Most recently discovered active pharmaceutical molecules and market-approved medicines are poorly soluble in water, resulting in limited drug bioavailability and therapeutic effectiveness. The application of coformers in a multicomponent crystal method is one possible strategy to modulate a drug’s solubility. A multicomponent crystal is a solid phase formed when several molecules of different substances crystallize in a crystal lattice with a certain stoichiometric ratio.
1. Introduction
Solubility is the phenomenon of dissolving a solute in a solvent to form a homogeneous system. Solubility is critical to achieving the desired drug concentration in systemic circulation to provide desired pharmacological effects. When a drug is administered orally, it must first dissolve in the gastrointestinal fluids before it can be absorbed into the bloodstream and reach the site of action
[1][2].
The newly discovered drugs with low solubility and a high permeability (about 60% to 70%) belong to class II BCS (biopharmaceutical classification system), and those with a low solubility profile and low permeability are part of class IV BCS
[3][4]. When administered orally, low-solubility drugs require higher doses to reach therapeutic plasma concentrations, which can cause problems associated with the drug delivery system, bioavailability, and therapeutic effectiveness
[2]. The new compounds that were found in
Pinus armandii Franch, namely 4′-methoxy-2-hydroxystilbene, 7,8-dihydro-2,4 (1h,3h)-pteridinedione, and 9h-pyrido [3,4-b] indole,7-methoxy-1,9-dimethyl-, among others, have potential to be used as biological substances in the pharmaceutical industry or as drugs, owing to their anti-inflammatory, anticancer, and anti-HIV activities
[5]. Further research is needed to evaluate the role of these compounds as either active ingredients or coformers.
Multicomponent crystals are solid phases that form when more than one molecule of different substances crystallize together in a crystal lattice with a certain stoichiometric ratio. In salt-form drugs, the components are arranged in a crystal lattice based on their ion pairs, whereas in cocrystals, the components are arranged through weak interactions, such as hydrogen bonds, −π arrangement, or van der Waals interactions
[6].
Coformers are cocrystallizing agents that interact non-ionically with active pharmaceutical ingredients in a crystal lattice; they have high water solubility and are usually non-volatile. Active ingredients and suitable coformers cause interactions through hydrogen bonds, van der Waals interaction, and -π stacking
[7]. The selection of coformers is a significant obstacle in the formation of multicomponent pharmaceutical crystals suited for specific active medicinal components. The ability of the coformer to create hydrogen bonds with the medicinal molecule is usually used to select a coformer. Coformers also need to be GRAS (generally recognized as safe) in order to confirm their non-toxicity and safety
[8].
2. Coformer Selection
The selection of coformers is a major challenge the development of pharmaceutical multicomponent crystals that are compatible with pharmaceutically active substances. The general strategy used in selecting coformers is tactless experimental approaches whereby a predetermined number of candidates from compounds listed in the generally regarded as safe (GRAS) list are used to form multicomponent crystals
[9]. The requirements for a substance to be used as a coformer are that it must be safe and non-toxic and have no side effects. Coformers must be on the FDA’s “Everything added to food in the United States” (EAFUS) list or on the generally regarded as safe (GRAS) list.
[10].
Several approaches can be adopted to select coformers, including the molecular synthon approach using the Cambridge Structural Database (CSD). The CSD provides information about the molecular associations of drugs and coformers based on functional groups bound to supramolecular synthons. A suitable coformer library can be prepared by CSD for the API. CSD is a computer-based approach used to find pairs of coformers and active pharmaceutical ingredients to form multicomponent crystals, as well as reduce costs and experimental time to increase research efficiency
[7][11].
pKa is a simple approximation used to predict the formation of multicomponent crystals. In general, salt formation occurs when there is a difference between the pKa of a base and the pKa of an acid (ΔpKa) > 3, whereas pKa < 0 results in the formation of cocrystals. The value of pKa and the probability of proton transfer between acid–base pairs was found to show a linear relationship. Cruz-Cabeza et al. analyzed the differences in pKa (ΔpKa) values for 6465 crystalline complexes containing acid–base pairs and separated the complexes into three zones based on pKa values. In zone 1 (ΔpKa < −1), approximately 99.1% of crystal complexes were observed to be non-ionized, i.e., no proton transfer, whereas, in zone 3 (ΔpKa > 4), 99.2% of crystal complexes were observed as both ionized and non-ionized crystal structures, indicating that ionization occurs, namely the complete transfer of protons. However, in zone 2 (−1 pKa ≥ 4), the ionized complex increased linearly with ab increasing pKa value
[12].
The Hansen solubility parameter is another important approach used to measure drug and coformer miscibility for crystallization under a multicomponent approach. According to the Hansen solubility parameter, the total cohesive energy of the liquid is divided into contributions from atomic dispersion (δd), dipole–dipole/polar interactions (δp), and hydrogen bonding (δh). Cohesive energy parameters can predict physicochemical properties such as melting point and solubility of a compound. Cohesive energy is the sum of van der Waals bonding forces, covalent bonds, hydrogen bonds, and ionic bonds. Cohesive energy is the energy required to break all these interactions and allow atoms or molecules to detach, resulting in the transformation of solids to liquid/gas or liquids to gas. If the cutoff value of the active compound and the coformer is less than 7 MPa
0.5, it is considered miscible and capable of forming cocrystals
[1][13]. Cocrystals can be formed depending on the cutoff value and radius of the active compound and the conformer
[13].
3. Multicomponent Crystal Formation
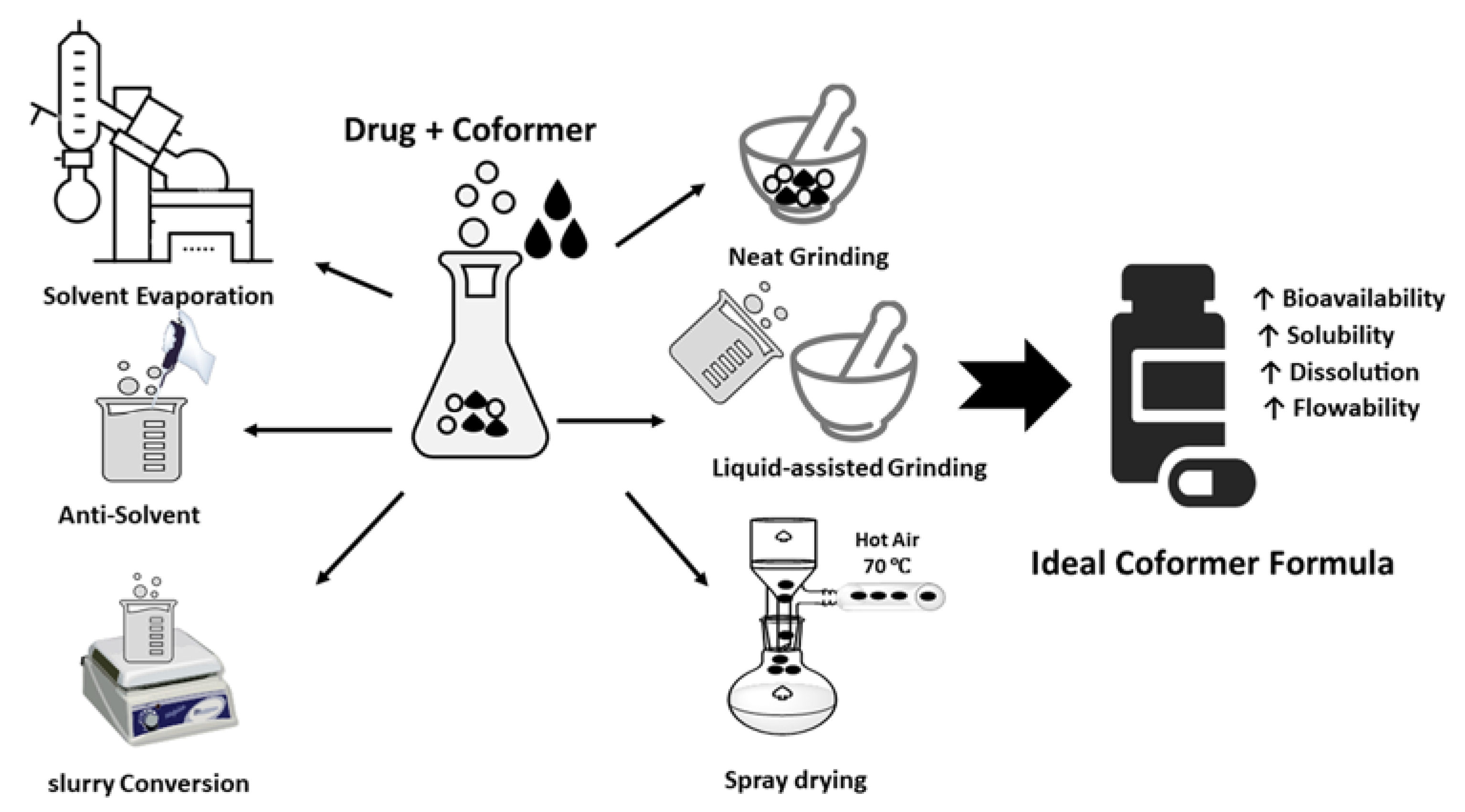
Multicomponent Crystal formed by different ways such as solvent evaporation, slurry conversion, Cooling cocrystallization, neat grinding, Liquid-assisted grinding, and spray drying (Figure 1). Each method was chosen depending on the characteristics both of the drug and coformer.
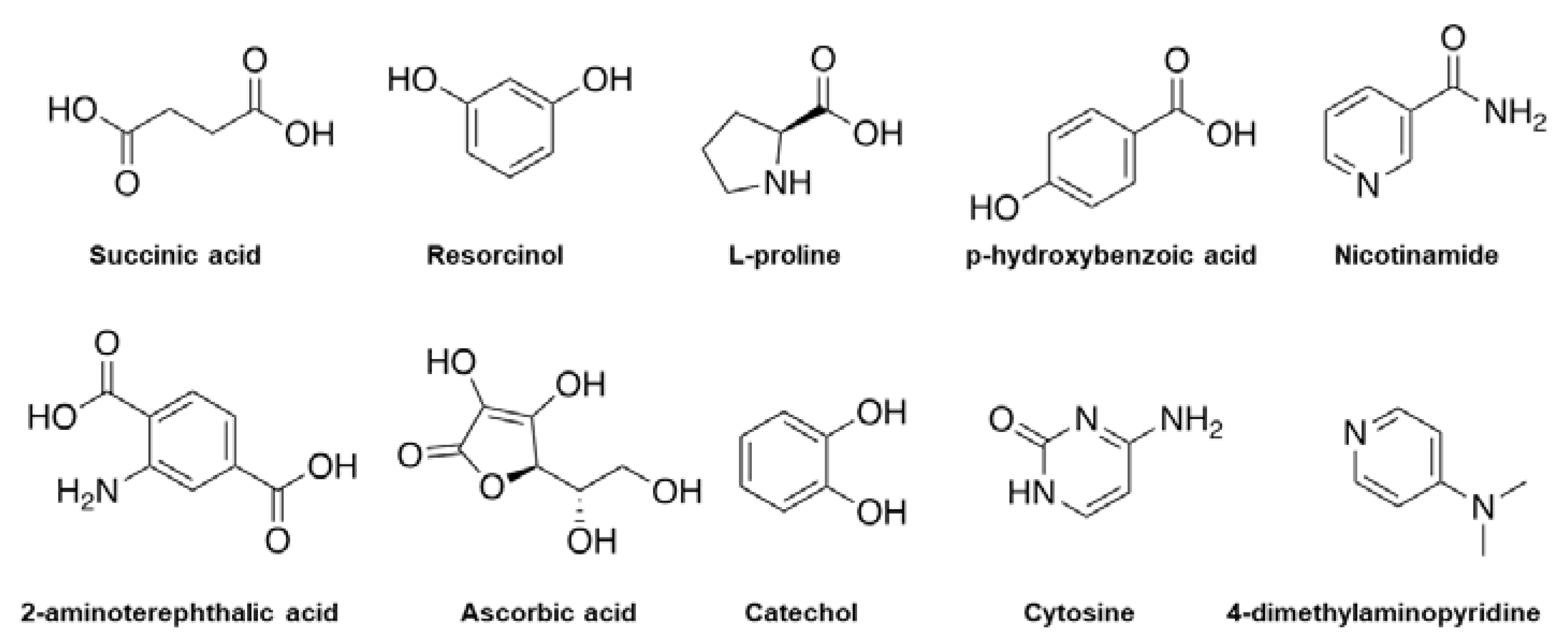
Figure 1. Examples of chemical molecules used for coformer formation.
Methods of forming multicomponent crystals can be classified as either thermodynamic or kinetic methods (
Figure 2). Kinetic method deals with non-equilibrium conditions depending on the energy of the system and the duration of the reaction. These methods are derived primarily from metastable cocrystal forms, which have higher Gibbs free energies than stable forms. Examples of kinetically based multicomponent crystal formation methods include grinding technology, spray drying, slurry sonication, and supercritical fluid technology. Using the thermodynamic method, the reaction occurs under equilibrium conditions and usually takes a long time. An example is the solvent evaporation method. Another classification of multicomponent crystal formation methods relates to the use of solvents. Multicomponent crystal formation techniques are also classified as solvent-based methods or solvent-free methods
[14].
Figure 2. Coformer synthesis techniques.
3.1. Solvent Evaporation
Solvent evaporation is the most commonly used method for laboratory-scale multicomponent crystal formation, during which the drug and coformer are dissolved at a given stoichiometric ratio in a suitable solvent. Stirring is carried out under constant conditions to facilitate molecular interactions between the drug and the conformer, and then the solvent is allowed to evaporate, forming a solid, namely crystals. The main requirement of this method is that the components in forming multicomponent crystals must be completely soluble in the solvent [15].
3.2. Antisolvent
This method utilizes a continuous co-crystallization process to optimize the quality, particle size, and cocrystal characteristics. The addition of an antisolvent reduces the solubility of the cocrystal until it reaches a supersaturated condition, resulting in cocrystal precipitation. As a result, the choice of solvent combination is critical, as the cocrystal must have poor solubility when little solvent is used. Solvent/antisolvent mixtures used to generate cocrystals include ethanol/water, ethanol/acetonitrile, and ethanol/ethyl acetate [16][17].
3.3. Cooling Co-Crystallization
Cooling co-crystallization is based on temperature variations and generally uses a reactor to mix components and solvents. The system is then heated to achieve dissolution of the two components. Saturation is achieved by reducing the temperature. This approach can be used with a complementary phase diagram to identify thermodynamically stable regions and predict potential cocrystal formation. In addition, by analyzing the kinetic pathways of the compounds and the degree of saturation, it is possible to determine the optimal conditions
[14].
3.4. Slurry Conversion
This method involves the formation of cocrystals by adding excess cocrystal components to the solvent. In this method, each component dissolves slowly to form a complex, which may result in the formation of cocrystals. Slurry conversion is an alternative method of producing cocrystals, the coformers of which are insufficiently soluble. The slurry conversion method is simple and includes the crystallization of pure cocrystals with a small quantity of solvent. This approach can be used to produce cocrystals only if the cocrystal is the most thermodynamically stable form relative to other crystalline forms. As a result, this approach can also be used to screen for the most stable crystal formations
[18].
3.5. Neat Grinding
Neat grinding, also called dry grinding or solid grinding, consists of mixing the stoichiometric cocrystal components together in a solid state and grinding them either manually using a mortar and pestle or mechanically using a ball mill or vibratory mill. Manual net grinding is associated with some reproducibility problems, so the mechanical method is more efficient. Mefenamic acid cocrystals with nicotinamide coformers, itraconazole co-crystals with succinic acid coformers, aspartic acid, serine, proline, and glycine were obtained by this technique
[19][20].
3.6. Liquid-Assisted Grinding
Liquid-assisted grinding involves the addition of a small amount of solvent to a dry solid prior to the start of the grinding process. The solvent acts as a catalyst, assisting in the formation of multicomponent crystals and must persist throughout the milling process. Liquid-assisted grinding results in more efficient multicomponent crystal formation and an increase in cocrystal formation kinetics compared to neat grinding
[14].
3.7. Spray Drying
Spray drying is a rapid method used to engineer a solid state, producing a dry powder from a solution or suspension using a stream of hot air. For coformer-pharmaceutically active substance solubility systems, in pure crystalline multicomponents cannot be formed using the solvent evaporation method, the spray-drying method can be used as an alternative. Carbamazepine–glutamic acid, theophylline–nicotinamide, urea succinic acid, and caffeine–glutamic acid cocrystals are examples of incongruent systems that cannot produce pure cocrystals by the solvent evaporation method but manage to form pure crystals when the spray drying-method is used
[21].
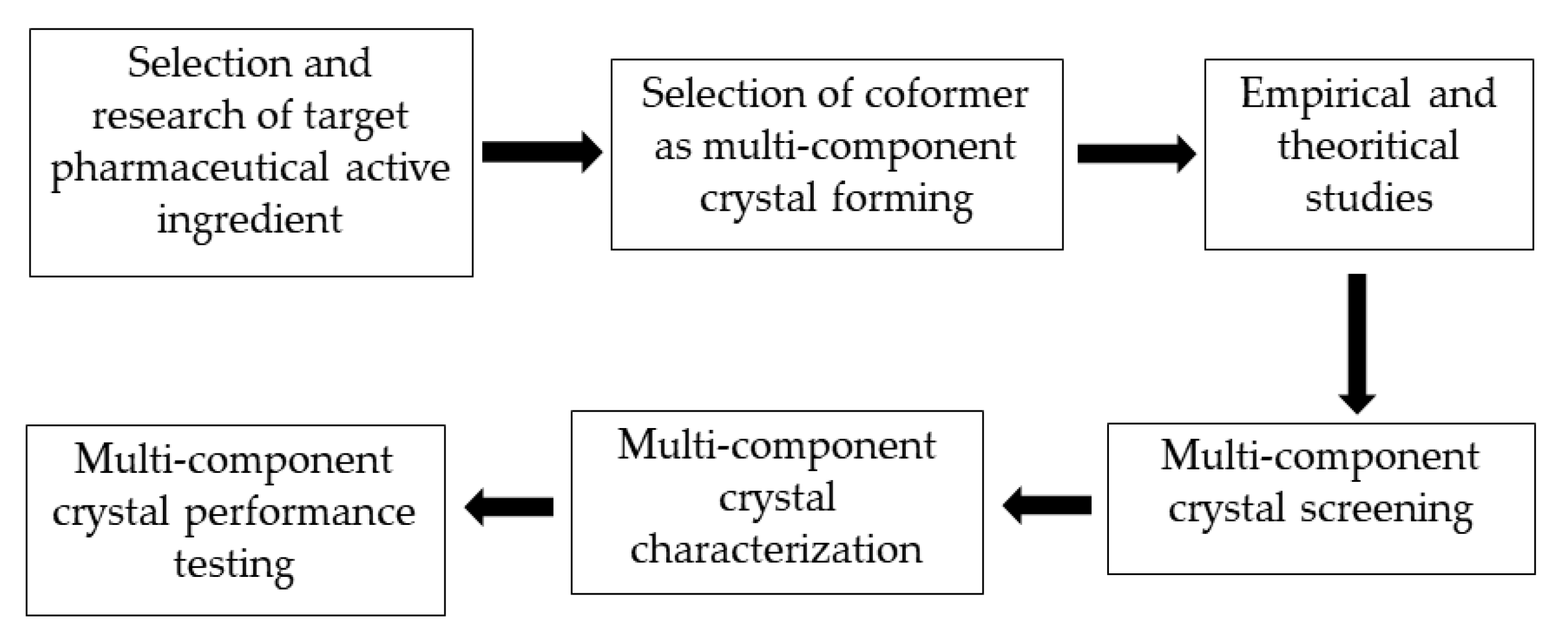
4. Multicomponent Crystal Development Strategy
The stages of a multicomponent crystal development strategy required to increase the solubility of a pharmaceutically active ingredient are presented in Figure 3. The first step in the development of a multicomponent crystal that sis compatible with a particular active substance is to study the molecular structure of the pharmaceutically active substance and identify functional groups that can form intermolecular interactions with appropriate coformers, such as van der Waals bonds, -π stacking, and hydrogen bond interactions. The next step is to select a multicomponent crystal-forming method, namely coformers.
Figure 3. Stages of a multicomponent crystal development strategy.
The main requirement for a coformer is that it must be safe and not cause side effects. Coformers must be registered as generally regarded as safe (GRAS), indicating their safety and non-toxicity. Coformer selection is an important step in the design of multicomponent crystals
[7][11].
During the coformer selection process in the design of multicomponent crystals, empirical and theoretical reference sources can be utilized, such as the Cambridge Structural Database (CSD) approach, Hansen’s solubility parameters, hydrogen bond theory, and pKa values. According to previous research, certain functional groups, such as alcohols, carboxylic acids, and amides, are suitable for the formation of supramolecular heterosynthone
[22].
This entry is adapted from the peer-reviewed paper 10.3390/molecules27248693