Potassium channels are widely distributed integral proteins responsible for the effective and selective transport of K+ ions through the biological membranes. According to the existing structural and mechanistic differences, they are divided into several groups. All of them are considered important molecular drug targets due to their physiological roles, including the regulation of membrane potential or cell signaling. Among the pharmaceuticals of plant origin, which are potassium channel modulators, flavonoids appear as a powerful group of biologically active substances. It is caused by their well-documented anti-oxidative, anti-inflammatory, anti-mutagenic, anti-carcinogenic, and antidiabetic effects on human health.
- potassium channels
- flavonoids
- potassium channel modulators
1. Introduction
Flavonoids comprise a wide group of polyphenolic compounds of plant origin, which exhibit anti-oxidative, anti-inflammatory, anti-mutagenic, and anti-carcinogenic properties, as well as the capability to regulate the functioning of key cellular enzymes [12][13]. Flavonoids can be divided into subclasses such as flavones, flavonols, flavanols, flavanones, isoflavones, anthocyanidins, and chalcones. Due to their ability to modulate cell physiology, they have a clinically proven positive impact in counteracting a plethora of health problems such as cardiovascular and metabolic diseases, or other inflammation-related pathologies, including cancers.
2. Kv Channels
2.1. Kv1.3 Channel
2.2. Kv1.5 Channel
2.3. Kv2.1 Channel
2.4. Kv4 Channels
2.5. hERG Channels
2.6. Further Kv Channels
The flavonoids also influence the Kv7.1 channel, which contributes to the regulation of the repolarization phase of the cardiac action potential. Puerarin is an isoflavonoid found in the root of Pueraria Lobata, which is known from its anti-inflammatory, immunomodulatory, anti-cancer and cardioprotective properties [52]. In [53], it was demonstrated that the isoflavone, puerarin, effectively downregulates the channel activity via direct interaction with the channel protein. It was reported that this inhibitory effect (along with the blockade of the slow delayed rectifier current ) contributed to the prolongation of action potential duration, which can be beneficial in the case of treatment of cardiovascular diseases. It turns out that also naringenin exerts an inhibitory effect on this channel, with a mild impact of this flavonoid on the current [51]. Eventually, the studies performed by Kang et al. [54] revealed that also (−)-epigallocatechin-3-gallate [54] is a potent inhibitor of the Kv1.7 ion channel.
3. Calcium-Activated Channels (KCa)
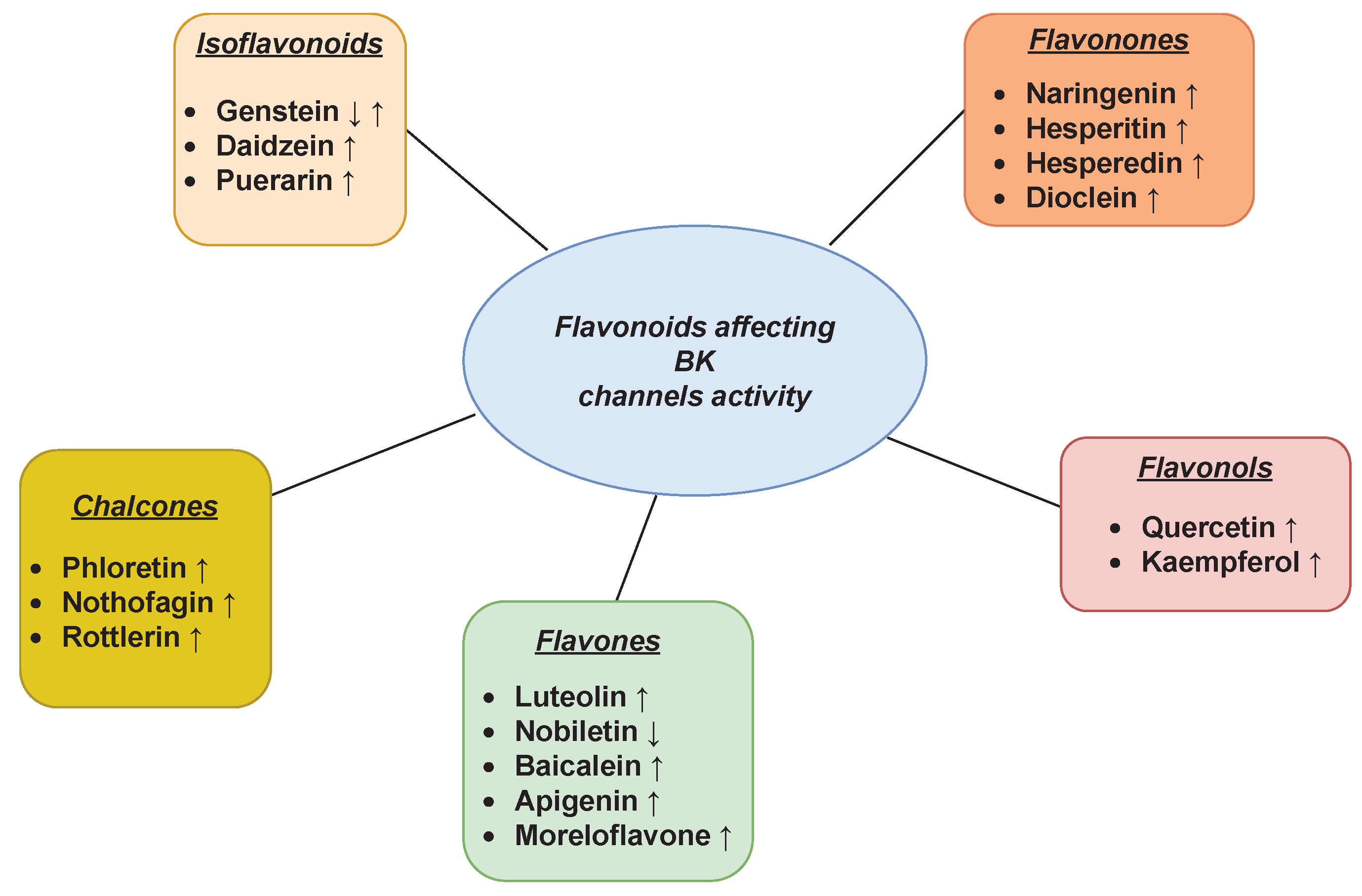
3.1. BK Channel
The large-conductance voltage- and Ca2+-activated channels (BK) are ubiquitously expressed K+ channels being characterized by a large single-channel conductance (150–300 pS) [56]. They are considered important drug targets due to their important roles in many physiological processes, such as neural transmission, hearing, endocrine secretion, and smooth muscle contraction [57]. In addition, the mitochondrial BK channel variants (mitoBK) received great scientific interest in terms of the possibilities of their chemical modulation because of the involvement of these channels in the regulation of metabolism, including ATP synthesis as well as the pro-life and pro-death processes [58][59][60].
3.2. IK and SK Channels
4. Inward Rectifying Potassium Channels (Kir)
The Inward Rectifying Potassium Channels (Kir) belong to one of the structurally simplest ion channels group containing four identical subunits, each containing two membrane-spanning alpha helices. These channels allow ions to be transported more effectively into than out of the cell. They are responsible for the regulation of resting membrane potential. Thus, their function is mostly related to the modulation of cardiac and neural cells activity, insulin secretion, or epithelial K+ transport [72]. The Kir channels are expressed in many cell types: myocytes, neurons, blood cells, endothelial, glial cells, or oocytes [73]. The classification of the Kir channels family covers the groups Kir1–Kir7 together with their respective subgroups. Among the Kir channels, one can also distinguish the adenosine triphosphate (ATP)-dependent K+ channels (KATP, Kir6) and the G-protein regulated K+ channels (GIRK, Kir3). The structure of Kir channels lacks a proper voltage-sensing domain. Nevertheless, some representatives of the Kir family exert a bit stronger ‘’voltage dependence” than the others. In that aspect, Kir 2 channels, which are strongly rectifying ones (and consequently more sensitive to extracellular K+), deserve to be distinguished.
The flavonols represented by quercetin and rutin exert significant impact on Kir channels. Trezza et al. [74] examined the both modulators and 5-hydroxyflavone in the context of their possible impact on the ATP-sensitive Kir6.1 channel. They compared the experimental results of channels from Rat norvegicus aorta cells with molecular dynamics and docking calculations. All the compared results suggested that there was no effect on Kir6.1 caused by rutin, and significant downregulation in the case of quercetin and 5-hydroxyflavone, but only in the case of the closed channel conformation. The cardioprotective effect of flavonoids on rat myocytes through the regulation of mitochondrial ATP-sensitive potassium channels activity was also shown recently by Rameshrad et al. [75]. This research group studied a flavonol, morin, and postulated that its antioxidative effects are mediated by mitochondrial ATP-dependent potassium channels. The activity of Kir6.1 can be upregulated in the presence of isovitexin, which is obtained from the extract of Luehea divaricata Mart., according to [76]. This effect supports the regulation of mesenteric arteriolar tone.
Well-pronounced effects on the Kir channels (especially KATP channels) are also documented for a natural alkaloid, berberine (BBR), which is conditionally classified as an ’isoquinoline flavonoid’. BBR is frequently used in the Chinese and East Asian medicines [77]. Hua et al. confirmed the inhibition effect of BBR on ATP-sensitive channels [78]. The scholars postulated that the anti-arrhythmic and antidiabetic properties of berberine are related to the inhibition of potassium channels. The inhibitory effects of berberine were also investigated by Wang et al. [79]. The scholars characterized a similar anti-arrhythmic impact of BBR manifested by the reduction of action potential duration and the effective refractory period of ischemia. In contrast to BBR, a flavonoid from the anthocyanins-cyanidin caused the upreguation of Kir6.2 genes, which have potential implication in glucose sensitivity and its homeostasis [80].
Another isoflavonoid, puerarin, exerts an activating effect on the mitochondrial K+ ATP-regulated channels (mitoKATP), according to the results presented in [81]. That study concluded that the mitoKATP channel activation participated in the cardioprotection by puerarin. The activation of mitochondrial KATP channels also plays a crucial role in shaping the cardioprotective effects exerted by other flavonoids [82]. Among them, six natural compounds should be mentioned: (−)-epigallocatechin-3-gallate [83], theaflavin [84], proanthocyanidins [85], genistein [86], baicalein [87], and morin[75].
5. Two-Pore Domain Potassium Channels (K2P)
The two-pore domain potassium channels are widely distributed in excitable and non-excitable cells and are responsible for the background potassium conductance [88][89]. They are emerging drug targets in case of a.o. cardiovascular and neurological diseases [90][91][92][93].
Among the TREK subgroup of K2P channels, TREK-1 ( ) and TRAAK ) are mainly expressed in the central nervous system (CNS), and TREK-2 ( ) is expressed in both CNS and peripheral tissues [94][95]. TREK channels are activated by several stimuli, including biomolecules (e.g., riluzole, nitrous oxide, polyunsaturated fatty acids, and lysophospholipids). These modulators can contribute to the opening of TREKs under pathological conditions. Considering the effects of flavonoids’ administration, the neuroprotective properties of quercetin were demonstrated in [96].
6. Conclusions
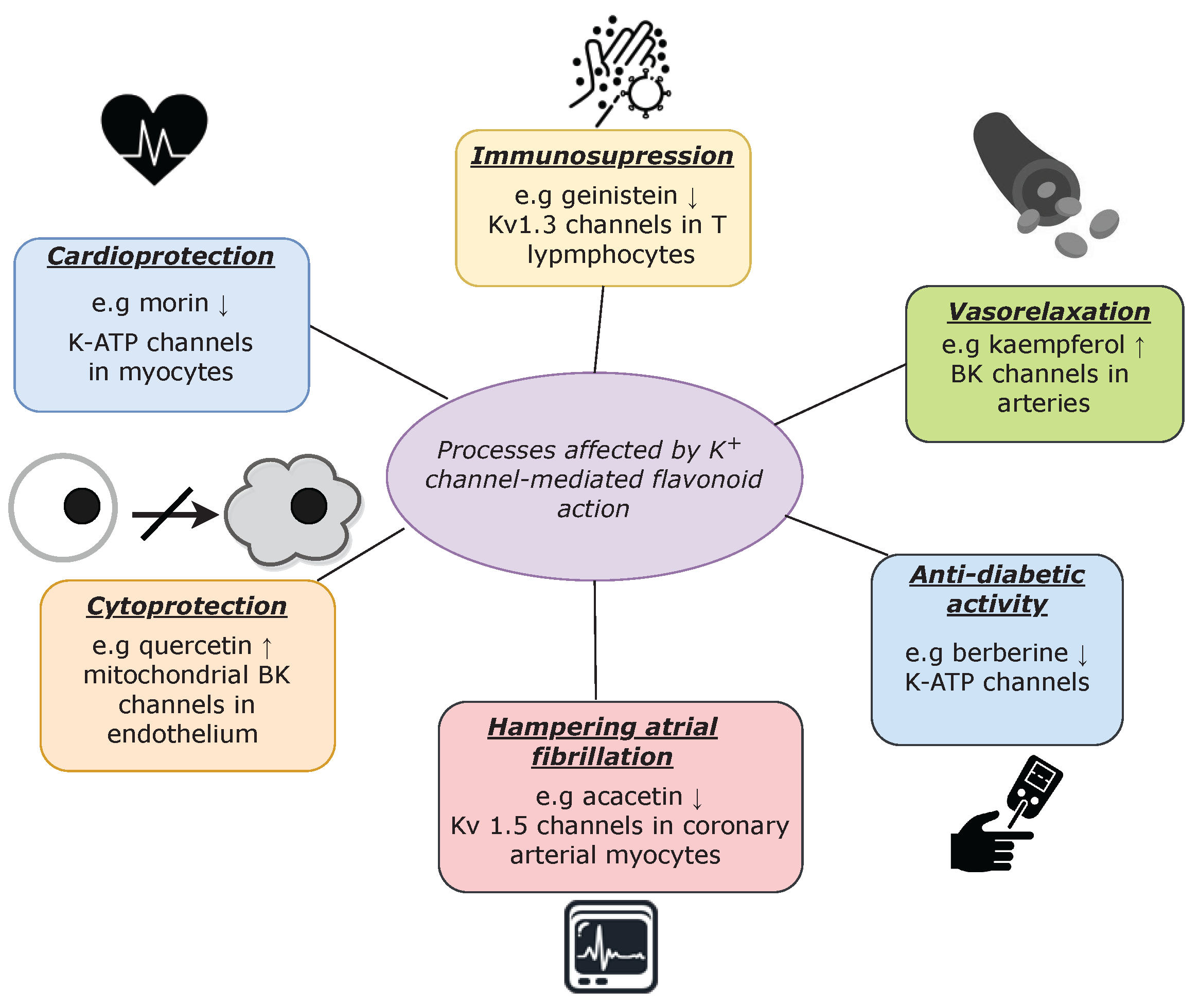
Flavonoids are widely known for their beneficial health effects, which involve complex biochemical interactions with specific molecular targets, including potassium channels. Due to the fact that these transport proteins play important roles in shaping cardiac action potential as well as smooth muscle tone, their stimulation by flavonoids yields vasorelaxant and cardioprotective effects [63][82][97][98][99][100]. Nevertheless, these effects are not the only examples of the K+ channel-mediated physiological processes that are regulated by flavonoids, as presented in Figure 2.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24021311
References
- Hille, B. Ionic channels in excitable membranes. Current problems and biophysical approaches. Biophys. J. 1978, 22, 283–294.
- Coetzee, W.A.; Amarillo, Y.; Chiu, J.; Chow, A.; Lau, D.; McCormack, T.; Morena, H.; Nadal, M.S.; Ozaita, A.; Pountney, D.; et al. Molecular diversity of K+ channels. Ann. N. Y. Acad. Sci. 1999, 868, 233–255.
- Miller, C. An overview of the potassium channel family. Genome Biol. 2000, 1, reviews0004.1.
- MacKinnon, R. Potassium channels. FEBS Lett. 2003, 555, 62–65.
- Kuang, Q.; Purhonen, P.; Hebert, H. Structure of potassium channels. Cell. Mol. Life Sci. 2015, 72, 3677–3693.
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Discov. 2009, 8, 982–1001.
- Hutchings, C.J.; Colussi, P.; Clark, T.G. Ion channels as therapeutic antibody targets. Proc. Mabs. Taylor Fr. 2019, 11, 265–296.
- Mathie, A.; Veale, E.L.; Cunningham, K.P.; Holden, R.G.; Wright, P.D. Two-pore domain potassium channels as drug targets: Anesthesia and beyond. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 401–420.
- Cui, M.; Cantwell, L.; Zorn, A.; Logothetis, D.E. Kir Channel Molecular Physiology, Pharmacology, and Therapeutic Implications. Pharmacol. Potassium Channels 2021, 267, 277–356.
- Dudem, S.; Sergeant, G.P.; Thornbury, K.D.; Hollywood, M.A. Calcium-activated K+ channels (KCa) and therapeutic implications. In Pharmacology of Potassium Channels; Springer: Berlin, Germany, 2021; pp. 379–416.
- Zúñiga, L.; Cayo, A.; González, W.; Vilos, C.; Zúñiga, R. Potassium Channels as a Target for Cancer Therapy: Current Perspectives. Oncotargets Ther. 2022, 15, 783–797.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750.
- Ranjan, R.; Logette, E.; Marani, M.; Herzog, M.; Tâche, V.; Scantamburlo, E.; Buchillier, V.; Markram, H. A kinetic map of the homomeric voltage-gated potassium channel (Kv) family. Front. Cell Neurosci. 2019, 13, 358.
- González, C.; Baez-Nieto, D.; Valencia, I.; Oyarzún, I.; Rojas, P.; Naranjo, D.; Latorre, R. K+ channels: Function-structural overview. Compr. Physiol. 2012, 2, 2087–2149.
- Cahalan, M.D.; Chandy, K.G. The functional network of ion channels in T lymphocytes. Immunol. Rev. 2009, 231, 59–87.
- DeCoursey, T.E.; Chandy, K.G.; Gupta, S.; Cahalan, M.D. Voltage-gated K+ channels in human T lymphocytes: A role in mitogenesis? Nature 1984, 307, 465–468.
- Panyi, G.; Possani, L.; De La Vega, R.R.; Gaspar, R.; Varga, Z. K+ channel blockers: Novel tools to inhibit T cell activation leading to specific immunosuppression. Curr. Pharm. Des. 2006, 12, 2199–2220.
- Teisseyre, A.; Gąsiorowska, J.; Michalak, K. Voltage-Gated Potassium Channels Kv1.3–Potentially New Molecular Target in Cancer Diagnostics and Therapy. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2015, 24, 517–524.
- Teisseyre, A.; Michalak, K. Genistein inhibits the activity of Kv1. 3 potassium channels in human T lymphocytes. J. Membr. Biol. 2005, 205, 71–79.
- Yu, Z.; Li, W.; Liu, F. Inhibition of proliferation and induction of apoptosis by genistein in colon cancer HT-29 cells. Cancer Lett. 2004, 215, 159–166.
- Teisseyre, A.; Duarte, N.; Ferreira, M.J.U.; Michalak, K. Influence of the multidrug transporter inhibitors on the activity of Kv1.3 voltage-gated potassium channels. Acta Physiol. Pol. 2009, 60, 69.
- Gasiorowska, J.; Teisseyre, A.; Uryga, A.; Michalak, K. The influence of 8-prenylnaringenin on the activity of voltage-gated kv1.3 potassium channels in human jurkat t cells. Cell. Mol. Biol. Lett. 2012, 17, 559–570.
- Gąsiorowska, J.; Teisseyre, A.; Uryga, A.; Michalak, K. Inhibition of Kv1.3 channels in human Jurkat T cells by xanthohumol and isoxanthohumol. J. Membr. Biol. 2015, 248, 705–711.
- Tamkun, M.M.; Knoth, K.M.; Walbridge, J.A.; Kroemer, H.; Roden, D.M.; Glover, D.M. Molecular cloning and characterization of two voltage-gated K+ channel cDNAs from human ventricle. FASEB J. 1991, 5, 331–337.
- Overturf, K.E.; Russell, S.N.; Carl, A.; Vogalis, F.; Hart, P.; Hume, J.; Sanders, K.; Horowitz, B. Cloning and characterization of a Kv1.5 delayed rectifier K+ channel from vascular and visceral smooth muscles. Am. J. Physiol. Cell Physiol. 1994, 267, C1231–C1238.
- Wang, Z.; Fermini, B.; Nattel, S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ. Res. 1993, 73, 1061–1076.
- Fedida, D.; Wible, B.; Wang, Z.; Fermini, B.; Faust, F.; Nattel, S.; Brown, A. Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ. Res. 1993, 73, 210–216.
- Wettwer, E.; Hála, O.; Christ, T.; Heubach, J.F.; Dobrev, D.; Knaut, M.; Varró, A.; Ravens, U. Role of I Kur in controlling action potential shape and contractility in the human atrium: Influence of chronic atrial fibrillation. Circulation 2004, 110, 2299–2306.
- Christophersen, I.E.; Olesen, M.S.; Liang, B.; Andersen, M.N.; Larsen, A.P.; Nielsen, J.B.; Haunsø, S.; Olesen, S.P.; Tveit, A.; Svendsen, J.H.; et al. Genetic variation in KCNA5: Impact on the atrial-specific potassium current I Kur in patients with lone atrial fibrillation. Eur. Heart J. 2013, 34, 1517–1525.
- Brendel, J.; Peukert, S. Blockers of the Kv1.5 channel for the treatment of atrial arrhythmias. Expert Opin. Ther. Patents 2002, 12, 1589–1598.
- Tamargo, J.; Caballero, R.; Gómez, R.; Delpón, E. IKur/Kv1.5 channel blockers for the treatment of atrial fibrillation. Expert Opin. Investig. Drugs 2009, 18, 399–416.
- Ford, J.W.; Milnes, J.T. New drugs targeting the cardiac ultra-rapid delayed-rectifier current (IKur): Rationale, pharmacology and evidence for potential therapeutic value. J. Cardiovasc. Pharmacol. 2008, 52, 105–120.
- Liu, Y.; Xu, X.H.; Liu, Z.; Du, X.L.; Chen, K.H.; Xin, X.; Jin, Z.D.; Shen, J.Z.; Hu, Y.; Li, G.R.; et al. Effects of the natural flavone trimethylapigenin on cardiac potassium currents. Biochem. Pharmacol. 2012, 84, 498–506.
- Murakoshi, H.; Trimmer, J.S. Identification of the Kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. J. Neurosci. 1999, 19, 1728–1735.
- Malin, S.A.; Nerbonne, J.M. Delayed rectifier K+ currents, IK, are encoded by Kv2 α-subunits and regulate tonic firing in mammalian sympathetic neurons. J. Neurosci. 2002, 22, 10094–10105.
- Trimmer, J.S. Immunological identification and characterization of a delayed rectifier K+ channel polypeptide in rat brain. Proc. Natl. Acad. Sci. USA 1991, 88, 10764–10768.
- Misonou, H.; Mohapatra, D.P.; Trimmer, J.S. Kv2.1: A voltage-gated k+ channel critical to dynamic control of neuronal excitability. Neurotoxicology 2005, 26, 743–752.
- Roe, M.W.; Worley, J.F.; Mittal, A.A.; Kuznetsov, A.; DasGupta, S.; Mertz, R.J.; Witherspoon, S.M.; Blair, N.; Lancaster, M.E.; McIntyre, M.S.; et al. Expression and function of pancreatic β-cell delayed rectifier K+ channels: Role in stimulus-secretion coupling. J. Biol. Chem. 1996, 271, 32241–32246.
- Aréchiga-Figueroa, I.A.; Morán-Zendejas, R.; Delgado-Ramírez, M.; Rodríguez-Menchaca, A.A. Phytochemicals genistein and capsaicin modulate Kv2.1 channel gating. Pharmacol. Rep. 2017, 69, 1145–1153.
- Baldwin, T.J.; Tsaur, M.L.; Lopez, G.A.; Jan, Y.N.; Jan, L.Y. Characterization of a mammalian cDNA for an inactivating voltage-sensitive K+ channel. Neuron 1991, 7, 471–483.
- Jerng, H.H.; Pfaffinger, P.J.; Covarrubias, M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol. Cell. Neurosci. 2004, 27, 343–369.
- Pak, M.D.; Baker, K.; Covarrubias, M.; Butler, A.; Ratcliffe, A.; Salkoff, L. mShal, a subfamily of A-type K+ channel cloned from mammalian brain. Proc. Natl. Acad. Sci. USA 1991, 88, 4386–4390.
- Guo, Y.; Zhang, C.; Ye, T.; Chen, X.; Liu, X.; Chen, X.; Sun, Y.; Qu, C.; Liang, J.; Shi, S.; et al. Pinocembrin ameliorates arrhythmias in rats with chronic ischaemic heart failure. Ann. Med. 2021, 53, 830–840.
- Chen, X.; Wan, W.; Ran, Q.; Ye, T.; Sun, Y.; Liu, Z.; Liu, X.; Shi, S.; Qu, C.; Zhang, C.; et al. Pinocembrin mediates antiarrhythmic effects in rats with isoproterenol-induced cardiac remodeling. Eur. J. Pharmacol. 2022, 920, 174799.
- Kim, H.J.; Ahn, H.S.; Choi, B.H.; Hahn, S.J. Inhibition of Kv4.3 by genistein via a tyrosine phosphorylation-independent mechanism. Am. J. Physiol. Cell Physiol. 2011, 300, C567–C575.
- Haverkamp, W.; Breithardt, G.; Camm, A.J.; Janse, M.J.; Rosen, M.R.; Antzelevitch, C.; Escande, D.; Franz, M.; Malik, M.; Moss, A.; et al. The potential for QT prolongation and pro-arrhythmia by non-anti-arrhythmic drugs: Clinical and regulatory implications: Report on a Policy Conference of the European Society of Cardiology. Cardiovasc. Res. 2000, 47, 219–233.
- Taglialatela, M.; Castaldo, P.; Pannaccione, A.; Giorgio, G.; Annunziato, L. Human ether-a-gogo related gene (HERG) K channels as pharmacological targets: Present and future implications. Biochem. Pharmacol. 1998, 55, 1741–1746.
- Zitron, E.; Scholz, E.; Owen, R.W.; Luück, S.; Kiesecker, C.; Thomas, D.; Kathoöfer, S.; Niroomand, F.; Kiehn, J.; Kreye, V.A.; et al. QTc prolongation by grapefruit juice and its potential pharmacological basis: HERG channel blockade by flavonoids. Circulation 2005, 111, 835–838.
- Scholz, E.P.; Zitron, E.; Kiesecker, C.; Lück, S.; Thomas, D.; Kathöfer, S.; Kreye, V.A.; Katus, H.A.; Kiehn, J.; Schoels, W.; et al. Inhibition of cardiac HERG channels by grapefruit flavonoid naringenin: Implications for the influence of dietary compounds on cardiac repolarisation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2005, 371, 516–525.
- Sanson, C.; Boukaiba, R.; Houtmann, S.; Maizières, M.A.; Fouconnier, S.; Partiseti, M.; Bohme, G.A. The grapefruit polyphenol naringenin inhibits multiple cardiac ion channels. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 735–740.
- Wang, S.; Zhang, S.; Wang, S.; Gao, P.; Dai, L. A comprehensive review on Pueraria: Insights on its chemistry and medicinal value. Biomed. Pharmacother. 2020, 131, 110734.
- Xu, H.; Zhao, M.; Liang, S.; Huang, Q.; Xiao, Y.; Ye, L.; Wang, Q.; He, L.; Ma, L.; Zhang, H.; et al. The effects of puerarin on rat ventricular myocytes and the potential mechanism. Sci. Rep. 2016, 6, 35475.
- Kang, J.; Cheng, H.; Ji, J.; Incardona, J.; Rampe, D. In vitro electrocardiographic and cardiac ion channel effects of (−)-epigallocatechin-3-gallate, the main catechin of green tea. J. Pharmacol. Exp. Ther. 2010, 334, 619–626.
- Ouadid-Ahidouch, H.; Ahidouch, A.; Pardo, L.A. Kv10.1 K+ channel: From physiology to cancer. PflÜGers-Arch. Eur. J. Physiol. 2016, 468, 751–762.
- Marty, A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature 1981, 291, 497–500.
- Cui, J.; Yang, H.; Lee, U.S. Molecular mechanisms of BK channel activation. Cell. Mol. Life Sci. 2009, 66, 852–875.
- Kulawiak, B.; Kudin, A.P.; Szewczyk, A.; Kunz, W.S. BK channel openers inhibit ROS production of isolated rat brain mitochondria. Exp. Neurol. 2008, 212, 543–547.
- Szabo, I.; Zoratti, M. Mitochondrial channels: Ion fluxes and more. Physiol. Rev. 2014, 94, 519–608.
- Krabbendam, I.E.; Honrath, B.; Culmsee, C.; Dolga, A.M. Mitochondrial Ca2+-activated K+ channels and their role in cell life and death pathways. Cell Calcium 2018, 69, 101–111.
- Nishida, S.; Satoh, H. Possible involvement of Ca2+ activated K+ channels, SK channel, in the quercetin-induced vasodilatation. Korean J. Physiol. Pharmacol. 2009, 13, 361–365.
- Maaliki, D.; Shaito, A.A.; Pintus, G.; El-Yazbi, A.; Eid, A.H. Flavonoids in hypertension: A brief review of the underlying mechanisms. Curr. Opin. Pharmacol. 2019, 45, 57–65.
- Calderone, V.; Chericoni, S.; Martinelli, C.; Testai, L.; Nardi, A.; Morelli, I.; Breschi, M.C.; Martinotti, E. Vasorelaxing effects of flavonoids: Investigation on the possible involvement of potassium channels. Naunyn-Schmiedeberg’S Arch. Pharmacol. 2004, 370, 290–298.
- Lopes, K.S.; Marques, A.A.M.; Moreno, K.G.T.; Lorençone, B.R.; Leite, P.R.T.; da Silva, G.P.; Dos Santos, A.C.; Souza, R.I.C.; Gasparotto, F.M.; Cassemiro, N.S.; et al. Small conductance calcium-activated potassium channels and nitric oxide/cGMP pathway mediate cardioprotective effects of Croton urucurana Baill. In hypertensive rats. J. Ethnopharmacol. 2022, 293, 115255.
- Oliani, J.; Ferreira, M.J.P.; Salatino, A.; Salatino, M.L.F. Leaf flavonoids from Croton urucurana and C. floribundus (Euphorbiaceae). Biochem. Syst. Ecol. 2021, 94, 104217.
- da Rocha Lapa, F.; Soares, K.C.; Rattmann, Y.D.; Crestani, S.; Missau, F.C.; Pizzolatti, M.G.; Marques, M.C.A.; Rieck, L.; Santos, A.R.S. Vasorelaxant and hypotensive effects of the extract and the isolated flavonoid rutin obtained from Polygala paniculata L. J. Pharm. Pharmacol. 2011, 63, 875–881.
- Nevala, R.; Paukku, K.; Korpela, R.; Vapaatalo, H. Calcium-sensitive potassium channel inhibitors antagonize genistein-and daidzein-induced arterial relaxation in vitro. Life Sci. 2001, 69, 1407–1417.
- Mahobiya, A.; Singh, T.U.; Rungsung, S.; Kumar, T.; Chandrasekaran, G.; Parida, S.; Kumar, D. Kaempferol-induces vasorelaxation via endothelium-independent pathways in rat isolated pulmonary artery. Pharmacol. Rep. 2018, 70, 863–874.
- Xu, Y.; Leung, G.; Wong, P.; Vanhoutte, P.; Man, R. Kaempferol stimulates large conductance Ca2+-activated K+ (BKCa) channels in human umbilical vein endothelial cells via a cAMP/PKA-dependent pathway. Br. J. Pharmacol. 2008, 154, 1247–1253.
- Xu, Y.; Leung, S.; Leung, G.; Man, R. Kaempferol enhances endothelium-dependent relaxation in the porcine coronary artery through activation of large-conductance Ca 2+-activated K+ channels. Br. J. Pharmacol. 2015, 172, 3003–3014.
- Marques, A.A.M.; da Silva, C.H.F.; de Souza, P.; de Almeida, C.L.; Cechinel-Filho, V.; Lourenço, E.L.; Junior, A.G. Nitric oxide and Ca2+-activated high-conductance K+ channels mediate nothofagin-induced endothelium-dependent vasodilation in the perfused rat kidney. Chem. Biol. Interact. 2020, 327, 109182.
- Reimann, F.; Ashcroft, F.M. Inwardly rectifying potassium channels. Curr. Opin. Cell Biol. 1999, 11, 503–508.
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 2010, 90, 291–366.
- Trezza, A.; Cicaloni, V.; Porciatti, P.; Langella, A.; Fusi, F.; Saponara, S.; Spiga, O. From in silico to in vitro: A trip to reveal flavonoid binding on the Rattus norvegicus Kir6.1 ATP-sensitive inward rectifier potassium channel. PeerJ 2018, 6, e4680.
- Rameshrad, M.; Omidkhoda, S.F.; Razavi, B.M.; Hosseinzadeh, H. Evaluating the possible role of mitochondrial ATP-sensitive potassium channels in the cardioprotective effects of morin in the isolated rat heart. Life Sci. 2021, 264, 118659.
- Tirloni, C.A.S.; Palozi, R.A.C.; Schaedler, M.I.; Guarnier, L.P.; Silva, A.O.; Marques, M.A.; Gasparotto, F.M.; Lourenço, E.L.B.; de Souza, L.M.; Junior, A.G. Influence of Luehea divaricata Mart. extracts on peripheral vascular resistance and the role of nitric oxide and both Ca+2-sensitive and Kir6. 1 ATP-sensitive K+ channels in the vasodilatory effects of isovitexin on isolated perfused mesenteric beds. Phytomedicine 2019, 56, 74–82.
- Teodoro, J.S.; Duarte, F.V.; Rolo, A.P.; Palmeira, C.M. Chapter 28—Mitochondria as a Target for Safety and Toxicity Evaluation of Nutraceuticals. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 387–400.
- Hua, Z.; Wang, X. Inhibitory effect of berberine on potassium channels in guinea pig ventricular myocytes. Yao Xue Xue Bao = Acta Pharm. Sin. 1994, 29, 576–580.
- Wang, Y.X.; Zheng, Y.M.; Zhou, X.B. Inhibitory effects of berberine on ATP-sensitive K+ channels in cardiac myocytes. Eur. J. Pharmacol. 1996, 316, 307–315.
- Suantawee, T.; Elazab, S.T.; Hsu, W.H.; Yao, S.; Cheng, H.; Adisakwattana, S. Cyanidin stimulates insulin secretion and pancreatic β-cell gene expression through activation of L-type voltage-dependent Ca2+ channels. Nutrients 2017, 9, 814.
- Yao, H.; Gao, Q.; Xia, Q. The role of mitochondrial K+ channels in the cardioprotection of puerarin against hypoxia/reoxygenation injury in rats. Zhongguo Ying Yong Sheng Xue Zhi = Zhongguo Yingyong Shenglixue Zazhi = Chin. J. Appl. Physiol. 2010, 26, 459–462.
- Kampa, R.P.; Sęk, A.; Bednarczyk, P.; Szewczyk, A.; Calderone, V.; Testai, L. Flavonoids as new regulators of mitochondrial potassium channels: Ccontribution to cardioprotection. J. Pharm. Pharmacol. 2022, rgac093.
- Song, D.K.; Jang, Y.; Kim, J.H.; Chun, K.J.; Lee, D.; Xu, Z. Polyphenol (−)-epigallocatechin gallate during ischemia limits infarct size via mitochondrial KATP channel activation in isolated rat hearts. J. Korean Med. Sci. 2010, 25, 380–386.
- Ma, H.; Huang, X.; Li, Q.; Guan, Y.; Yuan, F.; Zhang, Y. ATP-dependent potassium channels and mitochondrial permeability transition pores play roles in the cardioprotection of theaflavin in young rat. J. Physiol. Sci. 2011, 61, 337–342.
- Hu, Y.; Li, L.; Yin, W.; Shen, L.; You, B.; Gao, H. Protective effect of proanthocyanidins on anoxia-reoxygenation injury of myocardial cells mediated by the PI3K/Akt/GSK-3β pathway and mitochondrial ATP-sensitive potassium channel. Mol. Med. Rep. 2014, 10, 2051–2058.
- Couvreur, N.; Tissier, R.; Pons, S.; Chenoune, M.; Waintraub, X.; Berdeaux, A.; Ghaleh, B. The Ceiling Effect of Pharmacological Postconditioning with the Phytoestrogen Genistein Is Reversed by the GSK3β Inhibitor SB 216763 through Mitochondrial ATP-Dependent Potassium Channel Opening. J. Pharmacol. Exp. Ther. 2009, 329, 1134–1141.
- Tu, I.H.; Yen, H.T.D.; Cheng, H.W.; Chiu, J.H. Baicalein protects chicken embryonic cardiomyocyte against hypoxia–reoxygenation injury via μ-and δ-but not κ-opioid receptor signaling. Eur. J. Pharmacol. 2008, 588, 251–258.
- Lesage, F.; Lazdunski, M. Molecular and functional properties of two-pore-domain potassium channels. Am. J. Physiol. Ren. Physiol. 2000, 279, F793–F801.
- Feliciangeli, S.; Chatelain, F.C.; Bichet, D.; Lesage, F. The family of K2P channels: Salient structural and functional properties. J. Physiol. 2015, 593, 2587–2603.
- Herrera-Pérez, S.; Campos-Ríos, A.; Rueda-Ruzafa, L.; Lamas, J.A. Contribution of K2P potassium channels to cardiac physiology and pathophysiology. Int. J. Mol. Sci. 2021, 22, 6635.
- Wiedmann, F.; Frey, N.; Schmidt, C. Two-pore-domain potassium (K2P-) channels: Cardiac expression patterns and disease-specific remodelling processes. Cells 2021, 10, 2914.
- Andres-Bilbe, A.; Castellanos, A.; Pujol-Coma, A.; Callejo, G.; Comes, N.; Gasull, X. The background K+ channel TRESK in sensory physiology and pain. Int. J. Mol. Sci. 2020, 21, 5206.
- Luo, Y.; Huang, L.; Liao, P.; Jiang, R. Contribution of Neuronal and Glial Two-Pore-Domain Potassium Channels in Health and Neurological Disorders. Neural Plast. 2021, 2021, 8643129.
- Djillani, A.; Mazella, J.; Heurteaux, C.; Borsotto, M. Role of TREK-1 in health and disease, focus on the central nervous system. Front. Pharmacol. 2019, 10, 379.
- Medhurst, A.D.; Rennie, G.; Chapman, C.G.; Meadows, H.; Duckworth, M.D.; Kelsell, R.E.; Gloger, I.I.; Pangalos, M.N. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Mol. Brain Res. 2001, 86, 101–114.
- Ren, K.; Liu, H.; Guo, B.; Li, R.; Mao, H.; Xue, Q.; Yao, H.; Wu, S.; Bai, Z.; Wang, W. Quercetin relieves D-amphetamine-induced manic-like behaviour through activating TREK-1 potassium channels in mice. Br. J. Pharmacol. 2021, 178, 3682–3695.
- Saponara, S.; Fusi, F.; Iovinelli, D.; Ahmed, A.; Trezza, A.; Spiga, O.; Sgaragli, G.; Valoti, M. Flavonoids and hERG channels: Friends or foes? Eur. J. Pharmacol. 2021, 899, 174030.
- Fusi, F.; Trezza, A.; Tramaglino, M.; Sgaragli, G.; Saponara, S.; Spiga, O. The beneficial health effects of flavonoids on the cardiovascular system: Focus on K+ channels. Pharmacol. Res. 2020, 152, 104625.
- He, J.; Li, S.; Ding, Y.; Tong, Y.; Li, X. Research Progress on Natural Products’ Therapeutic Effects on Atrial Fibrillation by Regulating Ion Channels. Cardiovasc. Ther. 2022, 2022, 4559809.
- Scholz, E.P.; Zitron, E.; Katus, H.A.; Karle, C.A. Cardiovascular ion channels as a molecular target of flavonoids. Cardiovasc. Ther. 2010, 28, e46–e52.
