferrari, L.; Faou, A. Le Single wall and multiwall carbon nanotubes induce different toxicological responses in rat alveolar macrophages. J. Appl. Toxicol. 2019, 39, 764–772.
- Zeinabad, H.A.; Zarrabian, A.; Saboury, A.A.; Alizadeh, A.M.; Falahati, M. Interaction of single and multi wall carbon nanotubes with the biological systems: Tau protein and PC12 cells as targets. Sci. Rep. 2016, 6, 26508.
- Tripathi, N.; Mishra, P.; Harsh, H.; Islam, S.S. Fine-tuning control on CNT diameter distribution, length and density using thermal CVD growth at atmospheric pressure: An in-depth analysis on the role of flow rate and flow duration of acetylene (C2H2) gas. Appl. Nanosci. 2015, 5, 19–28.
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Release 2016, 238, 176–185.
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Aschberger, K.; Stone, V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physico-chemical characteristics. Nanotoxicology 2010, 4, 207–246.
- Kavosi, A.; Hosseini Ghale Noei, S.; Madani, S.; Khalighfard, S.; Khodayari, S.; Khodayari, H.; Mirzaei, M.; Kalhori, M.R.; Yavarian, M.; Alizadeh, A.M.; et al. The toxicity and therapeutic effects of single-and multi-wall carbon nanotubes on mice breast cancer. Sci. Rep. 2018, 8, 8375.
- Sato, Y.; Yokoyama, A.; Shibata, K.; Akimoto, Y.; Ogino, S.; Nodasaka, Y.; Kohgo, T.; Tamura, K.; Akasaka, T.; Uo, M.; et al. Influence of length on cytotoxicity of multi-walled carbon nanotubes against human acute monocytic leukemia cell line THP-1 in vitro and subcutaneous tissue of rats in vivo. Mol. Biosyst. 2005, 1, 176.
- Pu, J.; Wan, S.; Lu, Z.; Zhang, G.; Wang, L.; Zhang, X.; Xue, Q. Controlled water adhesion and electrowetting of conducting hydrophobic graphene/carbon nanotubes composite films on engineering materials. J. Mater. Chem. A 2013, 1, 1254–1260.
- Nguyen, K.C.; Ngoc, M.P.; Nguyen, M. Van Enhanced photocatalytic activity of nanohybrids TiO2/CNTs materials. Mater. Lett. 2016, 165, 247–251.
- Cai, R.; Chen, C. The Crown and the Scepter: Roles of the Protein Corona in Nanomedicine. Adv. Mater. 2019, 31, 1805740.
- Marangon, I.; Boggetto, N.; Ménard-Moyon, C.; Luciani, N.; Wilhelm, C.; Bianco, A.; Gazeau, F. Localization and Relative Quantification of Carbon Nanotubes in Cells with Multispectral Imaging Flow Cytometry. J. Vis. Exp. 2013.
- Jacobsen, N.R.; Møller, P.; Clausen, P.A.; Saber, A.T.; Micheletti, C.; Jensen, K.A.; Wallin, H.; Vogel, U. Biodistribution of Carbon Nanotubes in Animal Models. Basic Clin. Pharmacol. Toxicol. 2017, 121, 30–43.
- Chen, C.; Ogino, A.; Wang, X.; Nagatsu, M. Plasma treatment of multiwall carbon nanotubes for dispersion improvement in water. Appl. Phys. Lett. 2010, 96, 131504.
- Ruelle, B.; Bittencourt, C.; Dubois, P. Surface treatment of carbon nanotubes via plasma technology. In Polymer–Carbon Nanotube Composites; Elsevier: Amsterdam, The Netherlands, 2011; pp. 25–54.
- Spinato, C.; Perez Ruiz de Garibay, A.; Kierkowicz, M.; Pach, E.; Martincic, M.; Klippstein, R.; Bourgognon, M.; Wang, J.T.-W.; Ménard-Moyon, C.; Al-Jamal, K.T.; et al. Design of antibody-functionalized carbon nanotubes filled with radioactivable metals towards a targeted anticancer therapy. Nanoscale 2016, 8, 12626–12638.
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H. Carbon materials for drug delivery & cancer therapy. Mater. Today 2011, 14, 316–323.
- Sobhani, Z.; Behnam, M.A.; Emami, F.; Dehghanian, A.; Jamhiri, I. Photothermal therapy of melanoma tumor using multiwalled carbon nanotubes. Int. J. Nanomed. 2017, 12, 4509–4517.
- Yang, N.; Chen, X.; Ren, T.; Zhang, P.; Yang, D. Carbon nanotube based biosensors. Sens. Actuators B Chem. 2015, 207, 690–715.
- Veetil, J.V.; Ye, K. Tailored carbon nanotubes for tissue engineering applications. Biotechnol. Prog. 2009, 25, 709–721.
- Pantarotto, D.; Partidos, C.D.; Hoebeke, J.; Brown, F.; Kramer, E.; Briand, J.-P.; Muller, S.; Prato, M.; Bianco, A. Immunization with Peptide-Functionalized Carbon Nanotubes Enhances Virus-Specific Neutralizing Antibody Responses. Chem. Biol. 2003, 10, 961–966.
- Bianco, A.; Kostarelos, K.; Prato, M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005, 9, 674–679.
- Yaghoubi, A.; Ramazani, A. Anticancer DOX delivery system based on CNTs: Functionalization, targeting and novel technologies. J. Control. Release 2020, 327, 198–224.
- Behnam, M.A.; Emami, F.; Sobhani, Z.; Koohi-Hosseinabadi, O.; Dehghanian, A.R.; Zebarjad, S.M.; Moghim, M.H.; Oryan, A. Novel Combination of Silver Nanoparticles and Carbon Nanotubes for Plasmonic Photo Thermal Therapy in Melanoma Cancer Model. Adv. Pharm. Bull. 2018, 8, 49–55.
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon Nanotubes in Biomedical Applications: Factors, Mechanisms, and Remedies of Toxicity. J. Med. Chem. 2016, 59, 8149–8167.
- Firme, C.P.; Bandaru, P.R. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 245–256.
- Dong, J.; Ma, Q. Advances in mechanisms and signaling pathways of carbon nanotube toxicity. Nanotoxicology 2015, 9, 658–676.
- Phillips, A.; Borry, P.; Van Hoyweghen, I.; Vears, D.F. Disclosure of genetic information to family members: A systematic review of normative documents. Genet. Med. 2021, 23, 2038–2046.
- Inayat-Hussain, S.; Rajab, N.F.; Siew, E.L. Inayat-Hussain, S.; Rajab, N.F.; Siew, E.L. In vitro testing of biomaterials toxicity and biocompatibility. In Cellular Response to Biomaterials; Elsevier: Amsterdam, The Netherlands, 2009; pp. 508–537.
- Moll, J.; Colombo, R. (Eds.) Target Identification and Validation in Drug Discovery; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 986, ISBN 978-1-62703-310-7.
- Li, C.; Ouyang, L.; Armstrong, J.P.K.; Stevens, M.M. Advances in the Fabrication of Biomaterials for Gradient Tissue Engineering. Trends Biotechnol. 2021, 39, 150–164.
- Ruardy, T.G.; Schakenraad, J.M.; van der Mei, H.C.; Busscher, H.J. Preparation and characterization of chemical gradient surfaces and their application for the study of cellular interaction phenomena. Surf. Sci. Rep. 1997, 29, 3–30.
- Machnoor, M.; Iseri, E.; Shao, A.; Paknahad, J.; Gokoffski, K.K.; Lazzi, G. An Efficient, Large-Gradient, Electrical Stimulation System to Promote Directional Neural Growth. In Proceedings of the 2021 International Conference on Electromagnetics in Advanced Applications (ICEAA), Honolulu, Hawaii, USA, 9–13 August 2021; p. 406.
- Wu, J.; Mao, Z.; Tan, H.; Han, L.; Ren, T.; Gao, C. Gradient biomaterials and their influences on cell migration. Interface Focus 2012, 2, 337–355.
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2016, 14, 910–919.
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018, 19, 181.
- Rani, B. Role of the tissue microenvironment as a therapeutic target in hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 4128.
- Clause, K.C.; Barker, T.H. Extracellular matrix signaling in morphogenesis and repair. Curr. Opin. Biotechnol. 2013, 24, 830–833.
- Sutherland, R.M. Cell and Environment Interactions in Tumor Microregions: The Multicell Spheroid Model. Science 1988, 240, 177–184.
- Lee, G.Y.; Kenny, P.A.; Lee, E.H.; Bissell, M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 2007, 4, 359–365.
- Bich, L.; Pradeu, T.; Moreau, J.-F. Understanding Multicellularity: The Functional Organization of the Intercellular Space. Front. Physiol. 2019, 10.
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370.
- McClelland, R.E.; Dennis, R.; Reid, L.M.; Stegemann, J.P.; Palsson, B.; Macdonald, J.M. Tissue Engineering. In Introduction to Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2012; pp. 273–357.
- Al-Jamal, K.T.; Nerl, H.; Müller, K.H.; Ali-Boucetta, H.; Li, S.; Haynes, P.D.; Jinschek, J.R.; Prato, M.; Bianco, A.; Kostarelos, K.; et al. Cellular uptake mechanisms of functionalised multi-walled carbon nanotubes by 3D electron tomography imaging. Nanoscale 2011, 3, 2627.
- Liu, D.; Chen, S.; Win Naing, M. A review of manufacturing capabilities of cell spheroid generation technologies and future development. Biotechnol. Bioeng. 2021, 118, 542–554.
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a Type of Three-Dimensional Cell Cultures—Examples of Methods of Preparation and the Most Important Application. Int. J. Mol. Sci. 2020, 21, 6225.
- Sinha, S.; Malmi-Kakkada, A.N.; Li, X.; Samanta, H.S.; Thirumalai, D. Spatially heterogeneous dynamics of cells in a growing tumor spheroid: Comparison between theory and experiments. Soft Matter 2020, 16, 5294–5304.
- Heiss, M.; Hellström, M.; Kalén, M.; May, T.; Weber, H.; Hecker, M.; Augustin, H.G.; Korff, T. Endothelial cell spheroids as a versatile tool to study angiogenesis in vitro. FASEB J. 2015, 29, 3076–3084.
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324.
- Parfenov, V.A.; Koudan, E.V.; Bulanova, E.A.; Karalkin, P.A.; DAS Pereira, F.; Norkin, N.E.; Knyazeva, A.D.; Gryadunova, A.A.; Petrov, O.F.; Vasiliev, M.M.; et al. Scaffold-free, label-free and nozzle-free biofabrication technology using magnetic levitational assembly. Biofabrication 2018, 10, 034104.
- Kim, J.A.; Choi, J.-H.; Kim, M.; Rhee, W.J.; Son, B.; Jung, H.-K.; Park, T.H. High-throughput generation of spheroids using magnetic nanoparticles for three-dimensional cell culture. Biomaterials 2013, 34, 8555–8563.
- Li, Y.; Kumacheva, E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci. Adv. 2018, 4.
- Lu, H.; Stenzel, M.H. Multicellular Tumor Spheroids (MCTS) as a 3D In Vitro Evaluation Tool of Nanoparticles. Small 2018, 14, 1702858.
- Cong, Z.; Zhang, L.; Ma, S.-Q.; Lam, K.S.; Yang, F.-F.; Liao, Y.-H. Size-Transformable Hyaluronan Stacked Self-Assembling Peptide Nanoparticles for Improved Transcellular Tumor Penetration and Photo–Chemo Combination Therapy. ACS Nano 2020, 14, 1958–1970.
- Wang, Y.; Bahng, J.H.; Che, Q.; Han, J.; Kotov, N.A. Anomalously Fast Diffusion of Targeted Carbon Nanotubes in Cellular Spheroids. ACS Nano 2015, 9, 8231–8238.
- Voronin, D.V.; Abalymov, A.A.; Svenskaya, Y.I.; Lomova, M.V. Key Points in Remote-Controlled Drug Delivery: From the Carrier Design to Clinical Trials. Int. J. Mol. Sci. 2021, 22, 9149.
- Huo, S.; Ma, H.; Huang, K.; Liu, J.; Wei, T.; Jin, S.; Zhang, J.; He, S.; Liang, X.-J. Superior Penetration and Retention Behavior of 50 nm Gold Nanoparticles in Tumors. Cancer Res. 2013, 73, 319–330.
- Albanese, A.; Lam, A.K.; Sykes, E.A.; Rocheleau, J.V.; Chan, W.C.W. Tumour-on-a-chip provides an optical window into nanoparticle tissue transport. Nat. Commun. 2013, 4, 2718.
- Parakhonskiy, B.; Zyuzin, M.V.; Yashchenok, A.; Carregal-Romero, S.; Rejman, J.; Möhwald, H.; Parak, W.J.; Skirtach, A.G. The influence of the size and aspect ratio of anisotropic, porous CaCO3 particles on their uptake by cells. J. Nanobiotechnology 2015, 13, 53.
- Zhao, J.; Lu, H.; Wong, S.; Lu, M.; Xiao, P.; Stenzel, M.H. Influence of nanoparticle shapes on cellular uptake of paclitaxel loaded nanoparticles in 2D and 3D cancer models. Polym. Chem. 2017, 8, 3317–3326.
- Costa, P.M.; Bourgognon, M.; Wang, J.T.-W.; Al-Jamal, K.T. Functionalised carbon nanotubes: From intracellular uptake and cell-related toxicity to systemic brain delivery. J. Control. Release 2016, 241, 200–219.
- Cui, X.; Wan, B.; Yang, Y.; Ren, X.; Guo, L.-H. Length effects on the dynamic process of cellular uptake and exocytosis of single-walled carbon nanotubes in murine macrophage cells. Sci. Rep. 2017, 7, 1518.
- Kam, N.W.S.; Dai, H. Carbon Nanotubes as Intracellular Protein Transporters: Generality and Biological Functionality. J. Am. Chem. Soc. 2005, 127, 6021–6026.
- Yashchenok, A.M.; Bratashov, D.N.; Gorin, D.A.; Lomova, M.V.; Pavlov, A.M.; Sapelkin, A.V.; Shim, B.S.; Khomutov, G.B.; Kotov, N.A.; Sukhorukov, G.B.; et al. Carbon Nanotubes on Polymeric Microcapsules: Free-Standing Structures and Point-Wise Laser Openings. Adv. Funct. Mater. 2010, 20, 3136–3142.
- Shi, X.; von dem Bussche, A.; Hurt, R.H.; Kane, A.B.; Gao, H. Cell entry of one-dimensional nanomaterials occurs by tip recognition and rotation. Nat. Nanotechnol. 2011, 6, 714–719.
- Lacerda, L.; Russier, J.; Pastorin, G.; Herrero, M.A.; Venturelli, E.; Dumortier, H.; Al-Jamal, K.T.; Prato, M.; Kostarelos, K.; Bianco, A. Translocation mechanisms of chemically functionalised carbon nanotubes across plasma membranes. Biomaterials 2012, 33, 3334–3343.
This entry is offline, you can click here to edit this entry!
