Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
While pharmacotherapy of depression appears to have moved from the monoamine hypothesis to more fertile grounds of glutamatergic and GABAergic mechanisms, it has been challenging to shift the antipsychotic paradigm beyond the dopaminergic hypothesis of schizophrenia. Efforts to develop non-dopaminergic antipsychotic medications (APMs) have produced negative results; thus, there has been no effective APM without dopamine involvement during 70 years of antipsychotic drug development.
- seventy
- years
- antipsychotic
- development
1. Introduction
While pharmacotherapy of depression appears to have moved from the monoamine hypothesis to more fertile grounds of glutamatergic and GABAergic mechanisms, it has been challenging to shift the antipsychotic paradigm beyond the dopaminergic hypothesis of schizophrenia. Efforts to develop non-dopaminergic antipsychotic medications (APMs) have produced negative results; thus, there has been no effective APM without dopamine involvement during 70 years of antipsychotic drug development. The only exception has been the approval of pimavanserin, a selective 5HT2A receptor blocker, to treat Parkinson’s psychosis but not schizophrenic psychosis.
Although Federal Drug Agency (FDA) has approved multiple APMs in the last 70 years, most have been with modest variations in molecular targets to qualify for “me too” drugs. The lack of significant differences in mechanisms of action explains why current APMs are primarily effective for positive symptoms without any measurable improvements in negative and cognitive symptoms. The antipsychotic response has been defined as only a 20% reduction in total scores on the most commonly used scale to assess schizophrenia symptoms, the Positive and Negative Syndrome Scale (PANSS) [1]. This reduction in response criterion accommodated the limited efficacy of current APMs for negative and general schizophrenia symptoms, including some cognitive symptoms, such as orientation, attention, insight, and judgment. However, a 20% reduction in total PANSS scores may be mediated by a completely different set of schizophrenia symptoms from one to the other APM, making it difficult to interpret and compare antipsychotic efficacy with precision.
Major psychiatric disorders are diagnosed at a syndromic level, with various symptom domains having potentially different neurobiological underpinnings. Still, most psychiatric studies have used atheoretical diagnoses based on the Diagnostic Statistical Manual [2][3] or the International Statistical Classification of Diseases and Related Health Problems (https://icd.who.int, accessed on 6 December 2022), which could explain the failure to develop comprehensive treatments. In addition, inadequate sample sizes have failed to account for heterogeneity across schizophrenia patients, not allowing for sub-group analyses to generate testable hypotheses and guide future research. These diagnostic limitations were addressed by restricting federal funding for prespecified Research Domain Criteria (RDoC) [4], which were vague, poorly defined, and not scientifically rigorous. Unfortunately, this measure was also counterproductive in promoting neurobiological research that could help advance psychiatric treatments.
Furthermore, researchers have failed to develop early predictors or intermediate phenotypes for antipsychotic response or tolerability, which could guide treatment options for individual patients. In addition, there is almost no research on finding the maintenance dose and length of antipsychotic therapy in schizophrenia patients recovering from acute psychosis. Similarly, there is a lack of laboratory measures to optimize antipsychotic response or tolerability, except for hyperprolactinemia (indicating excessive D2 receptor occupancy), therapeutic drug monitoring (TDM) for clozapine, and pharmacogenetic testing for refractory patients. Even though these measures can enhance antipsychotic efficacy and tolerability, particularly in treatment-refractory schizophrenia, they are not frequently employed, resulting in suboptimal use of APMs [5]. In addition, some providers use high antipsychotic doses, especially in severely and persistently ill patients in state hospitals and prison systems, compromising tolerability and medication adherence and resulting in repeated hospitalizations [5]. Furthermore, the antipsychotic doses required to manage acute psychosis and behavioral dyscontrol are not reduced even after the patient stabilizes. Several clinicians do not employ therapeutic drug monitoring for clozapine before labeling patients as clozapine non-responders [6].
Since NIMH does not fund clinical trials, developing new APMs is primarily owned by the pharmaceutical industry. Although this process involves billions of dollars and may not be fundable by the NIMH, post-marketing and repurposing trials for already approved agents can be affordable, providing a less biased approach than industry-sponsored trials. Another limitation is that the results from the preclinical trials for drug approval cannot be applied to the general population as they are completed in a near-ideal patient population without comorbidities. Thus, it takes years to gather post-marketing effectiveness data applicable to the general patient population. Furthermore, prescriptions of newly approved APMs are too expensive to be covered by Medicaid to be used in the severely and persistently ill, homeless, and forensic population that needs these medications the most.
Although a detailed discussion of the statistical limitations of clinical trials is beyond the scope of this review, the statistics using p < 0.05 has failed to translate into clinically relevant results [7]. However, this trend is changing to more clinically applicable measures, such as confidence interval, effect size, number needed to treat (NNT) [8], and number needed to harm (NNH) [9]. In addition, an increasing level of placebo response may have also failed several promising psychotropics, including APMs [10]. In this context, a higher placebo response can lower the chances of finding a significant difference (i.e., signal) between placebo and active treatment. Several strategies have been proposed to reduce placebo response, but none have been foolproof [11].
However, despite all these obstacles in developing more effective APMs, currently available treatments have made some progress in tolerability, if not efficacy. Thus, relatively higher dose thresholds for adverse effects are observed with second-generation APMs, partial agonists, and especially the recently approved multimodal antipsychotic lumateperone. It is also worth mentioning that long-acting injectables (LAIs) have significantly impacted long-term clinical and functional outcomes in schizophrenia patients worldwide. This review provides a synopsis of different mechanism-based classes of antipsychotic medications approved to manage schizophrenia and other psychotic disorders over the last 70 years.
While pharmacotherapy of depression appears to have moved from the monoamine hypothesis to more fertile grounds of glutamatergic and GABAergic mechanisms, it has been challenging to shift the antipsychotic paradigm beyond the dopaminergic hypothesis of schizophrenia. Efforts to develop non-dopaminergic antipsychotic medications (APMs) have been disappointing, with none of the currently available APMs moving beyond the dopamine system during 70 years of antipsychotic drug development. The only exception has been the approval of pimavanserin, a selective 5HT2A receptor blocker, to treat Parkinson’s psychosis but not schizophrenic psychosis.
Although Federal Drug Agency (FDA) has approved multiple APMs over the last seven decades, most have been with modest variations in molecular targets to qualify for “me too” drugs. The lack of significant differences in mechanisms of action explains why current APMs are primarily effective for positive symptoms without any clinically meaningful improvements in negative and cognitive symptoms. Therefore, the antipsychotic response is defined as only a 20% reduction in total scores on the Positive and Negative Syndrome Scale (PANSS) [1] instead of a 50% reduction required for an antidepressant response. This reduction in response criterion accommodated the limited efficacy of current APMs for negative and some of the cognitive symptoms assessed with PANSS, such as orientation, attention, insight, and judgment. A 20% reduction in PANSS scores suggests an antipsychotic response may be defined by a completely different set of symptoms from one patient to the other, making it difficult to interpret and compare antipsychotic efficacy with precision.
Major psychiatric disorders are diagnosed at a syndromic level, with various symptom domains having potentially different neurobiological underpinnings. However, most diagnoses in psychiatric research are based on atheoretical diagnostic tools such as the Diagnostic Statistical Manual [2][3] or the International Statistical Classification of Diseases and Related Health Problems (https://icd.who.int, accessed on 6 December 2022). The lack of neurobiologically-oriented diagnoses could explain the failure to develop more effective and comprehensive treatments. In addition, relatively small sample sizes have failed to account for heterogeneity across schizophrenia patients, not allowing for sub-group analyses to generate testable hypotheses and guide future research. The relative failure of DSM-based research was addressed by restricting federal funding to prespecified Research Domain Criteria (RDoC) [4], which were also vague, poorly defined, and not scientifically rigorous. Unfortunately, this measure was also counterproductive in promoting neurobiological research that could help advance psychiatric treatments.
Thus, second-generation APMs, including partial agonists, may have higher dosing thresholds to cause adverse effects, such as extrapyramidal symptoms (EPS) and hyperprolactinemia. It is also worth mentioning that long-acting injectables (LAIs) have significantly impacted long-term clinical and functional outcomes in schizophrenia patients worldwide.



2. Older Antipsychotic Medications
Despite limited efficacy and frequent adverse effects, older or conventional APMs provided the first effective treatment for schizophrenia patients, who did not have any chance to live outside asylums and the prison systems. These APMs are further subclassified into high potency and low potency. The low-potency conventional APMs are generally less tolerable than the high-potency conventional APMs, due to multiple sites of action representing a “shotgun” approach [12]. These actions include muscarinic, histaminic, and alpha-1 adrenergic receptor blockade. Antimuscarinic effects include dry mouth, blurred vision, urinary retention, constipation, tachycardia, loss of sweating, confusion, and worsening of closed-angle glaucoma and cognitive function. Antihistaminic effects translate into sedation and short-term weight gain, and the alpha-1 adrenergic blockade mediates postural hypotension, dizziness, and sedation.
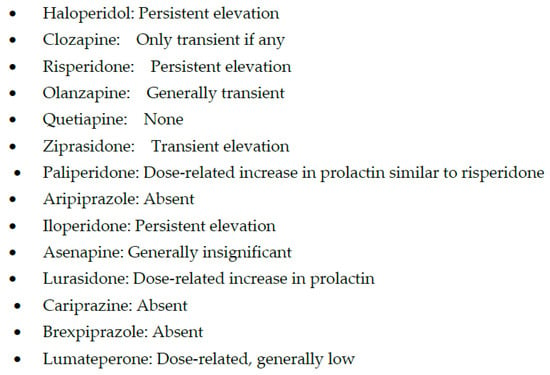
In contrast, high-potency APMs are relatively more selective for blocking dopamine-2 (D2) receptors, and the main adverse effects associated with them are due to D2 blockade, especially at high doses. These adverse effects include extrapyramidal symptoms (EPS), hyperprolactinemia (Figure 1 and Figure 2), and treatment-induced, or worsening of, pre-existing negative, affective or cognitive symptoms.

Figure 1. Extrapyramidal Symptoms (EPS) with antipsychotic medications.

Figure 2. Hyperprolactinemia with antipsychotic medications.
However, the most clinically-serious adverse effect of low-potency APMs is the effects on QTc prolongation, which can result in sudden cardiac death [13]. Although the high-potency APMs also prolong QTc interval, the most clinically serious effects include tardive dyskinesia [14] and neuroleptic malignant syndrome [15]. These adverse effects are generally reported with long-term use of high-dose antipsychotic pharmacotherapy [15]. However, some of the adverse effects of low-potency conventional APMs may be beneficial in few patients, including sedation and weight gain with the histamine-1 receptor blockade, reduction in pre-existing hypertension with the alpha-1 receptor blockade, and protection from EPS with the built-in anticholinergic effects. These adverse effects are also witnessed with newer APMs, such as olanzapine and clozapine [16]. Of note, none of the APMs is indicted for managing dementia-related psychosis due to increased risk for mortality in older adults [17].
There is one APM, loxapine, that is not only effective in schizophrenia but also in major depressive disorder because loxapine is demethylated to a tetracyclic metabolite, amoxapine, which has antidepressant properties [18]. Another worth-mentioning APM, molindone, has a moderate affinity for D2 receptors, with potential benefits in patients not responding to high-or low-affinity APMs [19]. It is also interesting that one of the older high-potency APM, pimozide is the only FDA-approved treatment for Tourette’s syndrome [20].
One of the most clinically significant advances in antipsychotic treatments has been the development of long-acting injectables (LAI) with high-potency APMs, haloperidol, and fluphenazine, followed by newer APMs, risperidone, paliperidone, and aripiprazole. Since medication nonadherence is commonly observed in schizophrenia subjects, the LAIs have improved the maintenance of antipsychotic response and prevention of psychotic relapse and rehospitalizations [21]. In addition, these LAIs are now also available with some of the newer generation APMs as discussed below.
In summary, serotonin and dopamine antagonism (SDA) with SGAPMs may have provided some cushion from dose-related adverse effects but without any significant benefits in efficacy. The only exception among SGAPMs is clozapine, which remains the gold standard for managing treatment-refractory schizophrenia (TRS). However, despite its unique efficacy, to our knowledge, clozapine has not been compared with other SGAMs or studied in post-marketing effectiveness or efficacy trials because it is only approved for patients with TRS.
3. Second-Generation Antipsychotic Medications (SGAPMs)
This class includes every APM developed after the conventional APMs despite significant differences in the mechanisms of action (Table 1), which has created confusion and misperceptions.
Table 1. Molecular targets and binding affinities (Ki, nM) for antipsychotic medications.
| Receptors | HAL | CLZ | RIS | OLZ | QUE | ZIP | PALI | ARIP | ILO | ASEN | LUR | CAR | BREX | LUMA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D2 | 0.7 | 126 | 4 | 11 | 160 | 5 | 4.6 | 0.45 | 6.3 | 1.3 | 1.68 | 0.49 | 0.3 | 32 |
| 5HT1A | >1000 | 875 | 210 | >10,000 | >1000 | 3 | 617 | 4.4 | 168 | 2.5 | 6.75 | 2.6 | 0.12 | - |
| 5HT2A | 45 | 16 | 0.5 | 4 | 295 | 0.4 | 1.1 | 3.4 | 5.6 | 0.06 | 2.03 | 18.8 | 0.47 | 0.54 |
| 5HT2C | >10,000 | 16 | 25 | 23 | >1000 | 1 | 48 | 15 | - | 0.03 | 415 | 134 | 12–34 | - |
| D3 | - | - | - | - | - | - | 3.5 | - | 7.1 | - | 15.7 | 0.08 | 1.1 | -- |
| 5HT7 | - | 18 | 6.6 | 110 | 310 | 6 | 2.7 | 10 | 22 | 0.13 | 0.5 | 111 | 3.7 | - |
| α1 | 6 | 7 | 0.7 | 19 | 7 | 10 | 2.5 | 57 | 0.36 | 1.2 | 47.9 | 155 | 0.17 | 73 |
| α2 | - | 16 | 11 | 210 | 350 | 400 | 3.9 | 38 | - | 1.2 | 10.8 | - | 0.59 | - |
| H1 | 440 | 6 | 20 | 7 | 11 | 47 | 19 | 61 | 437 | 1.0 | >1000 | 23.2 | 19 | >1000 |
| M1 | >1000 | 1.9 | >10,000 | 1.9 | 120 | >1000 | >10,000 | >10,000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. HAL = Haloperidol; CLZ = Clozapine; RIS = Rsiperidone; OLZ = Olanzapine; QUE = Quetiapine; ZIP = Ziprasidone; PALI = Palipreidone; ARIP= aripiprazole; ASEN = Asenapine; LUR = Lurasidone; CAR = cariprazine; BREX = Brexpiprazole; LUMA = Lumateperone. Arnt & Skarsfeld, 1998 [22]; Bymaster et al. 1996 [23]; Seeger et al. 1995 [24]; Schotte et al. 1996 [25], Maeda et al. 2014 [26]; Roth & Driscol, 2021 https://pdsp.unc.edu/databases/pdsp.php, accessed on 6 December 2022; Herman et al. 2018 [27]; Citrome 2010 [28] ; Citrome 2013 [29]; Corena-McLeod 2015 [30].
In addition, assigning clozapine to the newer APMs is not accurate, as clozapine was approved for clinical use in 1971 [31], even before the discovery of some of the older or first-generation APMs. However, it was not until 1989 that the FDA approved clozapine [31], perhaps due to the risk of bone marrow toxicity resulting in an earlier discontinuation in Europe. Similarly, the older versus new classification of APMs is not such a black-and-white concept. One of the older APM, loxapine, has been found to have more potent D2 than the 5HT2A receptor blockade, especially at lower doses (Table 1) [32], a profile that distinguishes older from newer APMs [33]. Risperidone, one of the earliest atypical after approval of clozapine, offers a similar challenge as risperidone loses its atypicality at higher doses and becomes a more potent D2 than 5HT2A receptor blocker [33]. At higher doses, risperidone has been shown to have an even higher level of prolactin (a surrogate for D2 receptor occupancy) than the prototypical high-potency APM, haloperidol [34].
Even within the second-generation APMs, there are significant differences despite their classification in the same group. Starting with clozapine is still one of the unique APM and the only one with proven efficacy in treatment-refractory schizophrenia (TRS) and anti-suicidal effects [35]. If there was no bone marrow toxicity, clozapine could be initiated early as the first line of treatment, improving clinical outcomes and prognosis at the onset of first-break psychosis. However, clozapine is not an easy APM to use, and several providers in the United States remain hesitant to initiate clozapine treatment even in TRS, explaining the underutilization of clozapine treatment in the United States among most developed countries in the world [36]. Clozapine’s toxicity to the neutrophils, which may result in agranulocytosis, explains why the regulatory authorities require registration with the Risk Evaluation and Mitigation Strategy (REMS) to monitor WBC count before a pharmacy can prescribe clozapine for the patients. Unfortunately, this blood test requirement also appears to make some patients hesitant to use clozapine treatment due to fear of needles. However, some of the needle-associated fear is overblown, and if patients are adequately educated about the risks and benefits of clozapine, a significantly large number may agree to use clozapine. At the same time, some European countries, such as the UK, have been adopting increasingly less stringent interval requirements for repeating blood tests required for clozapine treatment (http://www.medicines.org.uk/emc/medicine/1277, accessed on 8 December 2022). This is because agranulocytosis decreases from 0.70/1000 patient-years in the second 6 months of treatment and, after the first year, 0.39/1000 patient-years [37]. Nevertheless, few cases of late-onset clozapine-induced agranulocytosis have been reported [38], requiring continued WBC monitoring. There are other clinically serious effects of clozapine, including myocarditis [39], megacolon, and lowering of the seizure threshold [40]. Another adverse effect that requires special mention is the frequently reported clozapine-induced drooling (sialorrhea) [41]. If not properly treated, it can significantly decline oral hygiene and negatively impact the quality of life. In contrast to all these adverse effects, EPS and hyperprolactinemia are relatively rare with clozapine, probably due to its low water dissociation constant and transient and loose binding affinity for D2 receptors (Table 1) [42]. Therefore, even doses around 900 mg/day of clozapine have not been reported to increase EPS and prolactin, adverse effects that are primarily mediated by D2 receptor blockade. This characteristic is unique to clozapine and another newer APM, quetiapine [43].
Although clozapine is the only effective APM in TRS, not all treatment-refractory patients are clozapine responders [44]. However, there may be many TRS patients who may not be genuine nonresponders to clozapine and may be potentially converted into responders or partial responders by utilizing laboratory tools, such as therapeutic drug monitoring (TDM), to optimize clozapine response [6]. Clozapine has multiple metabolic pathways provided by cytochrome P-450 (CYP) enzymes, CYP1A2, CYP2D6, and CYP3A4 [45]. Genetic polymorphism in these enzymes may alter the efficacy and/or tolerability of any APM metabolized by these enzymes, including clozapine [46]. The ratio between clozapine and its primary metabolite, norclozapine (Clz/Nclz), is one of the best clinical examples of using TDM to predict the activity of CYP1A2 [6]. Since clozapine is biologically different from its primary metabolite, Clz/Nclz ratio may provide some insights into clozapine’s efficacy, safety, and tolerability [47]. A higher Clz/Nclz ratio suggests a low activity of CYP1A2, perhaps due to drug interactions, and a low ratio may denote the increased activity of CYP1A2 resulting in faster conversion of clozapine to norclozapine, which can happen with smoking [48]. Case studies have reported the lower threshold for clinically effective clozapine levels is 350 ng/mL [49][50][51]. The upper limit ranges between 450 ng/mL and 600 ng/mL, but levels at or above 1000 ng/mL can be toxic and should be avoided [52]. If these measures fail to increase clozapine response, some augmentation strategies can be effective, such as lamotrigine and aripiprazole at low doses [53].
All other SGAPMs do not appear to have the same efficacy profile as clozapine, despite some sharing similar but not identical adverse effect profiles. Olanzapine has some structural resemblance to clozapine, with a similar magnitude and frequency of weight gain but not its efficacy (Figure 3) [54][55].

Figure 3. Weight gain with antipsychotic medications.
Nevertheless, olanzapine continued to be taken by the study subjects the longest, despite no differences in efficacy in the large effectiveness trial with APMs, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) [56]. This study did not find any meaningful differences in efficacy between older (i.e., perphenazine) and newer APMs [57]. Although the FDA has approved a long-acting formulation of olanzapine, it is rarely used in clinical practice due to post-injection delirium sedation syndrome [58]. Despite multiple efforts, no significant breakthroughs have been achieved in reducing weight gain and metabolic syndrome with olanzapine and clozapine. However, the concomitant use of anti-diabetic medication, metformin, has helped reduce weight gain in some patients [59]. The recent approval of a fixed-dose combination of samidorphan, a mu-opioid receptor antagonist, and the second-generation antipsychotic drug, olanzapine, by the FDA has provided another option for managing olanzapine-induced weight gain [60][61]. This study reported 18% of patients gaining ≥ 10% weight in the olanzapine-samidorphan group as opposed to 30% in the olanzapine monotherapy group (Figure 3) [60].
The following SGAPM, quetiapine, has one of the lowest and most transient binding affinities for D2 receptors (Table 1) [43], to the extent that the preclinical trials showed even lower prolactin levels than the placebo [62]. Although some of this profile may be similar to clozapine, which it structurally resembles, quetiapine has not demonstrated that level of efficacy at the daily recommended dose of 300 mg [63]. Post-marketing data show that quetiapine doses between 600 to 900 mg a day may have better efficacy [63]. Nevertheless, quetiapine has offered a relatively benign option for managing secondary psychosis, especially in Parkinson’s disease [64] and dementias [65], where only a transient dopamine blockade may be desirable. Interestingly, a recently approved medication, pimavanserin for Parkinson’s disease psychosis, is the first treatment for secondary psychosis without direct dopamine involvement [66]. Interestingly, quetiapine has been one of the most frequently prescribed SGAPMs but at lower doses that are only effective for sedative effects and not psychosis [67].
The next APM, ziprasidone, is the first to report near weight-neutral effects and perhaps some reduction in low-density lipids and triglycerides as opposed to some of the other SGAPMs [68]. However, some initial concerns with the impact of QTc prolongation may have limited ziprasidone’s use in clinical practice (Figure 4) [69].

Figure 4. QTc prolongation with antipsychotic medications.
In addition to having less risk for metabolic syndrome, ziprasidone has also been one of the first lines of treatment for agitation and aggression, primarily due to a rapid onset of antipsychotic effects [70] without sedation [71], making it possible to interview an awake patient in the emergency department. In addition, ziprasidone may have some stimulating effects that may explain a few reports of manic conversions, perhaps due to the moderate blockade of norepinephrine and serotonin transporters [72]. These diverse effects of ziprasidone are probably due to its chimeric structure, which means that ziprasidone has two different moieties within the same molecule, resulting in 5HT2A inverse agonism, D2 receptor antagonism, 5HT1A receptor agonism, and a moderate serotonin and norepinephrine pump blockade [73]. This receptor action profile of ziprasidone may be responsible for its efficacy for depressive and negative symptoms in schizophrenia and schizoaffective disorders with fewer adverse effects, particularly EPS, hyperprolactinemia, and metabolic effects in comparison with conventional antipsychotics (Table 1; Figure 1, Figure 2 and Figure 3) [74][75][76]. Ziprasidone is primarily metabolized in the liver with a half-life of about 7 h at recommended doses. There is a low risk of pharmacokinetic drug interactions with ziprasione, as less than 1/3rd of ziprasidone’s metabolism is mediated by CYP enzymes [77][78]. However, ziprasidone must be given with food for effective absorption [79].
Paliperidone is a metabolite of risperidone (i.e., 9-OH risperidone) with a stronger binding affinity for D2 receptors than risperidone (Figure 1 and Figure 2) [30]. However, the higher affinity of paliperidone for D2 receptors does not result in any significant increase in prolactin elevation or EPS with paliperidone as compared to risperidone (Table 1). However, paliperidone has a longer half-life than risperidone and thus can be given once a day orally [30]. Since paliperidone is already a metabolite [80], it may be a safer option than other APMs in patients with liver dysfunction or polypharmacy due to the lower risk for drug interactions [81]. However, the most effective use of paliperidone has been its long-acting injectable (LAI), which provides the most flexibility regarding the duration of effects and LAI frequency. Paliperidone is now available as a monthly (Invega Sustenna), quarterly (Invega Trinza), and biannually LAI (Invega Hayfera), which significantly minimizes the number of patient visits to the clinic and can be cost-effective [82].
Iloperidone, the next SGAM, is one of the most potent blockers of noradrenergic α1 receptors (Table 1), which may expose schizophrenia subjects to significant postural hypotension and dizziness and requires a slow titration to avoid these adverse effects [83]. However, a potent noradrenergic α1 receptor antagonism may explain one of the lowest risks for akathisia (Figure 2), which, unlike other EPS, may be mediated by an increase in noradrenergic neurotransmission [84]. In addition, iloperidone may become a drug of choice if the posttraumatic disorder is associated with psychosis, as α1 receptor antagonism is the putative mechanism behind the efficacy of prazosin in improving sleep efficiency [85]. Similarly, iloperidone can be beneficial in managing comorbid hypertension in psychotic or posttraumatic patients [86]. Thus, overall, iloperidone may have one of the lowest risks of causing akathisia [84]. As can be seen in Figure 3, weight gain with iloperidone is also in the moderate range comparable with risperidone [84]. However, it does have some risk for QTc prolongation [87] (Figure 4).
Asenapine is the only SGAM approved as sublingual preparation and has been recently available as a transdermal treatment in the United States. The sublingual administration is reported to result in a more rapid onset of antipsychotic effects than orally administered APMs and, similar to the transdermal application, delivers a higher proportion of parent drug unaltered by first pass effect [88]. Unlike some of the other SGAPMs, asenapine does not require dose titration to avoid adverse effects, and starting dose can also be the effective dose and can be given once a day [89]. Weight gain is also less than olanzapine and risperidone (Figure 3) [89]. There has also been a suggestion that since asenapine antagonizes 5HT7 and adrenergic α2 receptors (Table 1), it may augment antidepressant response [90] and may have cognitive benefits [91].
Lurasidone followed the approval of asenapine as the next SGAPM. Food can affect the absorption of lurasidone but not as much as with ziprasidone [79]. Nevertheless, lurasidone exposure is increased twofold and maximum concentration is up to threefold when administered with food. Like asenapine, lurasidone also has a strong affinity for serotonin 5HT7 receptors (Table 1), which may have implications for cognition [92]. In addition, lurasidone is also a partial agonist at 5-HT1A; this may add further to the procognitive as well as potential antidepressant effects [93]. However, unlike iloperidone, lurasidone has minimal affinity for alpha-1 noradrenergic receptors and a low propensity for orthostatic hypotension (Table 1) [93]. More importantly, lurasidone has minimal affinity for 5HT2C receptors and virtually no affinity for histamine H1, predicting a low liability for weight gain (Figure 3) [94]. No affinity for histamine H1 predicts a lower risk for sedation compared with other APMs with potent histamine blockade (Table 1). Unlike olanzapine and clozapine, lurasidone’s lack of affinity for cholinergic M1 receptors would predict a low propensity for causing anticholinergic side effects (Table 1) [93]. Lurasidone’s dopamine D2 receptor occupancy at 40 mg/day ranges between 63% to 67%; assuming this is within the range of effective D2 receptor occupancy, an antipsychotic response could be potentially achieved with an oral dose of 40 mg/day [95], particularly in elderly subjects. The primary metabolic pathway for lurasidone is CYP3A4 [96], which has clinical implications regarding the use of lurasidone in the presence of an extensive list of inducers and inhibitors of CYP3A4.
4. Partial Agonists
Partial agonists of dopamine-2 (D2) receptors are also classified as the SGAPMs that are full antagonists of D2 receptors, which raises some questions. A partial agonist for D2 receptors is an entirely novel concept for which Arvid Carlsson received a Nobel prize in 2000 [97]. Often partial agonism is misperceived as an antipsychotic that only partially activates D2 receptors, which is far from the truth. A partial agonist’s action depends on the baseline neurotransmitter activity at its receptors, so a partial agonist will reduce an increased baseline receptor activity and increase a reduced baseline receptor activity, suggesting that a partial agonist is actually a system stabilizer [97][98]. Thus, a partial agonist at D2 receptors increases low dopamine activity in the mesocortical tract, which may potentially benefit effective, negative, and cognitive symptoms while reducing dopamine hyperactivity in the mesolimbic pathway required for antipsychotic effects [98][99]. In contrast, a full D2 receptor antagonist decreases dopamine activity globally to manage psychosis, including the hypoactive dopaminergic pathways, such as the mesocortical tract, thus increasing the risk for iatrogenically-induced affective, affective, or cognitive symptoms, especially with the high-dose antipsychotic pharmacotherapy [100]. Therefore, partial agonism brings a novel concept that is theoretically consistent with the basic pathophysiological model of schizophrenia with differential dopaminergic activity across dopaminergic pathways. Although this concept may not be clinically superior in managing positive symptoms, the adverse effect profile, particularly concerning EPS and hyperprolactinemia, may be relatively better than the previous classes of APMs, including the SGAPMs [101]. Thus, partial agonists appear to have a more favorable tolerability profile for adverse effects due to D2-receptor blockade, such as EPS and hyperprolactinemia [101]. One of the most significant differences between D2 receptor partial agonism and D2 receptor antagonism is the relative lack of dose-related adverse effects except for akathisia. However, like other APMs, partial agonists have also been associated with weight gain [102]. There are three partial agonist APMs approved by the FDA, aripiprazole, cariprazine, and brexpiprazole.
Aripiprazole was approved in 2002, even before some of the SGAPMs were approved. Although aripiprazole is a partial agonist, it has one of the most potent affinities for D2 receptors [98][99]. But since it is a system stabilizer and not a full antagonist at D2 receptors, the adverse effects and perhaps the antipsychotic efficacy may not always be dose-related. Lack of prolactin elevation and absence of EPS except for akathisia, irrespective of the dose, supports the partial agonism concept with aripiprazole (Figure 1 and Figure 2) [103][104]. Another benefit of aripiprazole and other partial agonists is a relatively lower incidence of weight gain and metabolic syndrome (Figure 3) [105]. Since aripiprazole is a partial agonist for D2 and the 5HT1A receptors, aripiprazole stabilizes two critical neurotransmitter systems in the brain, dopamine, and serotonin, which explains aripiprazole’s efficacy across several psychiatric disorders (Table 1) [106]. A partial agonist, such as aripiprazole, can also be helpful at a relatively low dose to reduce long-term adverse effects in patients receiving LAI, such as EPS and hyperprolactinemia [107]. Aripiprazole is the only partial agonist available as a long-acting injectable (LAI; Abilify Maintena and Aristada) [108][109]. One of the most significant clinical advances with aripiprazole is the approval of a unique delivery system, where a single oral dose of 30 mg aripiprazole can be given with 1064 mg of the LAI, aripiprazole lauroxil (Aristada®), along with an immediate release nanomolar preparation (Aristada Initio®) to achieve a clinically effective range of plasma levels of aripiprazole in a single day [110]. Administration of this aripiprazole combination ensures adequate aripiprazole levels in hospitalized patients at hospital discharge to prevent psychotic relapse during the most vulnerable post-hospital discharge. The real-world implication of LAIs is highlighted by two long-term outcome studies involving aripiprazole and paliperidone LAIs that reported reduced hospitalization rates [111][112]. Although some studies comparing oral APMs and LAIs have yielded some inconsistent results, the pooled data from a meta-analysis based on 42 studies showed the clinical superiority of the two LAIs over oral APMs in reducing hospitalization rates [113]. Based on findings from a large prospective study from Sweden over seven years, LAIs had the highest rates of relapse prevention and lowest rates of rehospitalizations in patients with schizophrenia [114]. Of note, only one oral APM, clozapine, could match these results from LAIs.
Cariprazine is indicated for treating schizophrenia and acute manic or mixed episodes associated with bipolar I disorder as a monotherapy [115][116]. It is the only antipsychotic medication that received approval for schizophrenia and bipolar I disorder at the same time [117]. Cariprazine is a novel antipsychotic with unique pharmacodynamic and pharmacokinetic properties [118][119]. Regarding pharmacodynamics, cariprazine is similar to other SGAMs exhibiting antagonistic activity at 5HT2A receptors [97]. In addition, it is similar to aripiprazole and brexpiprazole in demonstrating partial agonist activity at D2 and D3, and 5HT1A receptors (Table 1). However, cariprazine is the only partial agonist at D2 receptors that exhibits a higher affinity for the D3 than the D2 receptor. While this unique property continues to be highlighted throughout the literature, the clinical significance of this remains unknown. Cariprazine also has moderate histamine antagonism, low alpha-1 antagonism, and no significant affinity for muscarinic cholinergic receptors (Table 1) [119]. Cariprazine has two biologically active metabolites, desmethyl and didesmethyl cariprazine [119], of which the latter is primarily accountable for later and long-term efficacy and tolerability [117]. The most common treatment-emergent adverse events in the cariprazine group included insomnia, EPS, akathisia, sedation, nausea, dizziness, and constipation [118]. Cariprazine treatment was comparable to the placebo in weight gain and metabolic parameters (Figure 3), vital signs, sedation, prolactin, or QTc interval [118]. In addition, cariprazine-induced akathisia was reported as mild to moderate (Figure 1), which suggests that cariprazine may be slightly better than aripiprazole in metabolic adverse effects and akathisia [118]. However, akathisia was dose-related, and higher cariprazine doses than 3 mg/day resulted in a higher risk for akathisia. The only treatment-related serious adverse event was supraventricular tachycardia in one study subject, which resolved after the drug discontinuation [118].
Brexpiprazole is the latest D2 receptor partial agonist that received approval for the treatment of schizophrenia and for adjunctive use for MDD in patients who demonstrated inadequate response to standard antidepressant therapy [120][121]. The recommended dose for the treatment of schizophrenia is 2–4 mg/day [122]. Like aripiprazole, it is a partial agonist at D2 and 5HT1A receptors and an antagonist at serotonin 5HT2A receptors (Table 1) [123]. However, brexpiprazole displays less intrinsic activity at D2 receptors and, coupled with actions at 5HT1A, 5HT2A, and noradrenergic α1 receptors that are at least as potent as its action at D2 receptors, it is predicted to demonstrate a lower propensity to activating adverse events, such as akathisia and other EPS than aripiprazole (Figure 1) [123]. Clinically, brexpiprazole produced more statistically significant improvements in schizophrenia symptomatology and psychosocial functioning than placebo in adults with acute schizophrenia [124]. In long-term trials, brexpiprazole reduced the time to relapse compared with placebo, with continued improvement in symptoms and functioning [125]. Brexpiprazole was generally well tolerated with a relatively low incidence of activating and sedating adverse effects, small changes in QT interval and metabolic parameters that were not clinically significant, and moderate weight gain (Figure 3 and Figure 4) [124].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11010130
References
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276.
- Allsopp, K.; Read, J.; Corcoran, R.; Kinderman, P. Heterogeneity in psychiatric diagnostic classification. Psychiatry Res. 2019, 279, 15–22.
- Escobar, J.I. An insider’s view of the new diagnostic and statistical manual of North American psychiatry (DSM-5). Colomb. Médica 2013, 44, 129–131.
- Markowitz, J.C.; Milrod, B.L. Lost in Translation: The Value of Psychiatric Clinical Trials. J. Clin. Psychiatry 2022, 83, 43385.
- Shad, M.U. High-Dose Therapy in Treatment-Refractory Psychosis: A Retrospective Study. Prim. Care Companion CNS Disord. 2022, 24, 44191.
- Shad, M.U.; Felzien, E.; Roy, K.; Sethi, S. How to identify and manage non-response to clozapine? Asian J. Psychiatr. 2019, 45, 50–52.
- Grabowski, B. “P < 0.05” Might Not Mean What You Think: American Statistical Association Clarifies P Values. JNCI J. Natl. Cancer Inst. 2016, 108, djw194.
- Citrome, L. Number needed to treat: What it is and what it isn’t, and why every clinician should know how to calculate it. J. Clin. Psychiatry 2011, 72, 412–413.
- Andrade, C. The numbers needed to treat and harm (NNT, NNH) statistics: What they tell us and what they do not. J. Clin. Psychiatry 2015, 76, e330–e333.
- Kasper, S.; Dold, M. Factors contributing to the increasing placebo response in antidepressant trials. World Psychiatry 2015, 14, 304–306.
- Evans, K.; Colloca, L.; Pecina, M.; Katz, N. What can be done to control the placebo response in clinical trials? A narrative review. Contemp. Clin. Trials 2021, 107, 106503.
- Gardner, D.M.; Baldessarini, R.J.; Waraich, P. Modern antipsychotic drugs: A critical overview. Cmaj 2005, 172, 1703–1711.
- Preskorn, S.H. The evolution of antipsychotic drug therapy: Reserpine, chlorpromazine, and haloperidol. J. Psychiatr. Pract. 2007, 13, 253–257.
- Vardar, M.K.; Ceylan, M.E.; Unsalver, B.O. Assesment of Risk Factors for Tardive Dyskinesia. Psychopharmacol. Bull. 2020, 50, 36–46.
- Sarkar, S.; Gupta, N. Drug information update. Atypical antipsychotics and neuroleptic malignant syndrome: Nuances and pragmatics of the association. BJPsych Bull. 2017, 41, 211–216.
- Chew, M.L.; Mulsant, B.H.; Pollock, B.G.; Lehman, M.E.; Greenspan, A.; Kirshner, M.A.; Bies, R.R.; Kapur, S.; Gharabawi, G. A model of anticholinergic activity of atypical antipsychotic medications. Schizophr. Res. 2006, 88, 63–72.
- Jeste, D.V.; Blazer, D.; Casey, D.; Meeks, T.; Salzman, C.; Schneider, L.; Tariot, P.; Yaffe, K. ACNP White Paper: Update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008, 33, 957–970.
- Burch, E.A., Jr.; Goldschmidt, T.J. Loxapine in the treatment of psychotic-depressive disorders: Measurement of antidepressant metabolites. South. Med. J. 1983, 76, 991–995.
- Waugaman, R.M. Potential lower efficacy of molindone among first-generation antipsychotics. Am. J. Psychiatry 2009, 166, 491.
- Pringsheim, T.; Marras, C. Pimozide for tics in Tourette’s syndrome. Cochrane Database Syst. Rev. 2009, 2009, CD006996.
- Kane, J.M.; Leucht, S.; Carpenter, D.; Docherty, J.P. Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic D. The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: Methods, commentary, and summary. J. Clin. Psychiatry 2003, 64 (Suppl. 12), 5–19.
- Arnt, J.; Skarsfeldt, T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology 1998, 18, 63–101.
- Bymaster, F.P.; Calligaro, D.O.; Falcone, J.F.; Marsh, R.D.; Moore, N.A.; Tye, N.C.; Seeman, P.; Wong, D.T. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996, 14, 87–96.
- Seeger, T.F.; Seymour, P.A.; Schmidt, A.W.; Zorn, S.H.; Schulz, D.W.; Lebel, L.A.; McLean, S.; Guanowsky, V.; Howard, H.R.; Lowe, J.A. Ziprasidone (CP-88,059): A new antipsychotic with combined dopamine and serotonin receptor antagonist activity. J. Pharmacol. Exp. Ther. 1995, 275, 101–113.
- Schotte, A.; Janssen, P.F.M.; Gommeren, W.; Luyten, W.H.M.L.; Van Gompel, P.; Lesage, A.S.; De Loore, K.; Leysen, J.E. Risperidone compared with new and reference antipsychotic drugs: In vitro and in vivo receptor binding. Psychopharmacology 1996, 124, 57–73.
- Maeda, K.; Lerdrup, L.; Sugino, H.; Akazawa, H.; Amada, N.; McQuade, R.D.; Stensbøl, T.B.; Bundgaard, C.; Arnt, J.; Kikuchi, T. Brexpiprazole II: Antipsychotic-like and procognitive effects of a novel serotonin-dopamine activity modulator. J. Pharmacol. Exp. Ther. 2014, 350, 605–614.
- Herman, A.; El Mansari, M.; Adham, N.; Kiss, B.; Farkas, B.; Blier, P. Involvement of 5-HT(1A) and 5-HT(2A) Receptors but Not alpha (2)-Adrenoceptors in the Acute Electrophysiological Effects of Cariprazine in the Rat Brain In Vivo. Mol. Pharmacol. 2018, 94, 1363–1370.
- Citrome, L. Iloperidone: Chemistry, pharmacodynamics, pharmacokinetics and metabolism, clinical efficacy, safety and tolerability, regulatory affairs, and an opinion. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1551–1564.
- Citrome, L. A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: An evidence-based medicine approach. CNS Drugs 2013, 27, 879–911.
- Corena-McLeod, M. Comparative Pharmacology of Risperidone and Paliperidone. Drugs R D 2015, 15, 163–174.
- Wenthur, C.J.; Lindsley, C.W. Classics in chemical neuroscience: Clozapine. ACS Chem. Neurosci. 2013, 4, 1018–1025.
- Popovic, D.; Nuss, P.; Vieta, E. Revisiting loxapine: A systematic review. Ann. Gen. Psychiatry 2015, 14, 15.
- Farah, A. Atypicality of atypical antipsychotics. Prim. Care Companion CNS Disord. 2005, 7, 268–274.
- David, S.R.; Taylor, C.C.; Kinon, B.J.; Breier, A. The effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophrenia. Clin. Ther. 2000, 22, 1085–1096.
- Kerwin, R.W.; Bolonna, A.A. Is clozapine antisuicidal? Expert Rev. Neurother. 2004, 4, 187–190.
- Joober, R.; Boksa, P. Clozapine: A distinct, poorly understood and under-used molecule. J. Psychiatry Neurosci. 2010, 35, 147–149.
- Schulte, P. Risk of clozapine-associated agranulocytosis and mandatory white blood cell monitoring. Ann. Pharmacother. 2006, 40, 683–688.
- Patel, N.C.; Dorson, P.G.; Bettinger, T.L. Sudden late onset of clozapine-induced agranulocytosis. Ann. Pharmacother. 2002, 36, 1012–1015.
- Higgins, J.M.; San, C.; Lagnado, G.; Chua, D.; Mihic, T. Incidence and Management of Clozapine-Induced Myocarditis in a Large Tertiary Hospital. Can. J. Psychiatry 2019, 64, 561–567.
- De Fazio, P.; Gaetano, R.; Caroleo, M.; Cerminara, G.; Maida, F.; Bruno, A.; Muscatello, M.R.; Moreno, M.J.J.; Russo, E.; Segura-García, C. Rare and very rare adverse effects of clozapine. Neuropsychiatr. Dis. Treat. 2015, 11, 1995–2003.
- Maher, S.; Cunningham, A.; O’Callaghan, N.; Byrne, F.; Mc Donald, C.; McInerney, S.; Hallahan, B. Clozapine-induced hypersalivation: An estimate of prevalence, severity and impact on quality of life. Ther. Adv. Psychopharmacol. 2016, 6, 178–184.
- Seeman, P. Clozapine, a fast-off-D2 antipsychotic. ACS Chem. Neurosci. 2014, 5, 24–29.
- Kapur, S.; Zipursky, R.; Jones, C.; Shammi, C.S.; Remington, G.; Seeman, P. A positron emission tomography study of quetiapine in schizophrenia: A preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch. Gen. Psychiatry 2000, 57, 553–559.
- Siskind, D.; Siskind, V.; Kisely, S. Clozapine Response Rates among People with Treatment-Resistant Schizophrenia: Data from a Systematic Review and Meta-Analysis. Can. J. Psychiatry 2017, 62, 772–777.
- Shad, M.U. Clozapine toxicity: A discussion of pharmacokinetic factors. Asian J. Psychiatry 2008, 1, 47–49.
- Prior, T.I.; Baker, G.B. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J. Psychiatry Neurosci. 2003, 28, 99–112.
- Costa-Dookhan, K.A.; Agarwal, S.M.; Chintoh, A.; Tran, V.N.; Stogios, N.; Ebdrup, B.H.; Sockalingam, S.; Rajji, T.K.; Remington, G.J.; Siskind, D.; et al. The clozapine to norclozapine ratio: A narrative review of the clinical utility to minimize metabolic risk and enhance clozapine efficacy. Expert Opin. Drug Saf. 2020, 19, 43–57.
- Kroon, L.A. Drug interactions with smoking. Am. J. Health Pharm. 2007, 64, 1917–1921.
- Stieffenhofer, V.; Saglam, H.; Schmidtmann, I.; Silver, H.; Hiemke, C.; Konrad, A. Clozapine plasma level monitoring for prediction of rehospitalization schizophrenic outpatients. Pharmacopsychiatry 2011, 44, 55–59.
- Dettling, M.; Sachse, C.; Brockmöller, J.; Schley, J.; Müller-Oerlinghausen, B.; Pickersgill, I.; Rolfs, A.; Schaub, R.T.; Schmider, J. Long-term therapeutic drug monitoring of clozapine and metabolites in psychiatric in- and outpatients. Psychopharmacology 2000, 152, 80–86.
- Greenwood-Smith, C.; Lubman, D.I.; Castle, D.J. Serum clozapine levels: A review of their clinical utility. J. Psychopharmacol. 2003, 17, 234–238.
- Olesen, O.V.; Thomsen, K.; Jensen, P.N.; Wulff, C.H.; Rasmussen, N.-A.; Refshammer, C.; Sørensen, J.; Bysted, M.; Christensen, J.; Rosenberg, R. Clozapine serum levels and side effects during steady state treatment of schizophrenic patients: A cross-sectional study. Psychopharmacology 1995, 117, 371–378.
- Tiihonen, J.; Wahlbeck, K.; Kiviniemi, V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2009, 109, 10–14.
- Ma, X.; Maimaitirexiati, T.; Zhang, R.; Gui, X.; Zhang, W.; Xu, G.; Hu, G. HTR2C polymorphisms, olanzapine-induced weight gain and antipsychotic-induced metabolic syndrome in schizophrenia patients: A meta-analysis. Int. J. Psychiatry Clin. Pract. 2014, 18, 229–242.
- Grover, S.; Nebhinani, N.; Chakrabarti, S.; Avasthi, A.; Kulhara, P. Metabolic syndrome among patients receiving clozapine: A preliminary estimate. Indian J. Pharmacol. 2011, 43, 591–595.
- Lieberman, J.A.; Stroup, T.S.; McEvoy, J.P.; Swartz, M.S.; Rosenheck, R.A.; Perkins, D.O.; Keefe, R.S.E.; Davis, S.M.; Davis, C.E.; Lebowitz, B.D.; et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005, 353, 1209–1223.
- Meyer, J.M.; Nasrallah, H.A.; McEvoy, J.P.; Goff, D.C.; Davis, S.M.; Chakos, M.; Patel, J.K.; Keefe, R.S.; Stroup, T.S.; Lieberman, J.A. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia Trial: Clinical comparison of subgroups with and without the metabolic syndrome. Schizophr. Res. 2005, 80, 9–18.
- Lindenmayer, J.P. Long-acting injectable antipsychotics: Focus on olanzapine pamoate. Neuropsychiatr. Dis. Treat. 2010, 6, 261–267.
- De Silva, V.A.; Suraweera, C.; Ratnatunga, S.S.; Dayabandara, M.; Wanniarachchi, N.; Hanwella, R. Metformin in prevention and treatment of antipsychotic induced weight gain: A systematic review and meta-analysis. BMC Psychiatry 2016, 16, 341.
- Correll, C.U.; Newcomer, J.W.; Silverman, B.; DiPetrillo, L.; Graham, C.; Jiang, Y.; Du, Y.; Simmons, A.; Hopkinson, C.; McDonnell, D.; et al. Effects of Olanzapine Combined with Samidorphan on Weight Gain in Schizophrenia: A 24-Week Phase 3 Study. Am. J. Psychiatry 2020, 177, 1168–1178.
- Brunette, M.F.; Correll, C.U.; O’Malley, S.S.; McDonnell, D.; DiPetrillo, L.; Jiang, Y.; Simmons, A.; Silverman, B.L.; Citrome, L.; Green, A.I. Olanzapine Plus Samidorphan (ALKS 3831) in Schizophrenia and Comorbid Alcohol Use Disorder: A Phase 2, Randomized Clinical Trial. J. Clin. Psychiatry 2020, 81, 13176.
- Hamner, M. The effects of atypical antipsychotics on serum prolactin levels. Ann. Clin. Psychiatry 2002, 14, 163–173.
- Sparshatt, A.; Jones, S.; Taylor, D. Quetiapine: Dose-response relationship in schizophrenia. CNS Drugs 2008, 22, 49–68; discussion 69–72.
- Shotbolt, P.; Samuel, M.; David, A. Quetiapine in the treatment of psychosis in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2010, 3, 339–350.
- Zhong, K.X.; Tariot, P.N.; Mintzer, J.; Minkwitz, M.C.; Devine, N.A. Quetiapine to treat agitation in dementia: A randomized, double-blind, placebo-controlled study. Curr. Alzheimer Res. 2007, 4, 81–93.
- Patel, R.S.; Bhela, J.; Tahir, M.; Pisati, S.R.; Hossain, S. Pimavanserin in Parkinson’s Disease-induced Psychosis: A Literature Review. Cureus 2019, 11, e5257.
- Ohman, K.L.; Schultheis, J.M.; Kram, S.J.; Cox, C.E.; Gilstrap, D.L.; Yang, Z.; Kram, B.L. Effectiveness of Quetiapine as a Sedative Adjunct in Mechanically Ventilated Adults without Delirium. Ann. Pharmacother. 2021, 55, 149–156.
- Park, S.; Yi, K.K.; Kim, M.S.; Hong, J.P. Effects of ziprasidone and olanzapine on body composition and metabolic parameters: An open-label comparative pilot study. Behav. Brain Funct. 2013, 9, 27.
- Camm, A.J.; Karayal, O.N.; Meltzer, H.; Kolluri, S.; O’Gorman, C.; Miceli, J.; Tensfeldt, T.; Kane, J.M. Ziprasidone and the corrected QT interval: A comprehensive summary of clinical data. CNS Drugs 2012, 26, 351–365.
- Zimbroff, D.L.; Allen, M.H.; Battaglia, J.; Citrome, L.; Fishkind, A.; Francis, A.; Herr, D.L.; Hughes, D.; Martel, M.; Preval, H.; et al. Best clinical practice with ziprasidone IM: Update after 2 years of experience. CNS Spectr. 2005, 10, 1–15.
- Miller, D.D. Atypical antipsychotics: Sleep, sedation, and efficacy. Prim. Care Companion J. Clin. Psychiatry 2004, 6 (Suppl. 2), 3–7.
- Keating, A.M.; Aoun, S.L.; Dean, C.E. Ziprasidone-associated mania: A review and report of 2 additional cases. Clin. Neuropharmacol. 2005, 28, 83–86.
- Masand, P.S.; Nemeroff, C.B.; Newcomer, J.W.; Lieberman, J.A.; Schatzberg, A.F.; Weiden, P.J.; Kilts, C.D.; Harvey, P.D.; Daniel, D.G. From clinical research to clinical practice: A 4-year review of ziprasidone. CNS Spectr. 2005, 10 (Suppl. 17), 1–20.
- Carnahan, R.M.; Lund, B.C.; Perry, P.J. Ziprasidone, a new atypical antipsychotic drug. Pharmacotherapy 2001, 21, 717–730.
- Green, B. Focus on ziprasidone. Curr. Med. Res. Opin. 2001, 17, 146–150.
- Papakostas, G.I.; Fava, M.; Baer, L.; Swee, M.B.; Jaeger, A.; Bobo, W.V.; Shelton, R.C. Ziprasidone Augmentation of Escitalopram for Major Depressive Disorder: Efficacy Results from a Randomized, Double-Blind, Placebo-Controlled Study. Am. J. Psychiatry 2015, 172, 1251–1258.
- Shad, M.; Preskorn, S.; Miceli, J.; Wilner, K. Use of population pharmacokinetic modeling to characterize intramuscular pharmacokinetics for ziprasidone in schizophrenic patients. Clin. Pharmacol. Ther. 1999, 65, 171.
- Spina, E.; de Leon, J. Metabolic drug interactions with newer antipsychotics: A comparative review. Basic Clin. Pharmacol. Toxicol. 2007, 100, 4–22.
- Citrome, L. Using oral ziprasidone effectively: The food effect and dose-response. Adv. Ther. 2009, 26, 739–748.
- Urichuk, L.; Prior, T.I.; Dursun, S.; Baker, G. Metabolism of atypical antipsychotics: Involvement of cytochrome p450 enzymes and relevance for drug-drug interactions. Curr. Drug Metab. 2008, 9, 410–418.
- Amatniek, J.; Canuso, C.M.; Deutsch, S.I.; Henderson, D.C.; Mao, L.; Mikesell, C.; Rodriguez, S.; Sheehan, J.; Alphs, L. Safety of paliperidone extended-release in patients with schizophrenia or schizoaffective disorder and hepatic disease. Clin. Schizophr. Relat. Psychoses 2014, 8, 8–20.
- Chan, H.W.; Huang, C.Y.; Yen, Y.C. Clinical outcomes of paliperidone long-acting injection in patients with schizophrenia: A 1-year retrospective cohort study. BMC Psychiatry 2021, 21, 507.
- Caccia, S.; Pasina, L.; Nobili, A. New atypical antipsychotics for schizophrenia: Iloperidone. Drug Des. Dev. Ther. 2010, 4, 33–48.
- Citrome, L. Iloperidone: A clinical overview. J. Clin. Psychiatry 2011, 72 (Suppl. 1), 19–23.
- Shuman, M.D.; McGrane, I.R. Rationale for iloperidone in the treatment of posttraumatic stress disorder. Innov. Clin. Neurosci. 2014, 11, 23–25.
- Joshi, S.V.; Patel, E.P.; Vyas, B.A.; Lodha, S.R.; Kalyankar, G.G. Repurposing of Iloperidone: Antihypertensive and ocular hypotensive activity in animals. Eur. J. Pharm. Sci. 2020, 143, 105173.
- Llerena, A.; Berecz, R.; Dorado, P.; de la Rubia, A. QTc interval, CYP2D6 and CYP2C9 genotypes and risperidone plasma concentrations. J. Psychopharmacol. 2004, 18, 189–193.
- Pratts, M.; Citrome, L.; Grant, W.; Leso, L.; Opler, L.A. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr. Scand. 2014, 130, 61–68.
- Citrome, L. Iloperidone, asenapine, and lurasidone: A brief overview of 3 new second-generation antipsychotics. Postgrad. Med. 2011, 123, 153–162.
- McIntyre, R.S.; Wong, R. Asenapine: A synthesis of efficacy data in bipolar mania and schizophrenia. Clin. Schizophr. Relat. Psychoses 2012, 5, 217–220.
- Tarazi, F.I.; Neill, J.C. The preclinical profile of asenapine: Clinical relevance for the treatment of schizophrenia and bipolar mania. Expert Opin. Drug Discov. 2013, 8, 93–103.
- Ballaz, S.J.; Akil, H.; Watson, S.J. The 5-HT7 receptor: Role in novel object discrimination and relation to novelty-seeking behavior. Neuroscience 2007, 149, 192–202.
- Shayegan, D.K.; Stahl, S.M. Atypical antipsychotics: Matching receptor profile to individual patient’s clinical profile. CNS Spectr. 2004, 9 (Suppl. 11), 6–14.
- Kroeze, W.K.; Hufeisen, S.J.; Popadak, B.A.; Renock, S.M.; Steinberg, S.; Ernsberger, P.; Jayathilake, K.; Meltzer, H.Y.; Roth, B.L. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 2003, 28, 519–526.
- Potkin, S.G.; Keator, D.B.; Kesler-West, M.L.; Nguyen, D.D.; van Erp, T.G.M.; Mukherjee, J.; Shah, N.; Preda, A. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014, 19, 176–181.
- Meyer, J.M.; Loebel, A.D.; Schweizer, E. Lurasidone: A new drug in development for schizophrenia. Expert Opin. Investig. Drugs 2009, 18, 1715–1726.
- Grunder, G.; Carlsson, A.; Wong, D.F. Mechanism of new antipsychotic medications: Occupancy is not just antagonism. Arch. Gen. Psychiatry 2003, 60, 974–977.
- Stahl, S.M. Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 2: Illustrating their mechanism of action. J. Clin. Psychiatry 2001, 62, 923–924.
- Stahl, S.M. Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 1, “Goldilocks” actions at dopamine receptors. J. Clin. Psychiatry 2001, 62, 841–842.
- Nasrallah, H.A.; Mulvihill, T. Iatrogenic disorders associated with conventional vs. atypical antipsychotics. Ann. Clin. Psychiatry 2001, 13, 215–227.
- Kim, D.D.; Barr, A.M.; Lian, L.; Yuen, J.W.Y.; Fredrikson, D.; Honer, W.G.; Thornton, A.E.; Procyshyn, R.M. Efficacy and tolerability of aripiprazole versus D(2) antagonists in the early course of schizophrenia: A systematic review and meta-analysis. Npj Schizophr. 2021, 7, 29.
- Singh, T. Aripiprazole-induced weight gain. Psychiatry 2005, 2, 19.
- Kelly, D.L.; Powell, M.M.; Wehring, H.J.; Sayer, M.A.; Kearns, A.M.; Hackman, A.L.; Buchanan, R.; Nichols, R.B.; McEvoy, J.P.; Adams, H.A.; et al. Adjunct Aripiprazole Reduces Prolactin and Prolactin-Related Adverse Effects in Premenopausal Women with Psychosis: Results from the DAAMSEL Clinical Trial. J. Clin. Psychopharmacol. 2018, 38, 317–326.
- Yeager, A.; Shad, M.U. Aripiprazole for the Management of Antipsychotic-Induced Hyperprolactinemia: A Retrospective Case Series. Prim. Care Companion CNS Disord. 2020, 22, 26648.
- Nguyen, C.T.; Rosen, J.A.; Bota, R.G. Aripiprazole partial agonism at 5-HT2C: A comparison of weight gain associated with aripiprazole adjunctive to antidepressants with high versus low serotonergic activities. Prim. Care Companion CNS Disord. 2012, 14, 26654.
- Citrome, L. A review of aripiprazole in the treatment of patients with schizophrenia or bipolar I disorder. Neuropsychiatr. Dis. Treat. 2006, 2, 427–443.
- Fryefield, K.; Shad, M.U. Can low-dose aripiprazole reverse some of the adverse effects from a long-acting injectable? Schizophr. Res. 2019, 204, 417–418.
- Potkin, S.G.; Preda, A. Aripiprazole once-monthly long-acting injectable for the treatment of schizophrenia. Expert Opin. Pharmacother. 2016, 17, 395–407.
- Cruz, M.P. Aripiprazole Lauroxil (Aristada): An Extended-Release, Long-Acting Injection For the Treatment of Schizophrenia. Pharm. Ther. 2016, 41, 556–559.
- Ehret, M.J.; Davis, E.; Luttrell, S.E.; Clark, C. Aripiprazole Lauroxil NanoCrystal® Dispersion Technology (Aristada Initio®). Clin. Schizophr. Relat. Psychoses 2018, 12, 92–96.
- Di Lorenzo, R.; Ferri, P.; Cameli, M.; Rovesti, S.; Piemonte, C. Effectiveness of 1-year treatment with long-acting formulation of aripiprazole, haloperidol, or paliperidone in patients with schizophrenia: Retrospective study in a real-world clinical setting. Neuropsychiatr. Dis. Treat. 2019, 15, 183–198.
- Mason, K.; Barnett, J.; Pappa, S. Effectiveness of 2-year treatment with aripiprazole long-acting injectable and comparison with paliperidone palmitate. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211029490.
- Kishimoto, T.; Hagi, K.; Nitta, M.; Leucht, S.; Olfson, M.; Kane, J.M.; Correll, C.U. Effectiveness of Long-Acting Injectable vs Oral Antipsychotics in Patients with Schizophrenia: A Meta-analysis of Prospective and Retrospective Cohort Studies. Schizophr. Bull. 2018, 44, 603–619.
- Tiihonen, J.; Mittendorfer-Rutz, E.; Majak, M.; Mehtälä, J.; Hoti, F.; Jedenius, E.; Enkusson, D.; Leval, A.; Sermon, J.; Tanskanen, A.; et al. Real-World Effectiveness of Antipsychotic Treatments in a Nationwide Cohort of 29,823 Patients with Schizophrenia. JAMA Psychiatry 2017, 74, 686–693.
- Garnock-Jones, K.P. Cariprazine: A Review in Schizophrenia. CNS Drugs 2017, 31, 513–525.
- Campbell, R.H.; Diduch, M.; Gardner, K.N.; Thomas, C. Review of cariprazine in management of psychiatric illness. Ment. Health Clin. 2017, 7, 221–229.
- McCormack, P.L. Cariprazine: First Global Approval. Drugs 2015, 75, 2035–2043.
- Durgam, S.; Earley, W.; Guo, H.; Li, D.; Németh, G.; Laszlovszky, I.; Fava, M.; Montgomery, S.A. Efficacy and safety of adjunctive cariprazine in inadequate responders to antidepressants: A randomized, double-blind, placebo-controlled study in adult patients with major depressive disorder. J. Clin. Psychiatry 2016, 77, 371–378.
- Stahl, S.M. Mechanism of action of cariprazine. CNS Spectr. 2016, 21, 123–127.
- Thase, M.E.; Youakim, J.M.; Skuban, A.; Hobart, M.; Zhang, P.; McQuade, R.D.; Nyilas, M.; Carson, W.H.; Sanchez, R.; Eriksson, H. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: A phase 3, randomized, double-blind study. J. Clin. Psychiatry 2015, 76, 1232–1240.
- Maeda, K.; Sugino, H.; Akazawa, H.; Amada, N.; Shimada, J.; Futamura, T.; Yamashita, H.; Ito, N.; McQuade, R.D.; Mørk, A.; et al. Brexpiprazole I: In vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J. Pharmacol. Exp. Ther. 2014, 350, 589–604.
- Citrome, L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: A systematic review of the efficacy and safety profile for this newly approved antipsychotic-what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int. J. Clin. Pract. 2015, 69, 978–997.
- Stahl, S.M. Mechanism of action of brexpiprazole: Comparison with aripiprazole. CNS Spectr. 2016, 21, 1–6.
- Correll, C.U.; Skuban, A.; Ouyang, J.; Hobart, M.; Pfister, S.; McQuade, R.D.; Nyilas, M.; Carson, W.H.; Sanchez, R.; Eriksson, H. Efficacy and Safety of Brexpiprazole for the Treatment of Acute Schizophrenia: A 6-Week Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Psychiatry 2015, 172, 870–880.
- Ward, K.; Citrome, L. Brexpiprazole for the maintenance treatment of adults with schizophrenia: An evidence-based review and place in therapy. Neuropsychiatr. Dis. Treat. 2019, 15, 247–257.
This entry is offline, you can click here to edit this entry!
