Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Capsicum is one of the most economically important genera in the Solanaceae family. Capsicum fruits (peppers) are rich in phytochemicals with high nutritional value and significant health-promoting characteristics. The phytochemical profile of peppers consists of capsaicinoids, carotenoids, and phenolics, primarily.
- capsaicinoids
- carotenoids
- irradiance
- phenolic compounds
- plant secondary metabolites
1. Introduction
Capsicum is one of the most economically important genera in the Solanaceae family. This genus encompasses five domesticated species with more than 50,000 cultivars [1]. The fruits of Capsicum (peppers) are associated with significant health-promoting properties attributable to their nutritional composition and metabolite contents. These properties include analgesic, anti-obesity, cardioprotective, pharmacological, neurological, and dietetic, among others [2]. The specific phytochemicals associated with these properties include carotenoids (provitamin A), phenolic compounds, and capsaicinoids, primarily [3].
The phytochemical and secondary metabolite profiles of peppers are also a good source of nutrients and bioactive compounds [4][5]. Secondary metabolites are a large group of organic compounds with low molecular weight and specific physiological functions. These metabolites serve as chemical adaptations to stress conditions, or as defensive, protective, or offensive chemical agents against micro-organisms, insects, and herbivores [6].
The chemical composition of peppers is closely related to genotype, the process of fruit ripening [3][7], and environmental conditions [8][9]. The environmental factors that affect the biosynthesis, metabolism, and accumulation of phytochemicals in peppers include light, temperature, soil-water availability, and plant nutrition [10]. Thus, changes in environmental conditions can affect the biosynthesis of bioactive compounds in peppers [8].
Peppers vary in color, shape, and chemical composition [7]. Color properties vary by genotype and cultivar. Color changes occur during fruit maturation when the plastids transition from chloroplast to chromoplast in the fruits’ pericarp [3].
Currently, the production of peppers is carried out predominantly under protected horticulture conditions [11]. In particular, the manipulation of natural light by photo-selective netting or plastics, and supplemental lighting (artificial light) can be used to reduce heat and light stress and improve the yield and quality of horticultural crops [12]. These horticultural practices modify the light intensity and spectrum intercepted by the plants and may also affect the production levels of total phenols, ascorbic acid, and antioxidants due to the influence of modified light conditions on the metabolic pathways that lead to the formation of the phytochemicals [13]. Controlled growing conditions in glasshouses impacted the carotenoid contents in sweet peppers [14]. Thus, light intensity (irradiance) and spectrum are environmental factors that affect the phytochemical contents of peppers [15].
2. Light Interactions with Capsicum Plants
The growth and productivity of pepper crops are affected by environmental factors [16]. Among these factors, light is the principal source of energy that drives physiological processes, which include: photosynthesis, photomorphogenesis, fruit development, and maturation [17][18]. Plants interact with light through specific pigments that acquire light energy, and photoreceptors which are proteins that elicit different responses based on light conditions [19]. The most important plant photoreceptors reported for pepper plants include phytochromes, cryptochromes, phototropins, and UV-B-Resistance 8 (UVR8) photoreceptors (Figure 1) [20]. These photoreceptors have peak absorbance wavelengths for the induction of the responses.
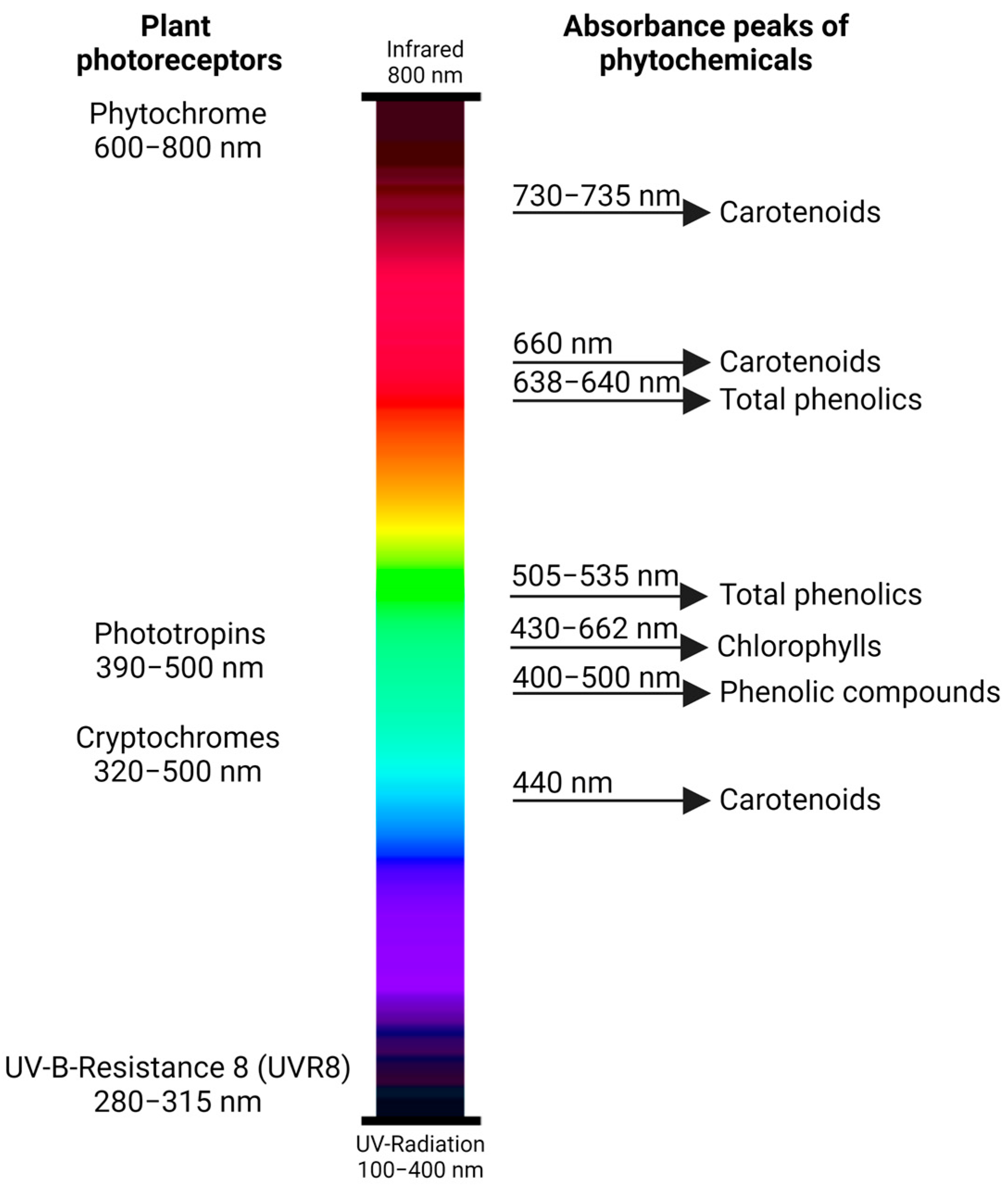
Figure 1. Plant photoreceptors (phytochrome, phototropins, cryptochromes, and UV-B-Resistance 8 (UVR8)) with the corresponding absorbance peaks (wavelengths of the electromagnetic spectrum) for each light-sensing photoreceptor protein. The light-responding groups of phytochemicals in plants in the specific wavelength ranges are provided on the right.
Currently, most of the horticultural production of peppers is carried out under protected agriculture conditions [21] primarily by the implementation of photo-selective shading nets [22], plastics [23], and, in some cases, artificial lighting [9][24] which includes ultraviolet radiation (UV), fluorescent lamps, and light-emitting diodes (LEDs) [25]. The active manipulation of light can improve plant productivity and the quality of peppers [26][27].
The biosynthesis of phytochemicals changes depending on light intensity and spectral quality. Plants accumulate phenolic compounds and other antioxidants such as carotenoids, flavonoids, and anthocyanins to protect against damaging high irradiance and UV radiation. Thus, spectral and irradiance manipulation could promote morphological and physiological responses and influence the biosynthesis, accumulation, and retention of phytochemicals [28][29]. UV radiation and excessive irradiance produced by different light sources may cause stress conditions and activate the defense response, changing a variety of bioactive compounds [25].
Shade nets and plastic covers reduce the light intensity (irradiance) and alter the light spectra that reach the crops. Reduced light intensity affects the physiological responses by decreasing photosynthetic rate and promoting an increase in leaf area [12], while scattering improves the penetration of spectrally modified light into the inner canopy of the crop [28][30]. Currently, the use of black shade nets is the predominant practice in the horticultural production of peppers. Black nets reduce light intensity and have a limited effect on light quality [31][32]. By contrast, colored shading nets selectively filter the solar radiation and promote specific wavelengths [33]. Colored shading nets could promote plants’ physiological and morphological responses [34]. Colored shading nets can selectively change the red to far-red ratios that are detected by the phytochromes, enhance the radiation available to activate the blue/ultraviolet-A photoreceptors, alter the blue light involved in phototropic responses mediated by phototropins, or enhance radiation at other wavelengths that influence plant response [35].
The traditional supplemental light sources used for greenhouse and in vitro applications include fluorescent, metal halide, high-pressure sodium, and incandescent lamps. These light sources have certain limitations as they produce an impractical mixture of wavelengths for plant growth [36], and their electricity consumption is high [37]. LEDs are considered improved light sources for greenhouse production as they can emit specific wavelengths aimed at increasing crop yield, higher quality yield, manipulation of harvest dates, and enhanced nutritional value in cultured plants [38]. Currently, these technologies are preferred for in vitro propagation and indoor plant growth, which are effective for the stimulation of plant phytochemicals during fruit development and postharvest [39].
3. Effects of Light Characteristics on the Phytochemicals of Capsicum Fruits
The most abundant secondary metabolites in Capsicum fruits include capsaicinoids, carotenoids, phenolic compounds, flavonoids, and a wide range of volatile compounds. The accumulation of phytochemicals in peppers is light-dependent, and the high variability of these compounds determines the diversity of aroma and flavor of peppers [40].
3.1. Capsaicinoids
Capsaicinoids are secondary metabolites biosynthesized exclusively by the fruits of Capsicum plants [41]. These metabolites are the bioactive compounds responsible for the pungent taste of peppers [42]. Capsaicinoids may occur in peppers in a wide range of contents from ‘Bell peppers’, where they are practically non-existent, to other high-pungency cultivars such as ‘Naga peppers’ [43]. Capsaicinoids are considered natural defense mechanisms against herbivores ranging from insects to rodents [1]. Capsaicinoids also mediate interactions with birds, who act as seed dispersers for wild peppers [44].
In recent years, capsaicinoid research has been influential in the development of innovative applications in the food and pharmaceutical industries [41] due to their value as antioxidants (free radical scavengers) [45], anti-arthritic [46], gastroprotective [47][48], anti-cancer [49], and analgesic agents [50], among others.
The most abundant capsaicinoids in peppers are capsaicin and dihydrocapsaicin [51][52]. Together, these compounds encompass more than 90% of the total capsaicinoid content of peppers [53]. Nonetheless, at least nine other capsaicinoids including nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicin have also been identified [43]. Capsaicinoid levels are influenced by the ontogenetic development of the peppers. The accumulation of capsaicinoids starts at the early stages of fruit development, followed by a high peak and a rapid decline [54].
3.1.1. Biosynthesis of Capsaicinoids
Capsaicinoid biosynthesis is derived from the phenylpropanoid pathway (Figure 2) [54][55][56] and occurs after the enzymatic condensation of a molecule of vanillylamine derived from phenylalanine, valine, or leucine to a branched-chain amino acid. The enzymes whose alleles determine pungency levels in peppers are CaMYB31, pAMT, CS/AT3/Pun1, and CaKR1 [57]. Capsaicin synthase (CS) is the last enzyme (encoded by the Pun1 gen) responsible for the condensation between vanillylamine and a fatty acid-CoA while the aromatic vanillylamine moiety is paired with many acyl groups, mostly medium-length (from 9 to 11 carbon atoms), giving the immediate reaction of capsaicin biosynthesis [58][59]. Capsaicinoids differ in their chemical structures, specifically in the side chain with a variable number of double bonds placed in different positions; the type of capsaicinoid depends on the products obtained from the different fatty acids in the dehydration synthesis reaction [55].
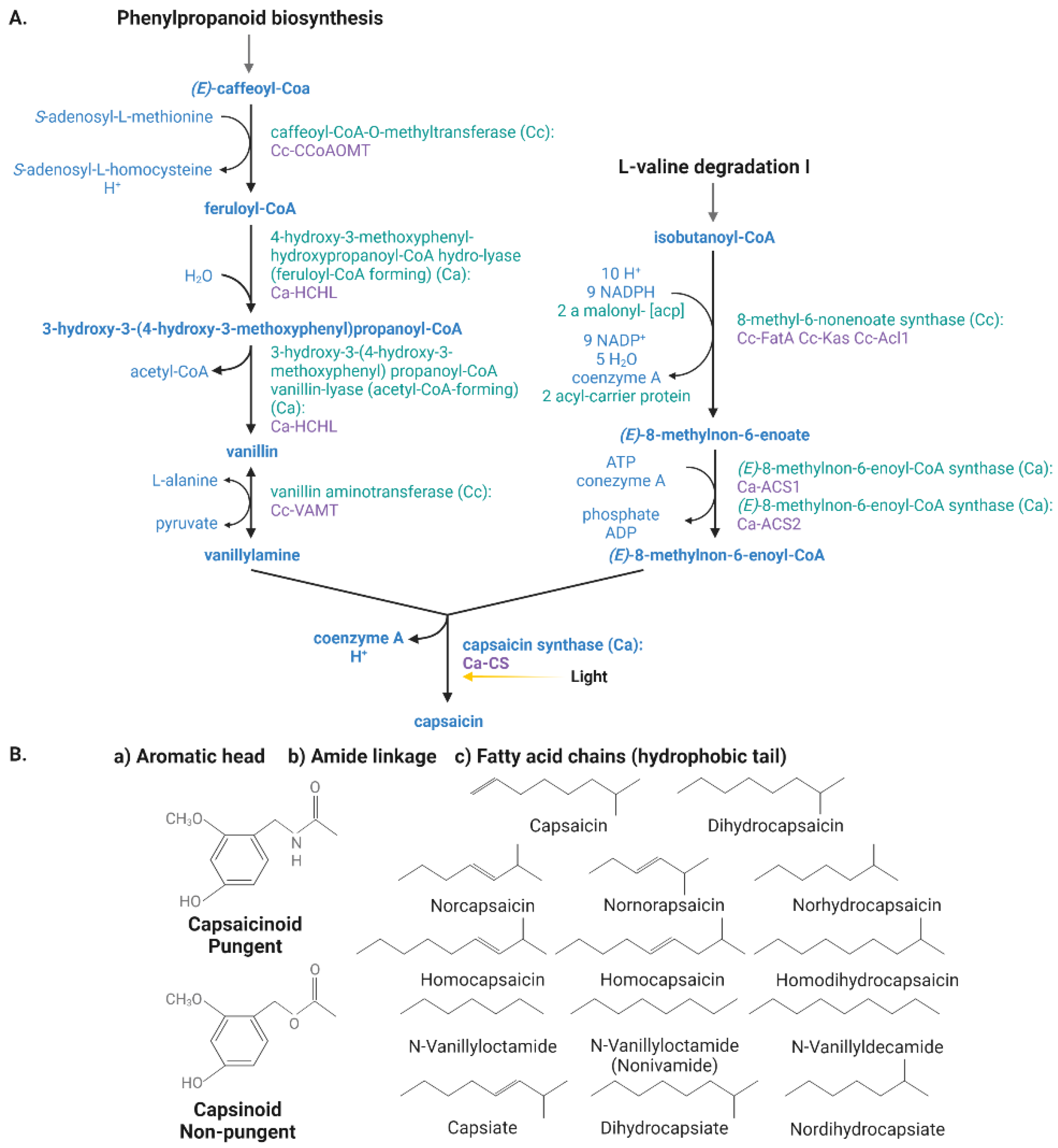
Figure 2. (A) Capsaicinoid biosynthetic pathway in peppers (Capsicum spp.) via phenylpropanoid and L-valine Degradation I. The yellow arrow indicates the light signal that regulates transcription factors at the molecular level. (B) Chemical structure of the most abundant capsaicinoids (pungent) and capsinoids (non-pungent) molecules of Capsicum fruits. Capsaicinoids and capsinoids differ in the R group (fatty acids) present.
Differences in capsaicinoid contents can be attributed to changes in the gene expression of the phenylpropanoid pathway. This biosynthetic pathway depends on the genotype and is affected by environmental conditions that include light, temperature, soil-water availability, and mineral nutrition [36][41]. Light intensity directly affects the biosynthesis and accumulation of capsaicinoids in peppers. Light exposure has a positive influence on the expression of the capsaicin synthase gene (CS) that has light-responsive motifs in its promoter region KAS (keto-acyl ACP synthase) and AMT (aminotransferase), with a negative effect through the induction of peroxidases that can degrade capsaicin. Currently, it is not well understood how this balance is controlled and adjusted [54]. The expression of the CaMYB31, KAS, and pAMT is affected in peppers of the C. annuum genus mainly by light but also by temperature, mechanical stress, and plant hormones [60]. The promoter of the Pun1 gene has light-responsive motifs and consensus elements that promote capsaicinoid biosynthesis [61].
3.1.2. Effects of Light on Capsaicinoids
In a study on bell pepper production, the optimum light intensity reported to obtain maximum fruit yield was estimated in the range of 1365 to 1470 µmol·m−2·s−1 [62]. Horticultural practices that modify irradiance may result in the enhancement or reduction of capsaicinoid contents (Table 1), depending on the species and the light modification mechanisms (e.g., color and degree of shading, or quality of light emitted by artificial illumination) [63].
Capsaicinoid accumulation is affected by the interaction of light intensity with temperature and relative humidity. In high-pungency peppers (C. chinense Jacq.), reduced light intensity and temperature caused lower capsaicinoid production of 4.82 and 3.49 mg plant−1 when plants were grown under 50% and 70% shade, respectively [63]. Reduced capsaicinoid accumulation also occurred at high irradiance levels and high temperatures. In addition, environments with reduced light intensity (713–783 µmol·m−2·s−1) and higher relative humidity increased capsaicinoid production [64]. Thus, the researchers suggest an optimum light intensity of 700 to 950 µmol·m−2·s−1 for capsaicinoid production in these cultivars [63].
Total capsaicinoid contents were significantly affected by the interaction of reduced light intensity using different color shades and harvest time in C. annuum ‘Star flame’ and ‘Fire flame’ [65]. The capsaicinoid contents of peppers grown under colored shading net treatments (white, red, and green) were higher than the unshaded treatment. Of those, the green shade treatment had a considerably higher capsaicinoid content at the first harvest time. This effect could be related to a higher average temperature (22–28 °C) during the cycle. However, other studies showed that higher average temperature and increased solar radiation were associated with lower capsaicinoid contents [41].
Exposure of pepper plants (C. chinense Jacq.) to reduced light intensities using shade nets increased the contents of secondary metabolites, including capsaicinoids and other phenolic compounds [63]. Reduced light intensities increased the contents of the phenylalanine ammonia-lyase (PAL) enzyme, which plays a vital role in capsaicinoid biosynthesis. Thus, an increase in the contents of PAL may also cause an increase in capsaicinoids in peppers [66]. Currently, there is not a full understanding of how capsaicinoid accumulation relates to the relevant biochemical reactions with precursors and environmental factors [58].
As for supplemental light, pepper fruits accumulated more capsaicinoids in plants grown in a closed environment under continuous fluorescent illumination (150–350 µmol·m−2·s−1) and constant temperature (28 °C) than pepper fruits grown under greenhouse conditions during the summer season [67].
Table 1. Effect of light condition treatments on the capsaicinoid content in Capsicum species.
| Capsicum spp. | Light Treatment | Effects on Capsaicinoids Compared to Control |
Biosynthetic Effect |
|---|---|---|---|
| C. chinense Jacq. Seven hot hybrid peppers | Light intensities (1200, 1313, 713, 1112, 774, and 783 μmol·m−2 ·s−1) in different locations with shading net with 50% shade | Reduced light intensity (713–783 µmol·m−2·s−1) and higher relative humidity increased capsaicinoid production in cultivars | Not reported [64] |
| C. chinense Jacq. ‘Bhut Jolokia’ ‘Akanee Pirote’ ‘Habanero’ |
Shading nets with 50%, and 70% shade, and unshaded as control | ‘Bhut Jolokia’ showed the highest capsaicinoid yield under 70% shading, ‘Akanee Pirote’ under 50% shading, and habanero peppers showed the lowest capsaicinoid content under shading treatments | Levels of phenylalanine ammonia-lyase (PAL) increased under low light intensities [63] |
| C. annuum ‘Star flame’ ‘Fire flame’ |
Colored shading nets: white, red, and green with 40% shade, and unshaded as control | Capsaicinoid content increased in color-shading treatments, specifically in green treatment in both cultivars | A high average temperature of 22–28 °C may have promoted capsaicinoid biosynthesis [65] |
| C. annuum ‘Super hot’ |
Greenhouse conditions with LED lighting treatments: blue, red, and a mixture of blue and red light, and 12 h of sunlight as control | Blue LEDs significantly increased nordihydrocapsaicin, capsaicin, dihydrocapsaicin, homocapsaicin, and homodihydrocapsaicin contents by 57, 43, 56, 28, and 54%, respectively | Capsaicin and dihydrocapsaicin accumulation helped in oxidative stress defense. Valine and phenylalanine increased in blue LED lights contributing to a higher content of capsaicinoids [68] |
| C. annuum ‘Cheonyang’ | LED lighting treatments: red, blue, and red plus blue, and fluorescent lamps as control |
Blue LEDs increased capsaicinoid contents, red LEDs reduce two times the capsaicinoid content compared to fluorescent light | Not reported [36] |
| C. annuum ‘Shishito pepper’ |
Continuous fluorescent illumination (150–350 µmol·m−2·s−1) at constant temperature (28 °C), and greenhouse conditions as control | Fewer seeds and higher concentration of capsaicin in fruits under continuous fluorescent illumination | There is a negative correlation between seed formation and capsaicin biosynthesis [67] |
| C. annuum Serrano ‘Tampiqueño 74′ Sweet pepper ‘California wonder’ |
Artificial light in postharvest (50 µmol·m−2·s−2) and dark conditions as control | Light factors increased capsaicin content in ‘Tampiqueño 74′ | CaMYB31-expression analysis from placental tissue of pungent and non-pungent fruits showed a positive correlation with the structural genes Ca4H, Comt, KAS, pAMT, and AT3 expression, and with the content of capsaicin and dihydrocapsaicin during fruit development [60] |
Differences in light spectral quality can also affect the accumulation of capsaicinoids in peppers. Peppers produced under blue spectrum light-emitting diodes (LEDs) increased capsaicinoid contents in comparison to plants exposed to fluorescent lights [36]. In a similar study under greenhouse conditions, supplemental blue light LEDs placed at the top and between plant rows also increased capsaicinoid levels in peppers. This was attributed to the blue wavelength, which is near the UV spectra, and causes the same oxidative stress response during the biosynthesis of capsaicin. Blue light also plays a role in chloroplast development, chlorophyll formation, and stomatal opening [68]. In postharvest, Serrano pepper fruits (‘Tampiqueño 74′) treated with light or dark conditions with varying exposure times, the expression of the structural genes KAS, pAMT, and the transcription factor gene CaMYB31 was higher under the light stimulus than fruits stored in the dark [60].
3.2. Carotenoids
Carotenoids are a numerous family of more than 850 naturally occurring lipophilic isoprenoid compounds widely distributed in nature [69]. All photosynthetic organisms, including plants, algae, and cyanobacteria, and some non-photosynthetic micro-organisms, including fungi and bacteria, synthesize carotenoids [70]. In plants, the principal function of carotenoids is the protection of cells and organelles against oxidative damage. Carotenoids prevent the accumulation of harmful oxygen species by interacting with singlet oxygen molecules and scavenging peroxy radicals [71]. Carotenoids are also involved in the photosynthetic process and play a role in photo-protection, photo-morphogenesis, and plant development. Carotenoids also promote the biosynthesis of other essential compounds and play a role in the attraction of insects for pollination and seed dispersal [4][71][72].
Carotenoids have several important essential functions in human nutrition and health. This group of compounds can prevent and protect from cardiovascular diseases, inhibit carcinogenic cells, macular degeneration, and cataracts [73]. Carotenoids are considered the most effective antioxidant compounds found in peppers, besides phenolic and flavonoid compounds, which act synergistically as efficient free radical scavengers [74][75]. Carotenoids deactivate free radicals and quench reactive oxygen species due to the presence of conjugated double bonds [42][76]. In addition, plant carotenoids are endogenous isoprenoid precursors of vitamin A, β-carotene, α-carotene, γ-carotene, and β-cryptoxanthin which can be converted into retinol, the assimilable form of vitamin A in the human body [77].
Capsicum fruits are rich sources of carotenoids. The wide range of colors in peppers is related to the stage of maturation and the differential accumulation of carotenoids [78][79]. Specifically, oxygenated carotenoids are responsible for the yellow, orange, and red colors of pepper fruits [80].
3.2.1. Biosynthesis of Carotenoids
Carotenoids are derived from the universal five-carbon precursor isopentenyl pyrophosphate (IPP, C5) [7]. In Capsicum, the plastidial isoprenoid biosynthesis pathway starts with the mevalonic acid which is entered into several reactions to produce the C5 building block precursors—isopentenyl diphosphate and dimethylallyl pyrophosphate. In plants, carotenoids are synthesized in the plastid using IPP generated from the methylerythritol-4-phosphate (MEP) pathway (Figure 3) [4][81]. The MEP pathway receives substrates, G3P and pyruvate, from primary metabolism and delivers IPP to the prenyl lipid pathway. Phytoene, the first carotenoid in the pathway, is synthesized from eight IPP units in the prenyl lipid pathway [72]. The carotenoid biosynthesis pathway is split into the α and β branches. The addition of a hydroxyl group to the end rings characterizes the transition from carotene to xanthophyll. The end-products found in red Capsicum fruits are the red pigments capsorubin and capsanthin with κ end groups, the latter being the most abundant [7].
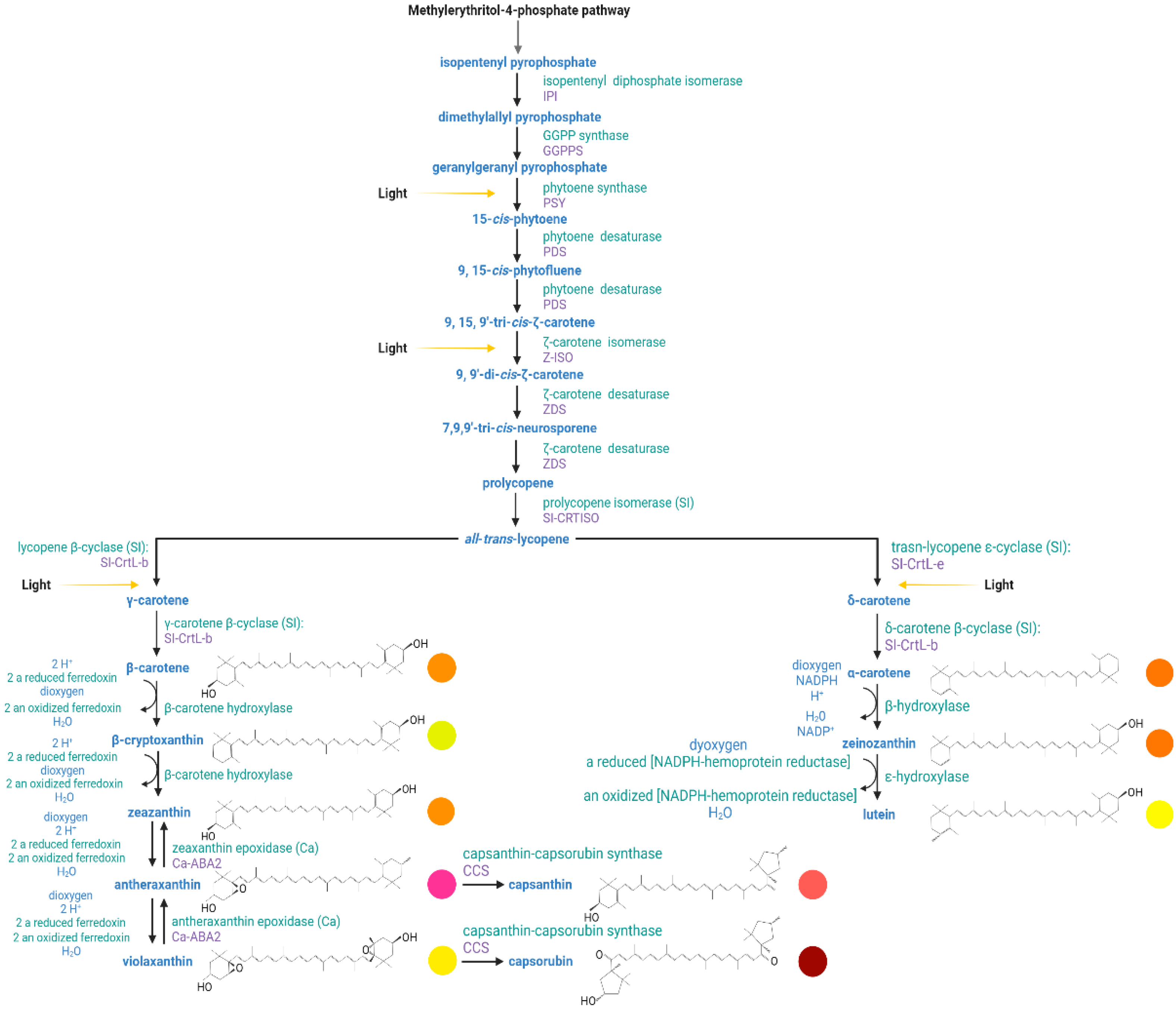
Figure 3. Carotenoid biosynthetic pathway in peppers (Capsicum spp.). Yellow arrows indicate the specific reaction steps at which light signal regulates the transcription factors at the molecular level. Chemical structures of the most abundant carotenoids present in Capsicum fruits. The circles indicate the color to which each carotenoid is associated in plant tissue.
In Capsicum fruits, carotenoid accumulation has been associated with the esterification of xanthophylls to allow for more efficient storage and increased stability, with the expression of a putative carotenoid acyl transferase, and an increased fibril content within the plastid [7].
This entry is adapted from the peer-reviewed paper 10.3390/horticulturae9010072
References
- Wahyuni, Y.; Ballester, A.R.; Tikunov, Y.; de Vos, R.C.H.; Pelgrom, K.T.B.; Maharijaya, A.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolomics and molecular marker analysis to explore pepper (Capsicum sp.) biodiversity. Metabolomics 2013, 9, 130–144.
- De Sá Mendes, N.; de Andrade Gonçalves, É.C.B. The role of bioactive components found in peppers. Trends Food Sci. Technol. 2020, 99, 229–243.
- Cisternas-Jamet, J.; Salvatierra-Martínez, R.; Vega-Gálvez, A.; Stoll, A.; Uribe, E.; Goñi, M.G. Biochemical composition as a function of fruit maturity stage of bell pepper (Capsicum annum) inoculated with Bacillus amyloliquefaciens. Sci. Hort. 2020, 263, 109107.
- Antonio, A.S.; Wiedemann, L.S.M.; Veiga Junior, V.F. The genus: Capsicum: A phytochemical review of bioactive secondary metabolites. RSC Adv. 2018, 8, 25767–25784.
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite biodiversity in pepper (Capsicum) fruits of thirty-two diverse accessions: Variation in health-related compounds and implications for breeding. Phytochemistry 2011, 72, 1358–1370.
- Namdeo, A.G. Plant cell elicitation for production of secondary metabolites: A Review. Pharmacogn. Rev. 2007, 1, 60–79.
- Berry, H.M.; Rickett, D.V.; Baxter, C.J.; Enfissi, E.M.A.; Fraser, P.D. Carotenoid biosynthesis and sequestration in red chilli pepper fruit and its impact on colour intensity traits. J. Exp. Bot. 2019, 70, 2637–2650.
- Lekala, C.S.; Madani, K.S.H.; Phan, A.D.T.; Maboko, M.M.; Fotouo, H.; Soundy, P.; Sultanbawa, Y.; Sivakumar, D. Cultivar-specific responses in red sweet peppers grown under shade nets and controlled-temperature plastic tunnel environment on antioxidant constituents at harvest. Food Chem. 2019, 275, 85–94.
- Pola, W.; Sugaya, S.; Photchanachai, S. Influence of postharvest temperatures on carotenoid biosynthesis and phytochemicals in mature green chili (Capsicum annuum L.). Antioxidants 2020, 9, 203.
- Ncise, W.; Daniels, C.W.; Nchu, F. Effects of light intensities and varying watering intervals on growth, tissue nutrient content and antifungal activity of hydroponic cultivated Tulbaghia violacea L. under greenhouse conditions. Heliyon 2020, 6, e03906.
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Barać, S.; Mastilović, J.; Kevrešan, Ž.; Fallik, E. Effect of shading by coloured nets on yield and fruit quality of sweet pepper. Zemdirbyste 2017, 104, 53–62.
- Valiente-Banuet, J.I.; Gutiérrez-Ochoa, A. Effect of irrigation frequency and shade levels on vegetative growth, yield, and fruit quality of piquin pepper (Capsicum annuum L. var. glabriusculum). HortScience 2016, 51, 573–579.
- Selahle, K.M.; Sivakumar, D.; Jifon, J.; Soundy, P. Postharvest responses of red and yellow sweet peppers grown under photo-selective nets. Food Chem. 2015, 173, 951–956.
- Russo, V.M.; Howard, L.R. Carotenoids in pungent and non-pungent peppers at various developmental stages grown in the field and glasshouse. J. Sci. Food. Agr. 2002, 82, 615–624.
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877.
- Shafiq, I.; Hussain, S.; Raza, M.A.; Iqbal, N.; Asghar, M.A.; Raza, A.; Fan, Y.-F.; Mumtaz, M.; Shoaib, M.; Ansar, M.; et al. Crop photosynthetic response to light quality and light intensity. J. Integr. Agric. 2021, 19, 2–21.
- Binotti, E.D.; Costa, E.; Binotti, F.F.D.S.; Batista, T.B. Chemical agents and shading levels for the production of pepper seedlings. Eng. Agric. 2018, 38, 450–456.
- Casierra-Posada, F.; Matallana-Díaz, Y.A.; Zapata-Casierra, E. Growth of bell pepper plants (Capsicum annuum) affected by coloured covers. Gesunde Pflanzen. 2014, 66, 149–155.
- Holopainen, J.K.; Kivimäenpää, M.; Julkunen-Tiitto, R. New light for phytochemicals. Trends Biotechnol. 2018, 36, 7–10.
- Jiao, Y.; Lau, O.S.; Deng, X.W. Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 2007, 8, 217–230.
- Jovicich, E.; VanSickle, J.J.; Cantliffe, D.J.; Stoffella, P.J. Greenhouse-grown colored peppers: A profitable alternative for vegetable production in Florida? Hort. Technol. 2005, 15, 355–369.
- Mashabela, M.N.; Selahle, K.M.; Soundy, P.; Crosby, K.M.; Sivakumar, D. Bioactive compounds and fruit quality of green sweet pepper grown under different colored shade netting during postharvest storage. J. Food Sci. 2015, 80, H2612–H2618.
- Zermeño-González, A.; Claveria-Cigarrero, G.L.; Melendres-Alvarez, A.I.; Ramírez-Rodriguez, H.; Munguía-López, J.P.; Campos-Magaña, S.G.; Cadena-Zapata, M. Colored plastic covers and its relationship with radiation, growth and yield of a sweet yellow pepper (Capsicum annuum L.) crop. Agrociencia 2019, 53, 709–723.
- Maroga, G.M.; Soundy, P.; Sivakumar, D. Different postharvest responses of fresh-cut sweet peppers related to quality and antioxidant and phenylalanine ammonia lyase activities during exposure to light-emitting diode treatments. Foods 2019, 8, 359.
- Hashim, M.; Ahmad, B.; Drouet, S.; Hano, C.; Abbasi, B.H.; Anjum, S. Comparative effects of different light sources on the production of key secondary metabolites in plants in vitro cultures. Plants 2021, 10, 1521.
- Dueck, T.; van Ieperen, W.; Taulavuori, K. Light perception, signalling and plant responses to spectral quality and photoperiod in natural and horticultural environments. Environ. Exp. Bot. 2016, 121, 1–150.
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90.
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Fallik, E. Effect of coloured shade-nets on plant leaf parameters and tomato fruit quality. J. Sci. Food Agr. 2015, 95, 2660–2667.
- Lester, G.E. Environmental regulation of human health nutrients (ascorbic acid, β-carotene, and folic acid) in fruits and vegetables. HortScience 2006, 41, 59–64.
- Shahak, Y. Photo-selective netting for improved performance of horticultural crops. A review of ornamental and vegetable studies carried out in Israel. Acta Hortic. 2008, 770, 161–168.
- Castellano, S.; Candura, A.; Scarascia Mugnozza, G. Relationship between solidity ratio, colour and shading effect of agricultural nets. Acta Hortic. 2008, 801, 253–258.
- Sivakumar, D.; Jifon, J.; Soundy, P. Spectral quality of photo-selective shade nettings improves antioxidants and overall quality in selected fresh produce after postharvest storage. Food Rev. Int. 2018, 34, 290–307.
- Castellano, S.; Mugnozza, G.S.; Russo, G.; Briassoulis, D.; Mistriotis, A.; Hemming, S.; Waajienberg, D. Plastic nets in agriculture: A general review of types and applications. Appl. Eng. Agric. 2008, 24, 799–808.
- Santana, J.Q.; Balbino, M.A.; Tavares, T.R.; Bezerra, R.S.; Farias, J.G.; Ferreira, R.C. Effect of photoselective screens in the development and productivity of red and yellow sweet pepper. Acta Hortic. 2012, 956, 493–500.
- Stamps, R.H. Use of colored shade netting in horticulture. HortScience 2009, 44, 239–241.
- Gangadhar, B.H.; Mishra, R.K.; Pandian, G.; Park, S.W. Comparative study of color, pungency, and biochemical composition in chili pepper (Capsicum annuum) under different light-emitting diode treatments. HortScience 2012, 47, 1729–1735.
- Al Murad, M.; Razi, K.; Jeong, B.R.; Samy, P.M.A.; Muneer, S. Light emitting diodes (LEDs) as agricultural lighting: Impact and its potential on improving physiology, flowering, and secondary metabolites of crops. Sustainability 2021, 13, 1985.
- Neugart, S.; Baldermann, S.; Hanschen, F.S.; Klopsch, R.; Wiesner-Reinhold, M.; Schreiner, M. The intrinsic quality of brassicaceous vegetables: How secondary plant metabolites are affected by genetic, environmental, and agronomic factors. Sci. Hortic. 2018, 233, 460–478.
- Jung, W.S.; Chung, I.M.; Hwang, M.H.; Kim, S.H.; Yu, C.Y.; Ghimire, B.K. Application of light-emitting diodes for improving the nutritional quality and bioactive compound levels of some crops and medicinal plants. Molecules 2021, 26, 1477.
- Vera-Guzmán, A.M.; Chávez-Servia, J.L.; Carrillo-Rodríguez, J.C.; López, M.G. Mexico evaluación fitoquímica en chile (Capsicum annuum L. and C. pubescens Ruiz & Pav.) silvestre y cultivado en Oaxaca, México. Chil. J. Agr. Res. 2011, 71, 578–585.
- Gurung, T.; Techawongstien, S.; Suriharn, B.; Techawongstien, S. Impact of environments on the accumulation of capsaicinoids in Capsicum spp. HortScience 2011, 46, 1576–1581.
- Batiha, G.E.S.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. And capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179.
- Forero, M.D.; Quijano, C.E.; Pino, J.A. Volatile compounds of chile pepper (Capsicum annuum L. var. Glabriusculum) at two ripening stages. Flavour. Frag. J. 2009, 24, 25–30.
- Mares-Quiñones, M.D.; Valiente-Banuet, J.I. Horticultural aspects for the cultivated production of piquin peppers (Capsicum annuum L. var. Glabriusculum) a review. HortScience 2019, 54, 70–75.
- Reddy, K.K.; Ravinder, T.; Prasad, R.B.N.; Kanjilal, S. Evaluation of the antioxidant activity of capsiate analogues in polar, nonpolar, and micellar media. J. Agric. Food Chem. 2011, 59, 564–569.
- Sarwa, K.K.; Das, P.J.; Mazumder, B. A nanovesicle topical formulation of Bhut Jolokia (hottest Capsicum): A potential anti-arthritic medicine. Expert Opin. Drug Del. 2014, 11, 661–676.
- Almeida, M.; Nadal, J.; Klein, T.; De Paula, J.; Budel, J.; Novatski, A.; Campessato, E.A.; Farrago, P.V. Innovative phytoformulation containing capsaicinoids: Microparticles development, analytical method validation, and anti-ulcer effect. Pharmacogn. Mag. 2018, 14, 290–296.
- Kuzma, M.; Fodor, K.; Almási, A.; Mózsik, G.; Past, T.; Perjési, P. Toxicokinetic study of a gastroprotective dose of capsaicin by HPLC-FLD method. Molecules 2019, 24, 2848.
- Friedman, J.R.; Nolan, N.A.; Brown, K.C.; Miles, S.L.; Akers, A.T.; Colclough, K.W.; Seidler, J.M.; Rimoldi, J.M.; Valentovic, M.A.; Dasgupta, P. Anticancer activity of natural and synthetic capsaicin analogs. J. Pharmacol. Exp. Ther. 2018, 364, 462–473.
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 2016, 21, 844.
- Cheok, C.Y.; Sobhi, B.; Adzahan, N.M.; Bakar, J.; Rahman, R.A.; Ab Karim, M.S.; Ghazali, Z. Physicochemical properties and volatile profile of chili shrimp paste as affected by irradiation and heat. Food Chem. 2017, 216, 10–18.
- Sricharoen, P.; Lamaiphan, N.; Patthawaro, P.; Limchoowong, N.; Techawongstien, S.; Chanthai, S. Phytochemicals in Capsicum oleoresin from different varieties of hot chilli peppers with their antidiabetic and antioxidant activities due to some phenolic compounds. Ultrason. Sonochem. 2017, 38, 629–639.
- Nuñez-Palenius, H.G.; Ochoa-Alejo, N. Effect of phenylalanine and phenylpropanoids on the accumulation of capsaicinoids and lignin in cell cultures of chili pepper (Capsicum annuum L.). In Vitro Cell Dev. Biol. Plant. 2005, 41, 801–805.
- Naves, E.R.; de Ávila Silva, L.; Sulpice, R.; Araújo, W.L.; Nunes-Nesi, A.; Peres, L.E.; Zsögön, A. Capsaicinoids: Pungency beyond Capsicum. Trends Plant Sci. 2019, 24, 109–120.
- Aza-González, C.; Núñez-Palenius, H.G.; Ochoa-Alejo, N. Molecular biology of capsaicinoid biosynthesis in chili pepper (Capsicum spp.). Plant Cell Rep. 2011, 30, 695–706.
- Stewart, C.; Mazourek, M.; Stellari, G.M.; O’Connell, M.; Jahn, M. Genetic control of pungency in C. chinense via the Pun1 locus. J. Exp. Bot. 2007, 58, 979–991.
- Burgos-Valencia, E.; Echevarría-Machado, I.; Narváez-Zapata, J.A.; Martínez-Estévez, M. Gene expression related to the capsaicinoids biosynthesis in the Capsicum genus: Molecular and transcriptomic studies. Braz. J. Bot. 2020, 43, 201–212.
- Caicedo-Lopez, L.H.; Guevara-Gonzalez, R.G.; Ramirez-Jimenez, A.K.; Feregrino-Pérez, A.A.; Contreras-Medina, L.M. Eustress application trough-controlled elicitation strategies as an effective agrobiotechnology tool for capsaicinoids increase: A review. Phytochem. Rev. 2022, 21, 1941–1968.
- Díaz, J.; Pomar, F.; Bernal, Á.; Merino, F. Peroxidases and the metabolism of capsaicin in Capsicum annuum L. Phytochem. Rev. 2004, 3, 141–157.
- Arce-Rodríguez, M.L.; Ochoa-Alejo, N. An R2R3-MYB transcription factor regulates capsaicinoid biosynthesis. Plant Physiol. 2017, 174, 1359–1370.
- Kim, J.S.; Park, M.; Lee, D.J.; Kim, D. Characterization of putative capsaicin synthase promoter activity. Mol. Cells 2009, 28, 331–339.
- Díaz-Pérez, J.C. Bell pepper (Capsicum annum L.) crop as affected by shade level: Fruit yield, quality, and postharvest attributes, and incidence of phytophthora blight (caused by Phytophthora capsici Leon.). HortScience 2014, 49, 891–900.
- Jeeatid, N.; Techawongstien, S.; Suriharn, B.; Bosland, P.W.; Techawongstien, S. Light intensity affects capsaicinoid accumulation in hot pepper (Capsicum chinense Jacq.) cultivars. Hortic. Environ. Biotech. 2017, 58, 103–110.
- Jeeatid, N.; Suriharn, B.; Techawongstien, S.; Chanthai, S.; Bosland, P.W.; Techawongstien, S. Evaluation of the effect of genotype-by-environment interaction on capsaicinoid production in hot pepper hybrids (Capsicum chinense Jacq.) under controlled environment. Sci. Hortic. 2018, 235, 334–339.
- Nagy, Z.; Daood, H.; Neményi, A.; Ambrózy, Z.; Pék, Z.; Helyes, L. Impact of shading net color on phytochemical contents in two chili pepper hybrids cultivated under greenhouse conditions. Korean J. Hortic. Sci. 2017, 35, 418–430.
- Phimchan, P.; Chanthai, S.; Bosland, P.W.; Techawongstien, S. Enzymatic changes in phenylalanine ammonia-lyase, cinnamic-4-hydroxylase, capsaicin synthase, and peroxidase activities in Capsicum under drought stress. J. Agric. Food Chem. 2014, 62, 7057–7062.
- Murakami, K.; Ido, M.; Masuda, M. Fruit Pungency of “Shishito” pepper as affected by a dark interval in continuous fluorescent illumination with temperature alteration. J. Soc. High Tech. Agric. 2006, 18, 284–289.
- Yap, E.S.P.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Vaswani, A.; Magana, A.A.; Morre, J.; Maier, C.S. Plant growth and metabolic changes in ‘Super Hot’ chili fruit (Capsicum annuum) exposed to supplemental LED lights. Plant Sci. 2021, 305, 110826.
- Quian-Ulloa, R.; Stange, C. Carotenoid biosynthesis and plastid development in plants: The role of light. Int. J. Mol. Sci. 2021, 22, 1184.
- Botella-Pavía, P.; Rodríguez-Concepción, M. Carotenoid biotechnology in plants for nutritionally improved foods. Physiol. Plantarum. 2006, 126, 369–381.
- Gómez-García, M.D.R.; Ochoa-Alejo, N. Biochemistry and molecular Biology of carotenoid biosynthesis in chili peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053.
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant. 2015, 8, 68–82.
- Hernández-Pérez, T.; Gómez-García, M.D.R.; Valverde, M.E.; Paredes-López, O. Capsicum annuum (hot pepper): An ancient Latin-American crop with outstanding bioactive compounds and nutraceutical potential. A review. Compr. Rev. Food Sci. F 2020, 19, 2972–2993.
- Hassan, N.M.; Yusof, N.A.; Yahaya, A.F.; Rozali, N.N.M.; Othman, R. Carotenoids of Capsicum fruits: Pigment profile and health-promoting functional attributes. Antioxidants 2019, 8, 469.
- Mercy, E.R.; David, U. Potential health benefits of conventional nutrients and phytochemicals of Capsicum peppers. Pharm. Pharmacol. Int. J. 2018, 6, 62–69.
- Rodríguez-Rodríguez, E.; Sánchez-Prieto, M.; Olmedilla-Alonso, B. Assessment of carotenoid concentrations in red peppers (Capsicum annuum) under domestic refrigeration for three weeks as determined by HPLC-DAD. Food Chem. 2020, 27, 100092.
- Giuliano, G. Provitamin A biofortification of crop plants: A gold rush with many miners. Curr. Opin. Biotech. 2017, 44, 169–180.
- Pola, W.; Sugaya, S.; Photchanachai, S. Color development and phytochemical changes in mature green chili (Capsicum annuum L.) exposed to red and blue light-emitting diodes. J. Agric. Food Chem. 2020, 68, 59–66.
- Villa-Rivera, M.G.; Ochoa-Alejo, N. Chili pepper carotenoids: Nutraceutical properties and mechanisms of action. Molecules 2020, 25, 5573.
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49.
- Fraser, P.D.; Schuch, W.; Bramley, P.M. Phytoene synthase from tomato (Lycopersicon esculentum) chloroplasts-partial purification and biochemical properties. Planta 2020, 211, 361–369.
This entry is offline, you can click here to edit this entry!
