1. Anatomy of Horse Skin
In mammalian species, the integumentary system plays an essential role in forming a physical barrier that ensures protection of homeostasis. Despite the high susceptibility to skin-related diseases experienced by domesticated animals, there is incomplete investigation of horse dermatology, and this appears to be an ongoing challenge for pharmaceutical development [
100]. For that reason, the majority of the data and information related to equine skin features were extrapolated from other mammalian species, including humans, dogs, and cats [
100].
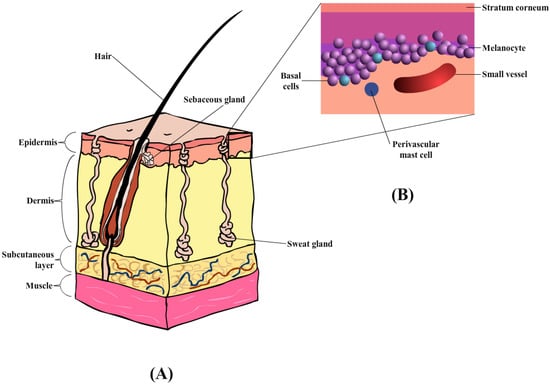
The skin comprises the epidermis and dermis, which provides a physical barrier between the internal and external environment (
Figure 3A) [
101]. Similarly, the skin also plays an important role in the horse, specifically thermoregulation, and provides pigmentation. The outermost layer of the skin in the equine is the epidermis [
100] containing multiple layers of cells, with an average thickness of about 0.0053 mm. Depending on the body region, the thickness of the epidermal layer is as thick as 6 mm, in areas such as the head, back, and rump [
100]. The epidermis layer (
Figure 3B) is composed of five layers of keratinocytes that undergoes constant proliferation, differentiation, and keratinization, contributing to the formation of the
stratum corneum (SC) or the outermost layer. These three processes occur at a reasonable rate to maintain the mechanical barrier. According to Buechner-Maxwell and colleagues [
100], the shedding cycle of a horse lasts for about 17 days, during which cell mitosis and superficial migration occur [
100]. The keratinocytes are also surrounded by the intercellular lipid bilayers which helps prevent fluid loss upon any transdermal penetration [
102]. Furthermore, the horse epidermis also consists of other cell types (or non-keratinocyte), namely Merkel cells, Langerhans’ cells, and Melanocytes. The roles of these specific cell types within the mammalian epidermis are generally the same across all species of mammals (e.g., Langerhans’ cells act as antigen-presenting cells that initiate cutaneous immune responses) [
100].
Horses share similar histological arrangements with other mammals regarding the dermal layer. In the majority of mammalian species the dermal layer is divided into the superficial and deep dermis, which are highly vascularized with collagen-rich connective tissues, and intensive nerve supply [
103]. The horse dermis also contains similar appendage features, such as hair follicles, sebaceous glands, sweat glands, and lymphatic vessels. Unlike other mammals, horses appear to have an extra layer that contributes to the increased thickness in the dorsal, croup, and back areas, where the thinnest (medial thigh and external genitalia) are on the ventral and medial surfaces of the limbs [
100]. Due to the variations within the dermal layer the choice of injection site and needle placement should be carefully considered when administering injectables by the subcutaneous or intramuscular routes.
Moreover, the horse integumentary structure also contains the subcutis layer (also known as hypodermis or subcutaneous), primarily formed by adipocytes, loose fibroblasts, and collagen that aids in anchoring the dermis to the underlying tissues [
102]. A typical structure of equine skin is illustrated in
Figure 3 below [
100].
Figure 3. (A) Diagrammatic illustration of horse skin, containing three main layers: epidermis, dermis, and subcutaneous layers, together with hair follicles and sweat glands. (B) Enlarged illustration of the epidermis layer, containing the stratum corneum, melanocyte, basal, and perivascular mast cells.
2. The Key Considerations for Topical and Intradermal Drug Delivery Development for the Horse
The skin is the major protecting interface between the internal organs and the external environment; it is also considered the largest organ and is highly innervated. A high level of innervation allows horses to respond to external stimuli, demonstrating implications for their general well-being. Several similar obstacles are faced in the development of both human and equine formulations for topical/transdermal administration. While the basic skin anatomy is similar in all mammals; the thickness of the epidermis and dermis layer varies between species. Hence, the rate of percutaneous absorption is different between humans and horses, causing more challenges in developing topical products for systemic delivery in the horse [
104].
Topically applied products come in different physical forms, including dusting powders (solid), creams ointments (semi-solids), and liquids (suspo-emulsion). One of the primary key concerns in equine topical/transdermal formulation development is the rate and extent to which active ingredients can penetrate into and through the skin [
105]. This varies among many intended applications of topical formulations, such as (i) locally acting (corticosteroids and ectoparasiticides), (ii) systemic acting (oestradiol and testosterone patches); (iii) surface(sunscreens), or (iv) deep tissue targeting (NSAIDs) [
105]. Hence, manufacturers must be aware of their intended indications for equine topical products, preparing an appropriate approach for a specific application.
Like the case of human products, for a systemic effect through topical formulation the drug needs to penetrate the SC or the major barrier of the skin [
105,
106]. Different regions of the equine body have different skin thicknesses, the density of hair follicles and glands, as well as vascularity and metabolic enzymes. In other words, differences in skin properties can result in regional differences in percutaneous drug penetration through equine skin [
105]. Therefore, the site of application for topically applied formulation plays an important role, and they should always be carefully considered. For example, topical application of anti-inflammatory hydrocortisone and methylsalicylate on equine legs was found to have a quicker rate and higher level of absorption, compared to when it was administrated in other parts of the body [
105].
Enhancing drug penetration through equine skin can be achieved using chemical and mechanical permeation enhancers. Chemical penetration accelerants interact with the lipid components of the skin, increasing its fluidity and causing the SC to swell [
107,
108]. These substances can also reduce the skin binding of drug molecules, hence, promoting their transportation through the skin. For a chemical to be considered a suitable penetration accelerant, the compound needs to be chemically stable; cutaneous waterproof, non-irritant, non-toxic, and compatible with other ingredients within the formulation [
107]. Stah and Kietzmann [
109] investigated the effects of six different permeation enhancers, as well as the effect of needle lengths on the delivery of transdermal lidocaine to equine skin. The study demonstrated the beneficial effects of equine skin micro-needle pretreatment, on topically applied lidocaine [
109].
For effective delivery of topical products, adequate contact time between the formulation and equine skin is required [
110]. To address issues with contact time, a few micro-vesicle formulations have been developed. For example, Novosomes
Vétoquinol was fabricated into an extremely stable micro-vesicle, with the ability to resist hydrolysis caused by enzymatic activities, and with improved stability at higher temperatures up to 80 °C. Moreover, the negatively charged
Vétoquinol product can form a stable attachment with the positively charged equine hairs, preventing the products from being removed due to rinsing or sweating [
110].
Spherulites® is an equine cleansing product that consists of 1 µm microparticles of plant-derived surfactants. This product contains chitosan and chitosanide which assist in forming a tightly bound film coating over the equine hair and skin. The tightly bound film increases the contact time of active ingredients with the skin, enhancing the topical drug delivery of active ingredients [
110].
As many of the USA FDA-approved equine products are systemically absorbed, veterinarians need to consider systemic side effects when prescribing a topically applied product [
111]. The systemic effect is a major challenge when obtaining the biowaiver/bioequivalent approval for topical products intended for localized effects [
111]. Drug transportation across various epidermal layers is also affected by the skin absorption mechanism. This can be different between species; and within a species, chemical absorption from the skin could vary between seasons, and is also induced by the effects of sex hormones [
104]. In other words, applying the percutaneous penetration data of one species to another without considering these interspecies variations is a risky and impractical approach. In addition to this, if the drug molecules from topical formulations are not well absorbed systemically, bioequivalence cannot be accessed from blood samples; instead, the in vivo efficacy and safety endpoints must be utilized. In this case, a higher number of test subjects need to be used to evaluate the dose–response relationship, instead of the typical dose–blood concentration relationship obtained from blood samples [
112]. A number of these preparations available in the market are presented in
Table 4 below.
Table 4. Examples of Marketed/Registered equine topical/transdermal products and their indications.
3. Novel Topical and Intradermal Drug Delivery Systems and Technologies Development for Horses
One of the most common forms of skin neoplasia in the horse is the Equine sarcoid—Bovine papillomavirus (BPV) induced tumours commonly found in horses [
113]. One of typical treatments for sarcoid involves the use of acyclovir topically [
113], as it is easy for the owner to apply, and the drug is known to have minimal side effects. The topical application of acyclovir in humans is well understood, however, in horses it is not well studied and documented. Haspeslagh et al. [
114] conducted a study to evaluate the transdermal delivery of acyclovir in both normal and sarcoid equine skin. The study revealed that drug penetration into the deeper dermal layers was significantly less in sarcoid skin, compared with normal skin [
114].
Mills and Cross [
115] conducted a study to evaluate the differences in drug penetration of fentanyl patches applied to different areas of equine skin. Skin samples were collected from the thorax, groin, and legs. It was demonstrated that drug penetration was better when the patch was applied to the thorax or the groin regions, compared to the leg. This could be due to the differences in cutaneous blood flow, appendageal density, and the thickness of the SC layer in the various regions of the skin [
115].
In other studies, penetration enhancers (PEs) are included in topically applied formulations, to increase the drug permeation rate through the barrier membrane. This effect can be achieved via interactions with different components of the skin, as PEs are thought to increase fluidity in the intercellular lipid lamellae, as well as cause the SC to swell [
109]. An example of a penetration enhancer is limonene, a substance that increases the percutaneous absorption of lipophilic and hydrophilic drugs. Ferrante et al. conducted a study evaluating the effects of 3 types of PEs–limonene, urea, and oleic acid, on diclofenac diethylamine (DD) permeation across equine skin [
116]. Results obtained from this study revealed a significant increase in DD permeation, and all 3 types are equally useful in enhancing DD transdermal absorption.
However, it is challenging to develop ideal topical DDSs for horses. One of the main obstacles associated with the use of topical medications is the uncertainty of drug penetrability, which influences the degree/extent of the drug that entered the systemic circulation. Drug penetration is dependent on several factors, such as the extent of perfusion of the skin, and the number of hair follicles [
117]. As a result, an important aspect to consider when applying topical formulation is the integrity of the horses’ SC layer. Pretreatments such as shaving, cleaning, or disinfection can irritate and damage the SC, which may cause changes to the drug efficacy, and systemic bioavailability and induce adverse effects. For example, Mills and Cross [
102] conducted a study to demonstrate the effects skin pretreatment has on drug penetration. It was concluded that the destruction of the SC will reduce the skin’s barrier function, leading to an increase in systemic exposure [
102]. Therefore, practitioners should be aware that the destruction of the SC layer increases systemic absorption, heightening equine pharmacological response, and increasing the risk of side effects.
Another potential issue is the extensive range of body sizes across and within equine species. Consequently, the ratio of the patch area to body weight restricts the number of drugs that can be delivered with this technology. As a result, equine patch development is not as feasible compared to patches devised for small animals like cats or dogs. However, patch development containing very potent drugs such as fentanyl, or other compounds where minimal exposure is required, might be possible for use in larger animals such as horses [
118]. Therefore, the choice of incorporated drugs is important and should be considered during the development of equine patches.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15010186