1. Introduction
Optical biosensors are analytical tools with optical detection systems that have been widely used in medicine, biomedical studies, the food industry, environmental monitoring, and pharmaceutical sciences. They consist of a biological recognition element integrated with an optical transducer to transmit changes in light responses and intensity in chemical and biochemical interactions between the measured substance and the probe. To be more specific, they excite analytes through a specific light wavelength to elevate the analytes’ energy levels. When they return to their normal level of energy, the surplus energy is freed in the form of photons. Owing to the existing optical approaches to detect and measure analytes, including luminescence and fluorescence, plasmon resonance, Raman scattering, ECL, and colorimetric methods, different classes of optical biosensors have been developed [
32,
33]. Due to their real-time and direct monitoring along with their ability to carry out multiplexed detection of many analytes, optical biosensors have replaced conventional methods of detecting and measuring CA-125 oncomarkers in biomedical research [
34,
35].
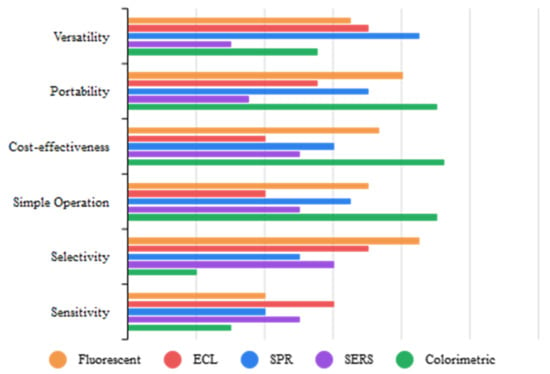
Figure 1 summarizes the advantages and limitations of optical methods used for biosensing cancer biomarker detection.
Table 1 reviews previous studies on the application of optical biosensors for CA-125 determination.
Figure 1. Comparison of optical methods.
2. Fluorscent-Based Biosensors
Fluorescence has become the leading optical method in biosensing owing to its low cost, simple operation, excellent selectivity, and high efficiency. The variations in the fluorescent characteristics of a bioreceptor, as a consequence of its interaction with an analyte, lead to its determination and detection [
74,
75]. Therefore, taking into account all these advantages and principles, researchers have proposed numerous fluorescent biosensors for the detection of CA-125 [
31,
32].
For example, Abou-Omar et al. [
44] provided a rapid, accurate, and sensitive nano-optical sensor based on a thin sol–gel film incorporating Au NPs for CA-125 detection in serum samples from normal women and patients diagnosed with OC. In this work, Au NPs were covered by a Schiff base ligand and then fixed into a sol–gel matrix to assess their optical properties using UV–vis spectrophotometry. Upon the addition of the cancer antigen, the absorbance intensity decreased. Then, the fluorescence emission spectra of this gold–Schiff base complex-doped sol–gel were investigated before and after the addition of CA-125. According to the results, the fabricated nano-optical sensor revealed a linearity of 2.0 to 127.0 U/mL with an LOD of 1.45 U/mL. In another study, Malsagova et al. [
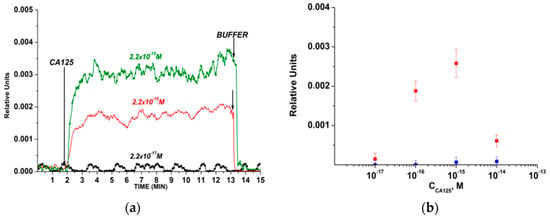
76] developed a silicon-on-insulator (SOI) nanowire biosensor and immobilized antibodies covalently on it to detect the CA-125 cancer antigen (
Figure 2). They concluded that as the protein concentration increases, the signal of the biosensor, which demonstrates the interaction among antibodies and CA-125, intensifies. Based on the results, they determined the minimum detectable concentration of the protein equals 1.5 × 10
−16 M.
Figure 2. The outcomes acquired from the detection of CA-125 protein in buffer solution while employing a silicon-on-insulator (SOI) nanowire biosensor with covalently immobilized antibodies: (
a) common sensorgrams acquired upon assessment of solutions with diverse concentrations of the target protein; (
b) dependencies of the level of the biosensor signal on the concentration of CA-125 in buffer solution [
76].
In another work conducted by Bahari et al. [
41], they measured CA-125 and CA15-3 tumor markers through an efficient immunosensor by applying the sensitivity of a fluorescence method and the great specificity of the synthesized magnetic molecularly imprinted polymers (MMIPs). In this study, they used noble Cd nanoclusters (NCs) and Ni NCs as effective and economic emitters along with magnetic graphene oxide (GO–Fe
3O
4) as a platform to support MMIP. The results revealed that by increasing concentrations of CA-125 and CA15-3, the fluorescence strength of the Ni NCs and Cd NCs was elevated. The fabricated optical sensor showed excellent properties in terms of linearity range (0.0005–40 U/mL) and LOD (50 μU/mL). This work claims that this imprinted immunosensor can be used as a clinical device for checking for breast cancer and OC.
Xu et al. [
43] constructed a double-color biosensor based on aptamers for the simultaneous determination of the carcinoembryonic antigen (CEA) and CA-125. They used salt-provoked Au NPs’ mass to light up the fluorescence of a dual-color DNA–silver NCs-aptamer (DNA-Ag NCs-Apt). Their color-based system comprised red-producing DNA-Ag NCs with the aptamer (rDNA1-AgNCs-Apt1) and green-emitting DNA-Ag NCs beside the CEA aptamer (gDNA2-AgNCs-Apt2). By applying this fluorescence aptasensor, an LOD of 0.015 U/mL was achieved for CA-125.
Fluorescence Resonance Energy Transfer (FRET)-Based Biosensors
This technique depends on nonradiative energy transmission between two fluorescent materials, a “donor” fluorophore to an “acceptor” fluorophore. Owing to its good sensitivity, fast sample analysis, and low background signal, it has been widely used as an important tool used to monitor protein interactions in biological studies [
45].
Omer and colleagues fabricated an ultrasensitive optical biosensor made up of carbon quantum dots (CQDs) for CA-125 detection in the early malignant stage. Their method relies on the quenching mechanism upon the interaction between CQDs and CA-125. The performance of the proposed optical sensor was remarkable due to its low LOD of 0.66 U/mL within the concentration range of 0.01 to 129 U/mL [
47].
3. Chemiluminescence-Based Biosensor
In this method, a chemical reaction between a biological recognition element and an analyte gives rise to produce a luminescence emission of light. By employing this energy, generated by returning an excited molecule to its ground state, researchers have proposed many CL biosensors as the most sensitive optical method [
77,
78]. Owing to their incredible sensitivity, simple instrumentation, and broad dynamic range, they have been widely used for the detection of various oncomarkers [
79]. Several CL biosensors have been familiarized as a sensitive means for the quantification of CA-125 oncomarkers, some of which are presented in
Table 1.
Al-Ogaidi et al. [
49] synthesized graphene quantum dots (GQDs) for chemiluminescent immune-chip fabrication. They transferred chemiluminescence resonance energy from CL reagents to GQDs. The proposed immunosensor could detect CA-125 at a concentration of 0.05 U/mL with a linear concentration range of 0.1 to 600 U/mL.
4. Fluorescent-Based Biosensors
This method uses an electrochemical process to trigger CL by which the radiated light is sensed in the presence of the desired voltage. Compared to other optical techniques, this method does not require an outside light source; thus, its major benefit over other techniques is the reduction in background signal. Moreover, ECL-based biosensors take advantage of the low cost of electrochemistry together with the sensitivity of luminescence [
80].
Babamiri et al. [
55] proposed an ultrasensitive immunosensor for the simultaneous measurement of cancer antigen 153 (CA15-3) and CA-125 tumor markers. They used dendrimer-sulfanilic acid-Ru(bpy)
32+ and polyamidoamine dendrimer-QDs along with Fe
3O
4–SiO
2 as an immunosensing platform and the carrier for reactants generating ECL. Their results reveal that the fabricated ECL immunosensor had an LOD of 0.1 µU/mL in the concentration range 1 µU/mL to 1 U/mL. The performance of the biosensor was evaluated in the human serum sample. According to the results, there was good harmony with the results obtained by the ELISA method.
In another study, Yin and colleagues [
57] designed a near-infrared (NIR) ECL immunosensor with the core/shell AgInS
2/ZnS nanocrystals (NCRs). By oxidizing the synthesized NCRs, both the monodispersed AgInS
2/ZnS NCRs and the surface-confined AgInS
2/ZnS NCRs formed sandwich-typed immuno-complexes with CA-125. Under physiological conditions, the designed immunoassay showed a low LOD (1 × 10
−6 U/mL) in a broad linear range (5 × 10
−6–5 × 10
−3 U/mL), and can thus eventually be used as an effective tool for CA-125 determination in the early diagnosis of OC.
5. Surface Plasmon Resonance (SPR)-Based Biosensor
This label-free optical method utilizes the affinity interaction between a probe and a target to increase the refractive indicator at the surface of SPR sensors. By observing changes in the refractive index, the reaction can be measured. This method provides researchers with a rapid and label-free tool to detect oncomarkers in clinical diagnosis [
81,
82].
Szymańska and coworkers [
65] used a thiol-modified gold surface for CA-125 detection via its antibody. In this work, the linear range was well-suited for use to determine the analyte in blood serum (2.2–150 U/mL). In the end, the designed sensor was successfully tested in real samples from patients diagnosed with OC.
Rebelo et al. [
66] developed an electrochemical sensor and an SPR optical sensor based on pyrrole (Py) electropolymerization on a Au screen-printed electrode (SPE). The SPR sensor provided a high-quality analysis of CA-125 while it was interacting with MMIP. The linear range and LOD of the SPR sensor for CA-125 determination were 0.1–300 U/mL and 0.1 U/mL, respectively.
6. Surface-Enhanced Raman Scattering (SERS)-Based Biosensor
This analytical method provides an enhanced Raman signal of molecules when they come to contact with nanostructured metallic surfaces. SERS-based biosensors have increasingly progressed in mapping and detecting oncomarkers. High resolutions and the possibility of multiplexed diagnosis make them a favorable tool for the simultaneous determination of several targets [
83,
84,
85].
Tunc et al. [
69] designed a sensing platform based on a self-assembled monolayer of Au to detect and determine the CA-125 biomarker. They localized highly enhanced electromagnetic fields near Au NPs and recorded CA-125 antibody and antigen couples. According to the results, there were major changes before and after CA-125 antibody–antigen bioconjugation in the SERS spectra and hot-spot SERS mapping, proving CA-125 binding.
7. Colorimetric Biosensor
Compared to previous optical biosensing methods, this technique is a simple method to detect a target by visual changes in color induced by the bioconjugation between the probe and the analyte. The low cost and simple instrumentation are the major advantages of this method, which make it promising for cheap and portable detection of oncomarkers [
86,
87].
Hosu et al. [
72] developed a colorimetric smartphone-enabled immunosensor based on a 3D nitrocellulose membrane and Au NPs for sandwich immobilization of the primary and secondary antibodies, respectively. The formation of an antibody–Au NPs complex caused Ag in an enhancer solution to be deposited and form Au/Ag nanocomposites in different gray colors based on the concentration of CA-125. They used an eight-megapixel camera for smartphones to determine image pixel intensity. The designed sensor revealed high sensitivity, and its LOD was 30 U/mL.
8. Brief Overview of Optical CA-125 Biosensors
In general, there have been various optical methods, including fluorescence, FRET, CL, ECL, SERS, SPR, and colorimetric, for CA-125 determination. The notable merits of optical biosensors, such as their great sensitivity, excellent selectivity, and easy instrumentation, make them a great alternative to conventional methods in the prognostication of OC. Photoluminescence [
48] and plasmon resonance scattering (PRS) [
71] are other techniques reported in recent studies, achieving LODs of 0.07 ng/mL and 0.4 U/mL, respectively, for CA-125 detection (
Table 1). Fluorescent and ECL-based biosensors are the most studied tool in the detection of CA-125 biomarkers due to their excellent characteristics, such as their low cost and high sensitivity, along with great selectivity. Among existing optical methods, fluorescence, FRET, and ECL have the best performances in the determination of CA-125 due to their low LOD. According to the LODs reported in previous works, sandwich nano-immunosensors and aptasensors demonstrate the highest sensitivity toward CA-125 determination. Interestingly, in a study conducted by Hamd-Ghadareh et al. [
45], an antibody–aptamer sandwich fluorescent immunosensor based on PAMAM-dendrimers/Au NPs was utilized for CA-125 detection. Their designed nanobiosensor achieved an LOD of 0.5 fg/mL. Moreover, some researchers have used multiplexed detection techniques for simultaneous optical measurement of CA-125 with other cancer biomarkers. Nano-biochips, disposable paper-based devices, and smartphone-based immunoassays are novel and attractive methods used for CA-125 detection (
Table 1). Despite all the advances in optical biosensors, there is an essential need to develop optical methods for point-of-care and commercial detection.
This entry is adapted from the peer-reviewed paper 10.3390/bios13010099