Many types of nanocarriers have been developed for treating brain disorders. Polymer-based therapeutic agents have been explored for the treatment of neurodegenerative diseases due to various fascinating advantages of polymers such as great biocompatibility, nontoxicity, controllable degradation rate, tunable architectures, the possibility of multiple interactions between amyloidogenic protein/peptide and polymer, and excellent in vivo stability. Some of the most commonly used coating polymers for neurodegenerative disorders are discussed.
- drug
- polymers
- neurodegenerative disorders
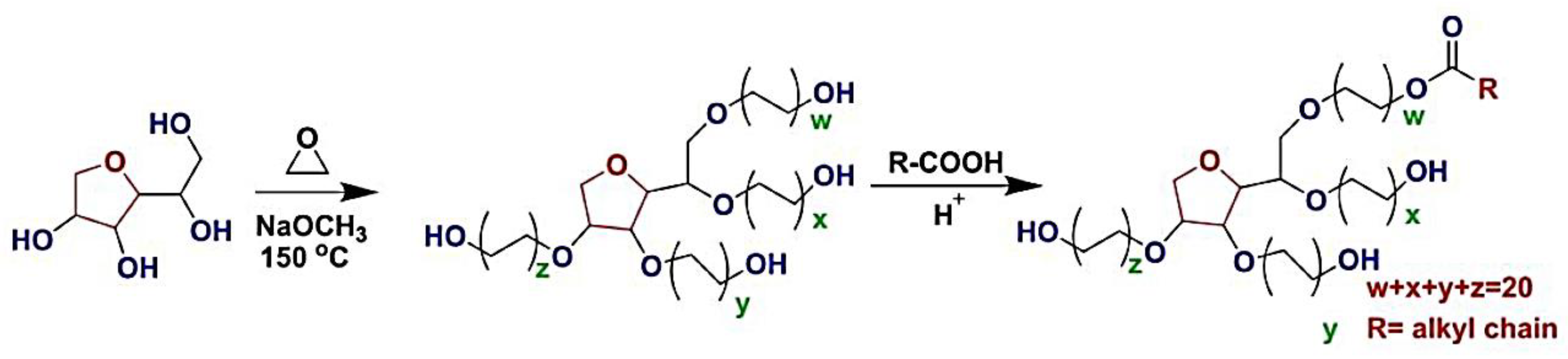
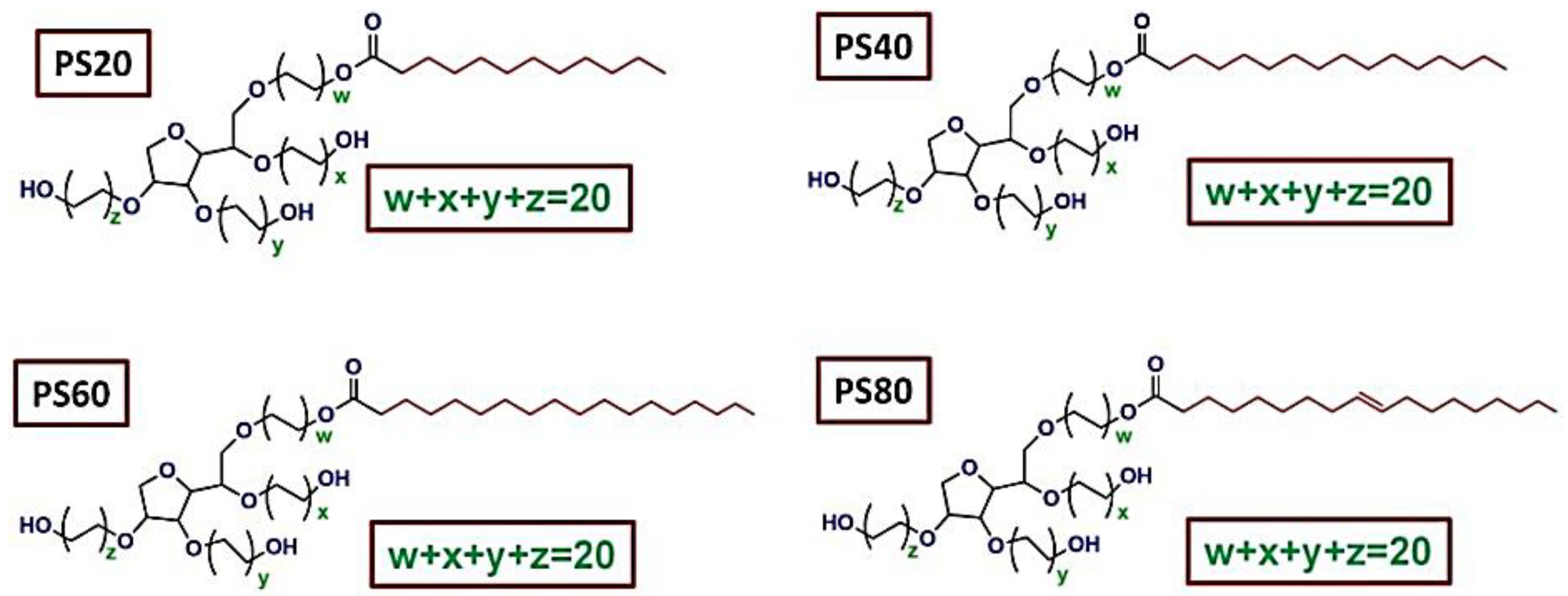
1. Polysorbate (PS)
Polysorbate (PSs) are nonionic synthetic surfactants. This group of surfactants with uncharged head groups is an important subgroup of surfactants made up of poly ethoxy Sorbitan fatty acid esters. PSs have long been used in pharmaceuticals and food additives like emulsifiers and stabilizers [1][2].
2. Polyethylene Glycol (PEG)
Polyethylene Glycols (PEGs) are also known as Macrogols [16]. PEG has become well known due to its great structural flexibility, lack of steric hindrances, amphiphilicity, biocompatibility, and high hydration capacity and has lots on interest in drug delivery applications [17][18]. PEGs have extremely active functional terminals and are electrically neutral at all pH levels [19].
3. Chitosan
4. Poly-Ɛ-Caprolactone (PCL)
Poly-Ɛ-Caprolactone (PCL) is a biodegradable, FDA-approved polyester that has been utilized in a variety of applications, including sutures, implants, contraception devices, and drug delivery systems [38][39]. PCL is made up of repeated hexanoate units and can be broken down in the body by hydrolysis into 6-hydroxy caproic acid [40], which can subsequently be converted into adipate [41] and catalyzed to CO2 [42]. The ring-opening polymerization of 𝜖-caprolactone or the condensation polymerization of 6-hydroxyhexanoic acid is used to prepare it. Due to PCL’s insolubility in water, di-block PEG-b-PCL copolymers are commonly used to prepare PCL-based nanoparticles. Standard procedures such as film dehydration, microfluidics, emulsion, and solvent displacement can be used to prepare these nanoparticles [43]. Drug delivery for neurological diseases has also been examined using PCL-based nanoparticles [44]. For example, in an intracranial glioma tumor-bearing in vivo model, peptide-functionalized PEG–PCL micelles demonstrated significantly better transport ratios and increased accumulation in an in vitro BBB model [45]. PCL, on the other hand, has a low degradation rate, making it inappropriate for use as a drug delivery method [46]. Changing the molar mass or coating it with alternative polymers, such as PLA, might alleviate this problem [47]. Hence, it has been used as a suitable coating in the design of the brain delivery system [48][49][50].
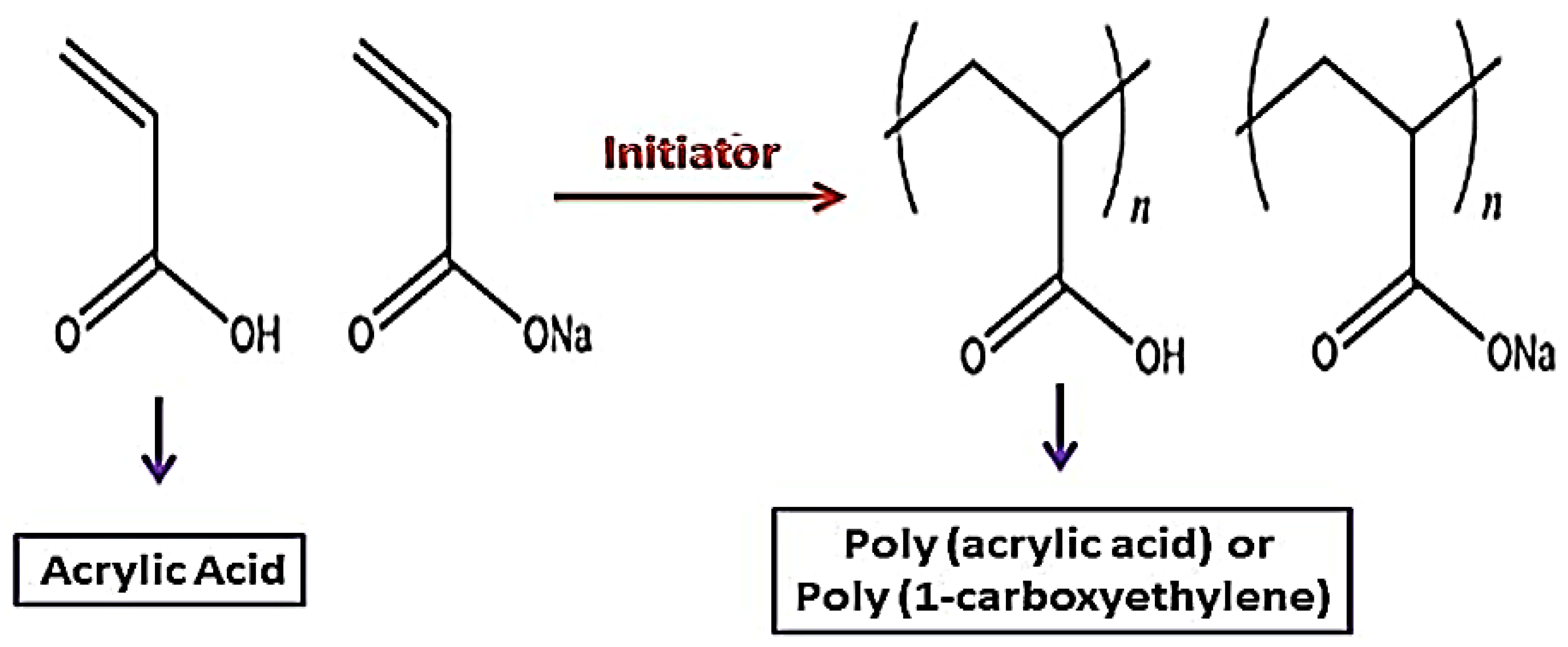
5. Polyacrylic Acid (PAA)
Polyacrylic Acid (PAA), also known as poly 1-carboxyethylene, is a high-molecular-weight synthetic polymer produced from acrylic acid monomers. PAA is typically formed with the use of an initiator and free radical polymerization (Figure 3). Poly (1-carboxyethylene) is a low-cost polymer that has been commercialized [51][52][53].
6. Poly (Lactic-co-Glycolic Acid) (PLGA)
Poly (Lactic-co-Glycolic Acid) (PLGA) is a type of linear copolymer that can be synthesized in a variety of ratios of the two monomers lactic acid and glycolic acid [64]. The FDA has approved PLGA for medical applications like drug delivery devices and biomaterials. The hydrolytic de-esterification of the PLGA copolymers is followed by the clearing of their monomeric anions, lactate, and glycolate, making them nontoxic and biodegradable [65][66]. The transformation of glycolic acid and lactic acid can alter the rate of degradation, degree of crystallinity, and mechanical strength, and therefore release kinetics and drug loading. Polylactic acid (PLA) is a crystalline hydrophobic polymer because of its methyl side chains, whereas polyglycolic acid (PGA) is a rigid and hydrophilic polymer with low mechanical strength [67]. As a result, PLGA copolymers with a higher PGA:PLA ratio are more hydrophobic, which means they degrade and release drugs at a slower rate [68]. PLGA can be prepared by using several methods: Segmer assembly polymerization [69], ring-opening polymerization [70], and the polycondensation process [68]. PLGA nanoparticles can be prepared from PLGA copolymers, utilizing processes like emulsion, nanoprecipitation, solvent co-evaporation, and spray-drying [71]. Soft lithography can also be used to synthesize non-spherical nanoparticles (e.g., cylindrical shapes) [72]. The terminal carboxylic acid groups can be used to introduce required surface modifications [73]. As a result, a wide range of pharmacological compounds, including anti-inflammatory medicines, antibiotics, chemotherapeutics, and proteins, have been integrated into PLGA nanoparticles [71]. For crossing the BBB, a variety of PLGA formulations have been investigated [74]. PLGA may be a well-known choice as a biodegradable medication carrier. The debasement rate of PLGA and the discharge of typified drugs can be controlled by the physicochemical properties of the polymer such as atomic weight, hydrophilicity, and lactide (LA) to glycolide (GA) proportion. So, PLGA-based nanoparticles have a higher effective half-life, bioavailability, and efficacy, and can be efficiently delivered to the brain by intranasal administration as well [75][76][77].
7. Hyaluronic Acid (HA)
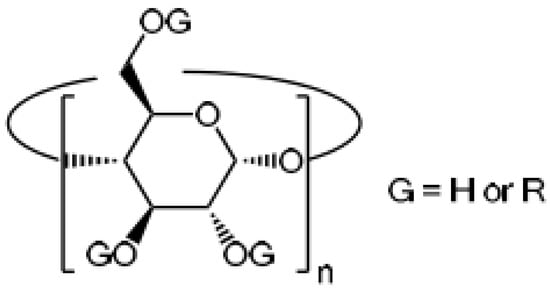
8. Cyclodextrins (CDs)
Cyclodextrins (CDs) are cyclic oligosaccharides made from starch by enzymatically cleaving the amylose helix. These ring-shaped molecules are very hydrophilic because their many hydroxyl moieties face outward. On the other hand, due to the glucosidic oxygen linkages, the inner side of the cavity is less hydrophilic. With the help of this structure, CDs can incorporate other less hydrophilic compounds (guests) into the cavity, creating what are known as host–guest inclusion complexes. Because of the CD cavity’s molecular size, the majority of medications, tastes, cosmetic compounds, insecticides, etc., can be molecularly enclosed there. The size of the guest affects the complex’s stoichiometry. Through particular synthetic processes, the many hydroxyl groups are easily changed to create different CD derivatives [85] (Figure 4).
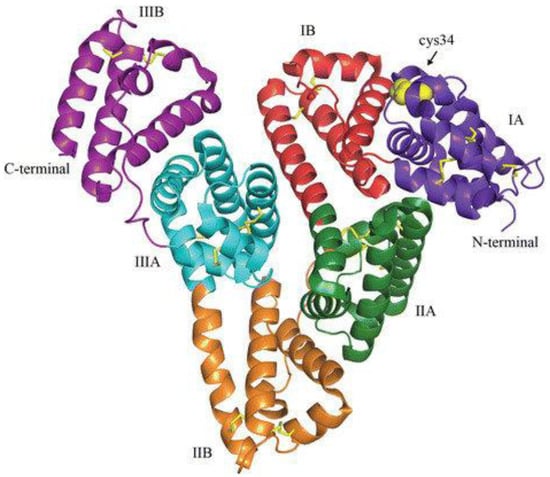
9. Human Serum Albumin (HSA)
This entry is adapted from the peer-reviewed paper 10.3390/molecules28020841
References
- Tao, X.; Li, Y.; Hu, Q.; Zhu, L.; Huang, Z.; Yi, J.; Yang, X.; Hu, J.; Feng, X. Preparation and drug release study of novel nanopharmaceuticals with polysorbate 80 surface adsorption. J. Nanomater. 2018, 2018, 4718045.
- Prieto, C.; Calvo, L. Performance of the biocompatible surfactant Tween 80, for the formation of microemulsions suitable for new pharmaceutical processing. J. Appl. Chem. 2013, 2013, 930356.
- Sahu, A.K.; Mishra, J.; Mishra, A.K. Introducing Tween-curcumin niosomes: Preparation, characterization and microenvironment study. Soft Matter 2020, 16, 1779–1791.
- Deng, L.-L.; Taxipalati, M.; Que, F.; Zhang, H. Physical characterization and antioxidant activity of thymol solubilized Tween 80 micelles. Sci. Rep. 2016, 6, 38160.
- Hekmat, A.; Attar, H.; Seyf Kordi, A.A.; Iman, M.; Jaafari, M.R. New oral formulation and in vitro evaluation of docetaxel-loaded nanomicelles. Molecules 2016, 21, 1265.
- Garidel, P.; Hoffmann, C.; Blume, A. A thermodynamic analysis of the binding interaction between polysorbate 20 and 80 with human serum albumins and immunoglobulins: A contribution to understand colloidal protein stabilisation. Biophys. Chem. 2009, 143, 70–78.
- Jiao, J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1663–1673.
- El-Setouhy, D.A.; Basalious, E.B.; Abdelmalak, N.S. Bioenhanced sublingual tablet of drug with limited permeability using novel surfactant binder and microencapsulated polysorbate: In vitro/in vivo evaluation. Eur. J. Pharm. Biopharm. 2015, 94, 386–392.
- Su, R.; Yang, L.; Wang, Y.; Yu, S.; Guo, Y.; Deng, J.; Zhao, Q.; Jin, X. Formulation, development, and optimization of a novel octyldodecanol-based nanoemulsion for transdermal delivery of ceramide IIIB. Int. J. Nanomed. 2017, 12, 5203.
- Ravichandran, V.; Lee, M.; Nguyen Cao, T.G.; Shim, M.S. Polysorbate-based drug formulations for brain-targeted drug delivery and anticancer therapy. Appl. Sci. 2021, 11, 9336.
- Torosantucci, R.; Furtmann, B.; Elshorst, B.; Pfeiffer-Marek, S.; Hartleb, T.; Andres, N.; Bussemer, T. Protein-excipient interactions evaluated via nuclear magnetic resonance studies in polysorbate-based multidose protein formulations: Influence on antimicrobial efficacy and potential study approach. J. Pharm. Sci. 2018, 107, 2531–2537.
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Miller, D.W. Salinomycin-loaded iron oxide nanoparticles for glioblastoma therapy. Nanomaterials 2020, 10, 477.
- Kreuter, J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev. 2001, 47, 65–81.
- Tröster, S.; Müller, U.; Kreuter, J. Modification of the body distribution of poly (methyl methacrylate) nanoparticles in rats by coating with surfactants. Int. J. Pharm. 1990, 61, 85–100.
- Ray, S.; Sinha, P.; Laha, B.; Maiti, S.; Bhattacharyya, U.K.; Nayak, A.K. Polysorbate 80 coated crosslinked chitosan nanoparticles of ropinirole hydrochloride for brain targeting. J. Drug Deliv. Sci. Technol. 2018, 48, 21–29.
- Thomas, A.; Müller, S.S.; Frey, H. Beyond poly (ethylene glycol): Linear polyglycerol as a multifunctional polyether for biomedical and pharmaceutical applications. Biomacromolecules 2014, 15, 1935–1954.
- Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Lohrasbi-Nejad, A.; Nematollahi, M.H. A new formulation of hydrophobin-coated niosome as a drug carrier to cancer cells. Mater. Sci. Eng. C 2020, 113, 110975.
- Davarpanah, F.; Khalili Yazdi, A.; Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M. Magnetic delivery of antitumor carboplatin by using PEGylated-Niosomes. DARU J. Pharm. Sci. 2018, 26, 57–64.
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275.
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728.
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly (ethylene glycol)-and poly (phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377.
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly (ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308.
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of polymeric nanoparticles for blood–brain barrier transfer—Strategies and challenges. Adv. Sci. 2021, 8, 2003937.
- Howard, M.D.; Jay, M.; Dziubla, T.D.; Lu, X. PEGylation of nanocarrier drug delivery systems: State of the art. J. Biomed. Nanotechnol. 2008, 4, 133–148.
- Rabiee, N.; Ahmadi, S.; Afshari, R.; Khalaji, S.; Rabiee, M.; Bagherzadeh, M.; Fatahi, Y.; Dinarvand, R.; Tahriri, M.; Tayebi, L. Polymeric nanoparticles for nasal drug delivery to the brain: Relevance to Alzheimer’s disease. Adv. Ther. 2021, 4, 2000076.
- Nguyen, T.T.; Nguyen, T.T.D.; Nguyen, T.K.O.; Vo, T.K. Advances in developing therapeutic strategies for Alzheimer’s disease. Biomed. Pharmacother. 2021, 139, 111623.
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11.
- Younes, I.; Hajji, S.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. Int. J. Biol. Macromol. 2014, 69, 489–498.
- Peluso, G.; Petillo, O.; Ranieri, M.; Santin, M.; Ambrosic, L.; Calabró, D.; Avallone, B.; Balsamo, G. Chitosan-mediated stimulation of macrophage function. Biomaterials 1994, 15, 1215–1220.
- Majedi, F.S.; Hasani-Sadrabadi, M.M.; VanDersarl, J.J.; Mokarram, N.; Hojjati-Emami, S.; Dashtimoghadam, E.; Bonakdar, S.; Shokrgozar, M.A.; Bertsch, A.; Renaud, P. On-chip fabrication of paclitaxel-loaded chitosan nanoparticles for cancer therapeutics. Adv. Funct. Mater. 2014, 24, 432–441.
- Mikušová, V.; Mikuš, P. Advances in chitosan-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2021, 22, 9652–9673.
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable polymeric nanoparticles for therapeutic cancer treatments. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 105–127.
- Monsalve, Y.; Tosi, G.; Ruozi, B.; Belletti, D.; Vilella, A.; Zoli, M.; Vandelli, M.A.; Forni, F.; Lopez, B.L.; Sierra, L. PEG-g-chitosan nanoparticles functionalized with the monoclonal antibody OX26 for brain drug targeting. Nanomedicine 2015, 10, 1735–1750.
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019, 560, 282–293.
- Pacheco, C.; Sousa, F.; Sarmento, B. Chitosan-based nanomedicine for brain delivery: Where are we heading? React. Funct. Polym. 2020, 146, 104430.
- Wilson, B.; Alobaid, B.N.M.; Geetha, K.M.; Jenita, J.L. Chitosan nanoparticles to enhance nasal absorption and brain targeting of sitagliptin to treat Alzheimer’s disease. J. Drug Deliv. Sci. Technol. 2021, 61, 102176.
- Manek, E.; Darvas, F.; Petroianu, G.A. Use of biodegradable, chitosan-based nanoparticles in the treatment of Alzheimer’s disease. Molecules 2020, 25, 4866.
- Sinha, V.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ϵ-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23.
- Woodruff, M.; Hutmacher, D. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256.
- Woodward, S.C.; Brewer, P.; Moatamed, F.; Schindler, A.; Pitt, C. The intracellular degradation of poly (ε-caprolactone). J. Biomed. Mater. Res. 1985, 19, 437–444.
- Kanehisa, M.; Goto, S.; Kawashima, S.; Nakaya, A. The KEGG databases at GenomeNet. Nucleic Acids Res. 2002, 30, 42–46.
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617.
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60.
- Varan, C.; Bilensoy, E. Cationic PEGylated polycaprolactone nanoparticles carrying post-operation docetaxel for glioma treatment. Beilstein J. Nanotechnol. 2017, 8, 1446–1456.
- Xin, H.; Jiang, X.; Gu, J.; Sha, X.; Chen, L.; Law, K.; Chen, Y.; Wang, X.; Jiang, Y.; Fang, X. Angiopep-conjugated poly (ethylene glycol)-co-poly (ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials 2011, 32, 4293–4305.
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864.
- Huang, M.-H.; Li, S.; Vert, M. Synthesis and degradation of PLA–PCL–PLA triblock copolymer prepared by successive polymerization of ε-caprolactone and dl-lactide. Polymer 2004, 45, 8675–8681.
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional polymeric nanoplatforms for brain diseases diagnosis, therapy and theranostics. Biomedicines 2020, 8, 13.
- Mohamadpour, H.; Azadi, A.; Rostamizadeh, K.; Andalib, S.; Saghatchi Zanjani, M.R.; Hamidi, M. Preparation, Optimization, and Evaluation of Methoxy Poly (ethylene glycol)-co-poly (ε-caprolactone) Nanoparticles Loaded by Rivastigmine for Brain Delivery. ACS Chem. Neurosci. 2020, 11, 783–795.
- Pohlmann, A.R.; Fonseca, F.N.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.; Guterres, S.S. Poly (ϵ-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 623–638.
- Pavlinec, J.; Novák, I.; Rychlý, J.; Kleinová, A.; Nógellová, Z.; Preťo, J.; Vanko, V.; Žigo, O.; Chodák, I. Hot melt adhesives prepared by grafting of acrylic and crotonic acids onto metallocene ethylene–octene copolymers. J. Plast. Film Sheet. 2019, 35, 239–259.
- Dashtizadeh, A.; Abdouss, M.; Khorassani, M.; Mahdavi, H. Preparation of silica-filled water-based acrylic nanocomposite paints with improved scratch resistance. J. Plast. Film Sheet. 2012, 28, 120–135.
- Foroushani, M.S.; Zahmatkeshan, A.; Arkaban, H.; Shervedani, R.K.; Kefayat, A. A drug delivery system based on nanocomposites constructed from metal-organic frameworks and Mn3O4 nanoparticles: Preparation and physicochemical characterization for BT-474 and MCF-7 cancer cells. Colloids Surf. B Biointerfaces 2021, 202, 111712.
- Mori, H.; Müller, A.H.; Klee, J.E. Intelligent colloidal hybrids via reversible pH-induced complexation of polyelectrolyte and silica nanoparticles. J. Am. Chem. Soc. 2003, 125, 3712–3713.
- Shimizu, T.; Masuda, M.; Minamikawa, H. Supramolecular nanotube architectures based on amphiphilic molecules. Chem. Rev. 2005, 105, 1401–1444.
- Xu, M.; Zhu, J.; Wang, F.; Xiong, Y.; Wu, Y.; Wang, Q.; Weng, J.; Zhang, Z.; Chen, W.; Liu, S. Improved in vitro and in vivo biocompatibility of graphene oxide through surface modification: Poly (acrylic acid)-functionalization is superior to PEGylation. ACS Nano 2016, 10, 3267–3281.
- Kausar, A. Poly (acrylic acid) nanocomposites: Design of advanced materials. J. Plast. Film Sheet. 2021, 37, 409–428.
- Jing, X.; Feng, P.; Chen, Z.; Xie, Z.; Li, H.; Peng, X.-F.; Mi, H.-Y.; Liu, Y. Highly stretchable, self-healable, freezing-tolerant, and transparent polyacrylic acid/nanochitin composite hydrogel for self-powered multifunctional sensors. ACS Sustain. Chem. Eng. 2021, 9, 9209–9220.
- Ebrahimi, A.K.; Sheikhshoaie, I.; Salimi, S.; Arkaban, H. In-situ facile synthesis of superparamagnetic porous core-shell structure for dye adsorption. J. Mol. Struct. 2021, 1228, 129797.
- Jiang, L.; Gao, L.; Wang, X.; Tang, L.; Ma, J. The application of mucoadhesive polymers in nasal drug delivery. Drug Dev. Ind. Pharm. 2010, 36, 323–336.
- Borkar, N.; Mu, H.; Holm, R. Challenges and trends in apomorphine drug delivery systems for the treatment of Parkinson’s disease. Asian J. Pharm. Sci. 2018, 13, 507–517.
- Durazo, S.A.; Kompella, U.B. Functionalized nanosystems for targeted mitochondrial delivery. Mitochondrion 2012, 12, 190–201.
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Pal Singh Chauhan, N.; Jadoun, S.; Dehghani Soltani, M.; Zarrintaj, P. Polyacrylic acid nanoplatforms: Antimicrobial, tissue engineering, and cancer theranostic applications. Polymers 2022, 14, 1259.
- Hines, D.J.; Kaplan, D.L. Poly (lactic-co-glycolic) acid-controlled-release systems: Experimental and modeling insights. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 257–276.
- Jose, S.; Sowmya, S.; Cinu, T.; Aleykutty, N.; Thomas, S.; Souto, E. Surface modified PLGA nanoparticles for brain targeting of Bacoside-A. Eur. J. Pharm. Sci. 2014, 63, 29–35.
- Arkaban, H.; Mirzaei, M.; Behzadi, M. Magnetic solid-phase extraction of lawsone using polyphenol-coated magnetic nanoparticles: Synthesis, characterization and examination. Chromatographia 2021, 84, 455–462.
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397.
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659.
- Stayshich, R.M.; Weiss, R.M.; Li, J.; Meyer, T.Y. Periodic incorporation of pendant hydroxyl groups in repeating sequence PLGA copolymers. Macromol. Rapid Commun. 2011, 32, 220–225.
- Choi, S.H.; Park, T.G. Synthesis and characterization of elastic PLGA/PCL/PLGA tri-block copolymers. J. Biomater. Sci. Polym. Ed. 2002, 13, 1163–1173.
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-based nanoparticles in cancer treatment. Front. Pharmacol. 2018, 9, 1260.
- Chu, K.S.; Hasan, W.; Rawal, S.; Walsh, M.D.; Enlow, E.M.; Luft, J.C.; Bridges, A.S.; Kuijer, J.L.; Napier, M.E.; Zamboni, W.C. Plasma, tumor and tissue pharmacokinetics of Docetaxel delivered via nanoparticles of different sizes and shapes in mice bearing SKOV-3 human ovarian carcinoma xenograft. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 686–693.
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG-PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86.
- Hoyos-Ceballos, G.P.; Ruozi, B.; Ottonelli, I.; Da Ros, F.; Vandelli, M.A.; Forni, F.; Daini, E.; Vilella, A.; Zoli, M.; Tosi, G. PLGA-PEG-ANG-2 Nanoparticles for Blood–Brain Barrier Crossing: Proof-of-Concept Study. Pharmaceutics 2020, 12, 72.
- Gambaryan, P.; Kondrasheva, I.; Severin, E.; Guseva, A.; Kamensky, A. Increasing the Efficiency of Parkinson’s Disease Treatment Using a poly (lactic-co-glycolic acid) (PLGA) Based L-DOPA Delivery System. Exp. Neurobiol. 2014, 23, 246.
- Huang, W.; Zhang, C. Tuning the size of poly (lactic-co-glycolic acid) (PLGA) nanoparticles fabricated by nanoprecipitation. Biotechnol. J. 2018, 13, 1700203.
- Zeb, A.; Gul, M.; Nguyen, T.-T.-L.; Maeng, H.-J. Controlled release and targeted drug delivery with poly (lactic-co-glycolic acid) nanoparticles: Reviewing two decades of research. J. Pharm. Investig. 2022, 52, 683–724.
- Popova, N.V.; Jücker, M. The role of mTOR signaling as a therapeutic target in cancer. Int. J. Mol. Sci. 2021, 22, 1743.
- Su, M.; Soomro, S.H.; Jie, J.; Fu, H. Effects of the extracellular matrix on myelin development and regeneration in the central nervous system. Tissue Cell 2021, 69, 101444.
- Bădilă, A.E.; Rădulescu, D.M.; Niculescu, A.-G.; Grumezescu, A.M.; Rădulescu, M.; Rădulescu, A.R. Recent advances in the treatment of bone metastases and primary bone tumors: An up-to-date review. Cancers 2021, 13, 4229.
- Voci, S.; Fresta, M.; Cosco, D. Gliadins as versatile biomaterials for drug delivery applications. J. Control. Release 2021, 329, 385–400.
- Browne, S.; Hossainy, S.; Healy, K. Hyaluronic Acid macromer molecular weight dictates the biophysical properties and in vitro cellular response to semisynthetic hydrogels. ACS Biomater. Sci. Eng. 2019, 6, 1135–1143.
- Zakusilo, F.T.; O’Banion, M.K.; Gelbard, H.A.; Seluanov, A.; Gorbunova, V. Matters of size: Roles of hyaluronan in CNS aging and disease. Ageing Res. Rev. 2021, 72, 101485.
- Grieco, M.; Ursini, O.; Palamà, I.E.; Gigli, G.; Moroni, L.; Cortese, B. HYDRHA: Hydrogels of hyaluronic acid. New biomedical approaches in cancer, neurodegenerative diseases, and tissue engineering. Mater. Today Bio 2022, 17, 100453.
- Coisne, C.; Tilloy, S.; Monflier, E.; Wils, D.; Fenart, L.; Gosselet, F. Cyclodextrins as emerging therapeutic tools in the treatment of cholesterol-associated vascular and neurodegenerative diseases. Molecules 2016, 21, 1748.
- Loftus, S.K.; Morris, J.A.; Carstea, E.D.; Gu, J.Z.; Cummings, C.; Brown, A.; Ellison, J.; Ohno, K.; Rosenfeld, M.A.; Tagle, D.A. Murine model of Niemann-Pick C disease: Mutation in a cholesterol homeostasis gene. Science 1997, 277, 232–235.
- Carstea, E.D.; Morris, J.A.; Coleman, K.G.; Loftus, S.K.; Zhang, D.; Cummings, C.; Gu, J.; Rosenfeld, M.A.; Pavan, W.J.; Krizman, D.B. Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science 1997, 277, 228–231.
- Fanali, G.; Di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290.
- Sebak, S.; Mirzaei, M.; Malhotra, M.; Kulamarva, A.; Prakash, S. Human serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: Preparation and in vitro analysis. Int. J. Nanomed. 2010, 5, 525.
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell. Ther. 2016, 4, 3.
