Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Energetic composite materials (ECMs) are the basic materials of polymer binder explosives and composite solid propellants, which are mainly composed of explosive crystals and binders. During the manufacturing, storage and use of ECMs, the bonding surface is prone to micro/fine cracks or defects caused by external stimuli such as temperature, humidity and impact, affecting the safety and service of ECMs. Therefore, substantial efforts have been devoted to designing suitable self-healing binders aimed at repairing cracks/defects.
- energetic composite materials
- cracks and defects
- self-healing
- binders
1. Introduction
Energetic composite materials (ECMs) are the basic materials of polymer binder explosives (PBXs) and composite solid propellants (CSPs), as well as the symbol and key technologies of upgraded weapons. They are a class of high-filled polymer composites, mainly composed of explosive crystals and a small number of polymer binders (5~20 wt%). Under external stimulation (temperature, humidity, vibration, etc.), the bonding surface is prone to form micro/fine cracks, defects and other damages [1][2]. On the one hand, the existence of microcracks is not conducive to the mechanical properties of ECMs. On the other hand, microcracks and defects tend to cause excessive hot spots, which increases the risk of explosion when subjected to external stimuli (such as impact or friction) [3]. Introducing the function of self-healing to ECMs will solve the problems mentioned above, and the stability, security and service life of ECMs will, therefore, be improved. It can be predicted that the research on self-healing ECMs will gain increasing attention in the coming future.
In order to repair hidden microcracks, increase safety and prolong the service life, the concept of self-healing polymers was proposed in the 1980s [4]. Conceptually, self-healing polymers have the inherent ability to substantially recover the load transfer capability after damage. This recovery can occur autonomously or by applying specific stimuli (such as radiation, heat, or water). Therefore, these materials are expected to make a remarkable difference in the improvement of the durability and safety of polymer materials without requiring extra external maintenance or expensive active monitoring [5]. In recent years, a large number of self-healing polymers have been developed [6][7][8].
According to the self-healing strategy, self-healing materials can be divided into external self-healing materials and intrinsic self-healing materials. The external self-healing process mainly depends on microcapsules [9][10][11], microvascular networks [12][13][14] or hollow fibers [15][16][17] that are added to the polymer matrix. Therefore, the external self-healing process requires a high cost of healing agents to allow limited repair times, which restricts the application in self-healing ECM systems. The intrinsic self-healing process is generally based on the dissociation and rearrangement of dynamic covalent or non-covalent bonds in materials, which can be spontaneous or driven by external stimuli (heat, light, pH, etc.). Dynamic covalent bonds include Diels–Alder bonds [18][19][20], disulfide bonds [21][22][23], acyl semicarbazides (ASCZ) [24], acylhydrazone bonds [25][26][27], imine bonds [28][29][30], borate ester bonds [31][32][33], diselenide bonds [34][35][36], etc. Dynamic non-covalent bonds include hydrogen bonds [37][38][39], metal–ligand coordination [40][41][42], host–guest interactions [43][44][45], donor–acceptor interactions [46][47][48], ionic bonds [49][50][51], etc.
In intrinsic self-healing materials, these dynamic covalent and non-covalent bonds have the potential to provide self-healing, which can both be autonomous and experience several healing cycles, inducing the fusion between material science and supramolecular chemistry [52]. Owing to the fact that the relevant interactions of self-healing are dynamically reversible, the repair of the intrinsic self-healing materials can be carried out many times compared to the external self-healing materials. Therefore, researchers tend to select the intrinsic self-healing binders to apply to ECMs. Currently, self-healing methods for ECMs mainly involve several dynamic chemistries (Figure 1). When ECMs are damaged, dynamic covalent or non-covalent bonds in the binders can be reconnected to repair cracks or defects.
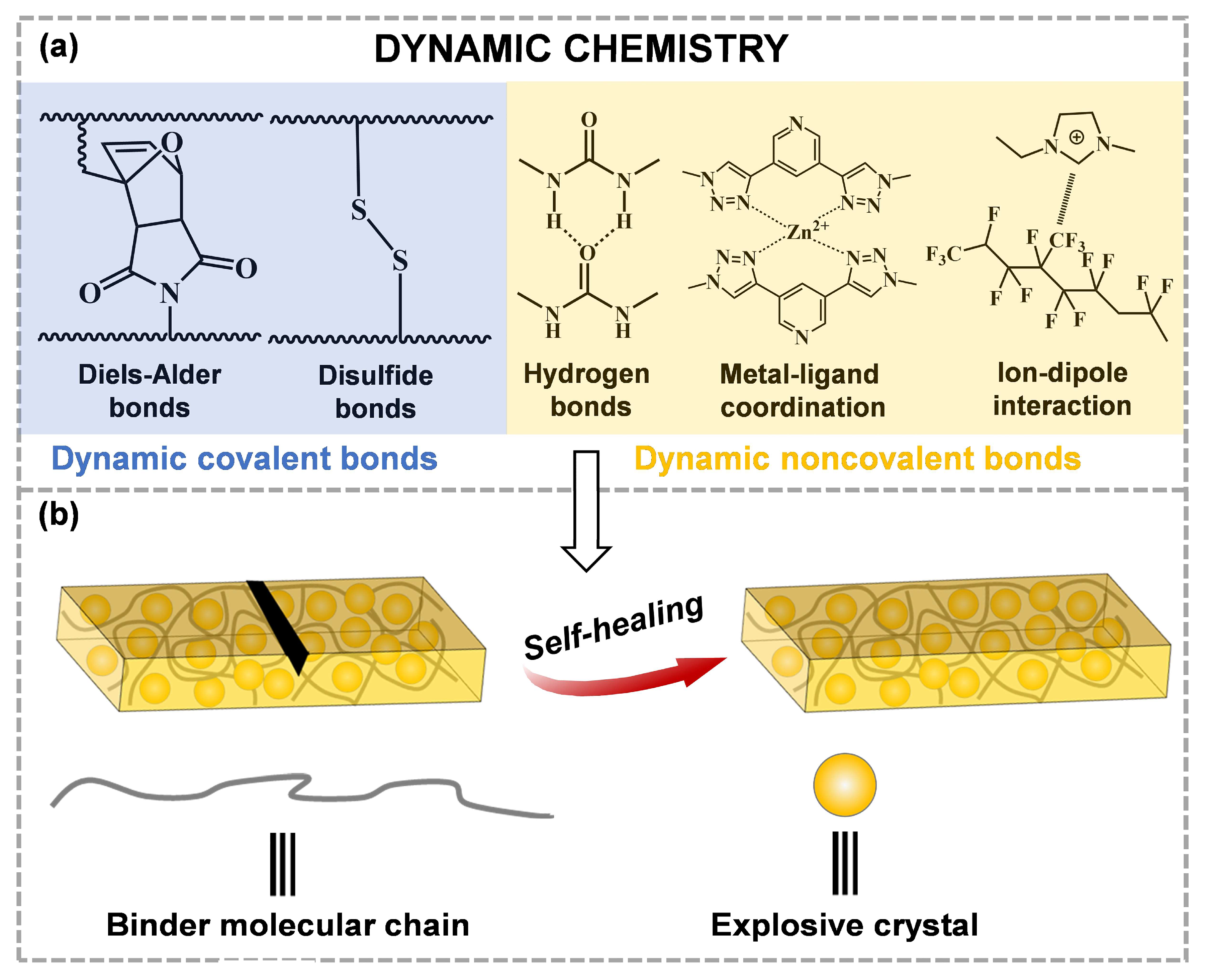
Figure 1. (a) Several typical self-healing methods for the crack-healing of ECMs, (b) the crack-healing process in ECMs.
2. Dynamic Covalent Bonds in Self-Healing ECMs
Covalent bonds possess higher fracture tolerance than non-covalent bonds, which can endow the polymer matrix with stronger mechanical properties. Self-healing polymers based on dynamic covalent bonds can undergo dynamic bond dissociation and rearrangement under external stimuli. Due to the stability of bonds and the high efficiency of the reversible reaction, self-healing polymers can heal the damaged parts independently.
2.1. Diels–Alder Reaction
The Diels–Alder reaction is a cycloaddition reaction, that is, the reaction of conjugated dienes with substituted olefins (dienophiles) to form cyclohexene adducts [53]. Common conjugated dienes include furan, furan amine, furan alcohol, furan mercaptan and tetrahydrofuran methacrylate, and frequent dienophiles include maleic anhydride, maleimide and bismaleimide [54][55][56]. The Diels–Alder reaction is thermoreversible, for which the degree of the reaction can be controlled using temperature. Therefore, this mechanism is suitable for self-healing polymers.
Liang et al. [57] designed a TFP (trifuryl propane)-FTPB (furyl terminated polybutadiene)-PDMI (N, N′-1,3-Phthalic maleimide) self-healing binder based on the Diels–Alder reaction. The synthesis methods of TFP and FTPB are shown in Figure 2a. The addition of TFP improved the perfection of the polymer cross-linking network, which is conducive to tensile strength. The existence of Diels–Alder bonds ensured the healing of cracks (Figure 2b). At 120 °C, the occurrence of the Retro-Diels–Alder reaction caused the separation of diene and dienophile, and the molecular mobility increased as the molecular chain became shorter. Active molecular diffusion is beneficial to the rearrangement of molecular chains. At 60 °C, the reassociation of Diels–Alder bonds further repaired the mechanical damages of materials. The self-healing process could be observed directly using the hot-stage optical microscope. As shown in Figure 2c, the crack width was significantly reduced compared with that before healing, which is mainly related to the thermal reversibility of Diels–Alder bonds.
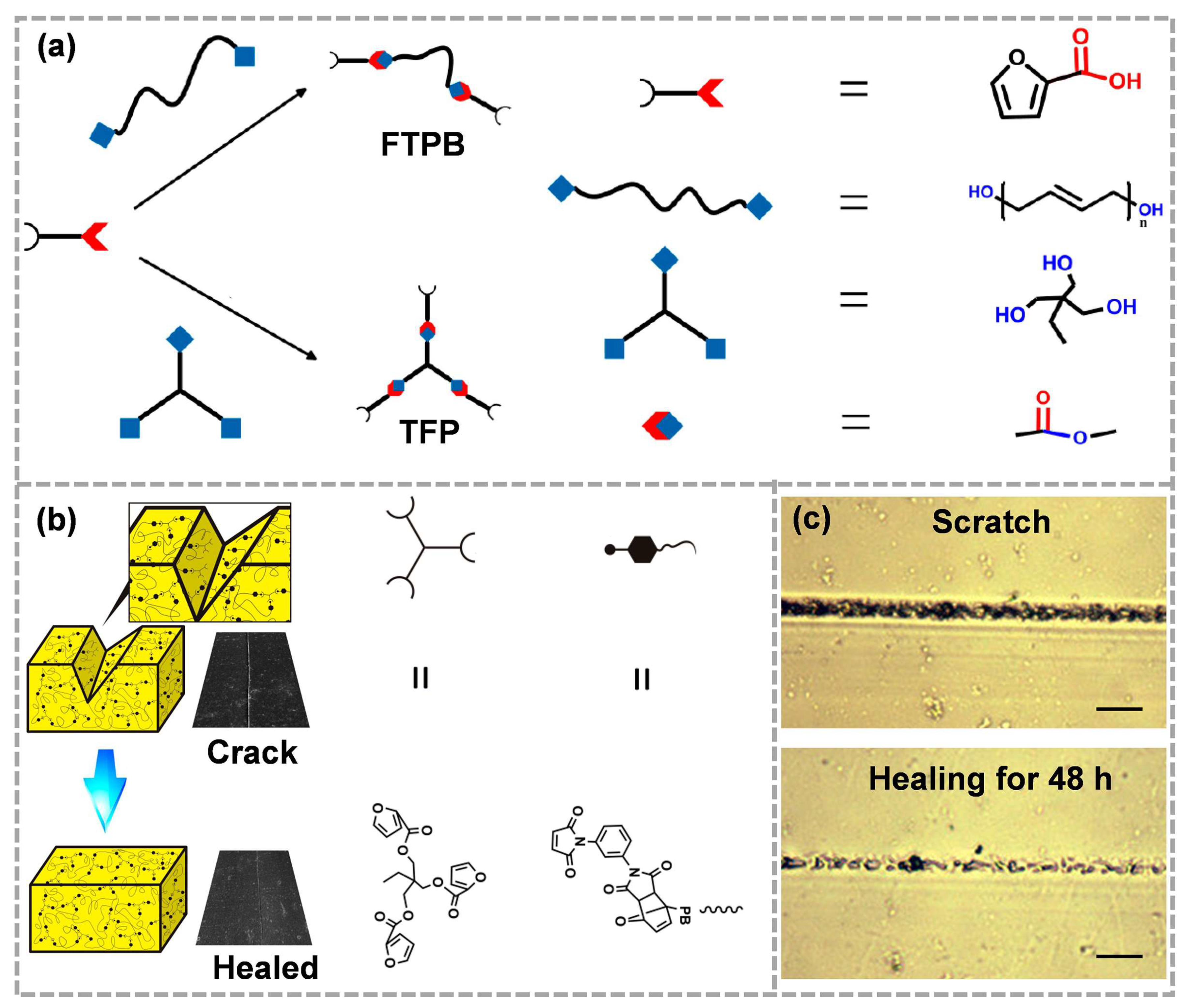
Figure 2. (a) Synthetic route of FTPB and TFP, (b) the self-healing process of TFP-FTPB-PDMI film and (c) the corresponding optical microscope images (reproduced from Ref. [57] with permission; Copyright MDPI, 2017).
Subsequently, Xia et al. [58], based on Liang’s work, synthesized a novel FTPB using the reaction of isocyanate terminated polybutadiene with fury amine, and then prepared self-healing binder films (FTPB-DAs) based on the Diels–Alder reaction system using the reaction of furan with bismaleimide. The FTPB-DA could be converted to FTPB and bismaleimide at 120 °C. When the temperature dropped to 60 °C, it would be re-crosslinked to form FTPB-DA, thus showing that it has a self-healing property (Figure 3a). In Xia’s strategy, the content of furan groups on the FTPB skeleton could be adjusted by changing the ratio of -NCO and -OH, so as to regulate the chemical crosslinking density of the binders, which is convenient for further balancing the mechanical properties and self-healing properties. Binders with thermal reversibility were further utilized with HMX to prepare PBX (DAPU-HMX) [59]. A CT test showed that, after impact, three cracks formed in the damaged sample, which gradually reduced or even disappeared after healing (Figure 3b). The Brazilian test results indicated that the mechanical properties of the damaged DAPU-HMX samples were seriously reduced, and the healed mechanical strength could recover to 85% of the initial (Figure 3c,d). Thermally reversible Diels–Alder bonds have been shown to be effective for the self-healing of ECMs. The rearrangement of molecular chains and the reassociation of Diels–Alder bonds at the crack repair the damaged network and form new “topological entanglements”. The “topological entanglements” further restore the mechanical properties of the materials. However, for the self-healing binders based on the Diels–Alder reaction, the activation temperature of the Retro-Diels–Alder reaction that is responsible for the repair process is as high as 100~130 °C. For sensitive energetic materials, safety needs further evaluation. Therefore, researchers tend to apply mild dynamic chemistries to ECMs.
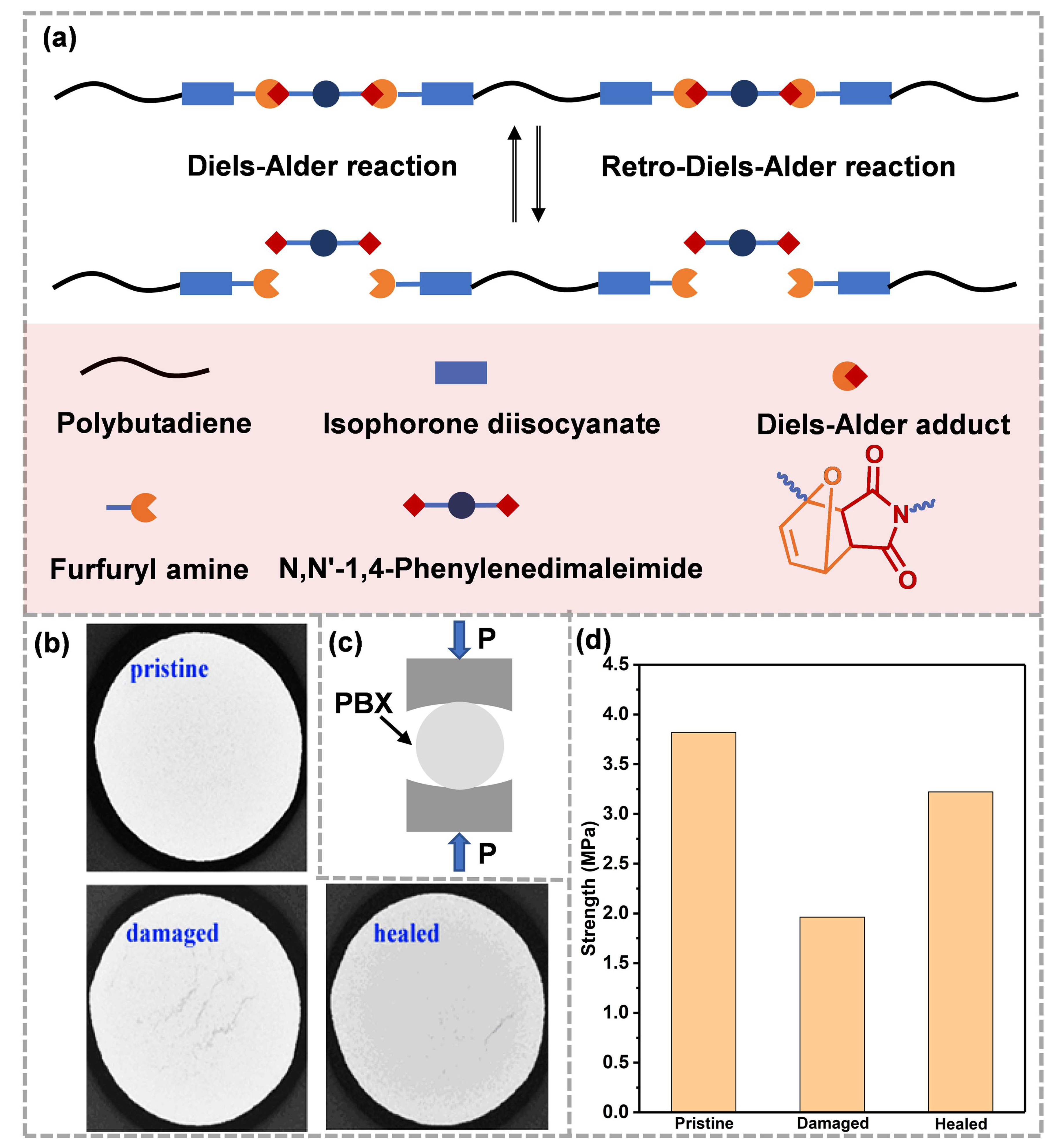
Figure 3. (a) Thermoreversible process of FTPB-DA for self-healing (reproduced from Ref. [58] with permission; Copyright Royal Society of Chemistry, 2021), (b) CT images, (c) Brazilian test method and (d) mechanical strength of DAPU-HMX (reproduced from Ref. [59] with permission; Copyright Elsevier, 2019).
2.2. Disulfide Exchange Reaction
Disulfide bonds are ubiquitous in organisms and are mainly used to maintain the tertiary structure of proteins, which can be dissociated under the action of light, heat or mechanical force [60][61], or be reformed and exchanged in the appropriate temperature and pH environment (Figure 4a) [62][63]. In addition, disulfide exchange provides significant advantages in self-healing, because S–S bonds can be combined into networks with low glass transition temperature, promoting low-temperature reversibility [64].
CSPs are mainly composed of polymer binders, oxidizers, combustion promoters, aluminum powder and other components [65][66]. In order to ensure high energy, the highly energetic crystals in CSPs usually exceed 80 wt%, while polymer binders are mostly less than 20 wt% [67]. In other words, CSPs belong to a kind of high-filling polymer composite. Such compositional characteristics usually result in low strength and high brittleness. In practical application, the crystal layer is easy to peel off from the coating due to the influence of a complex environment such as external temperature and stress, which leads to the generation of cracks/voids [68][69]. The rearrangement and exchange of disulfide bonds could promote the reattachment of coatings and grain layers, thus healing the debonding interface (Figure 4b) [70]. Furthermore, dynamic disulfide bonds can exhibit high dynamics at lower temperatures, so they are also suitable for repairing cracks/defects inside ECMs. Li et al. [71] prepared a new polyurethane binder (DSPU) with polycaprolactone glycol as the molecular skeleton and bis (4-aminophenyl) disulfide as the chain extender to solve the micro damage problem in PBXs (Figure 4c). Due to the covalent dynamic disulfide bonds in the binders, heat drives in situ healing at the crack/defect. The damaged CL-20-based PBXs just require being heated to 60 °C for several hours to repair, which is obviously related to the dynamic chemical properties of disulfide bonds under heating conditions.
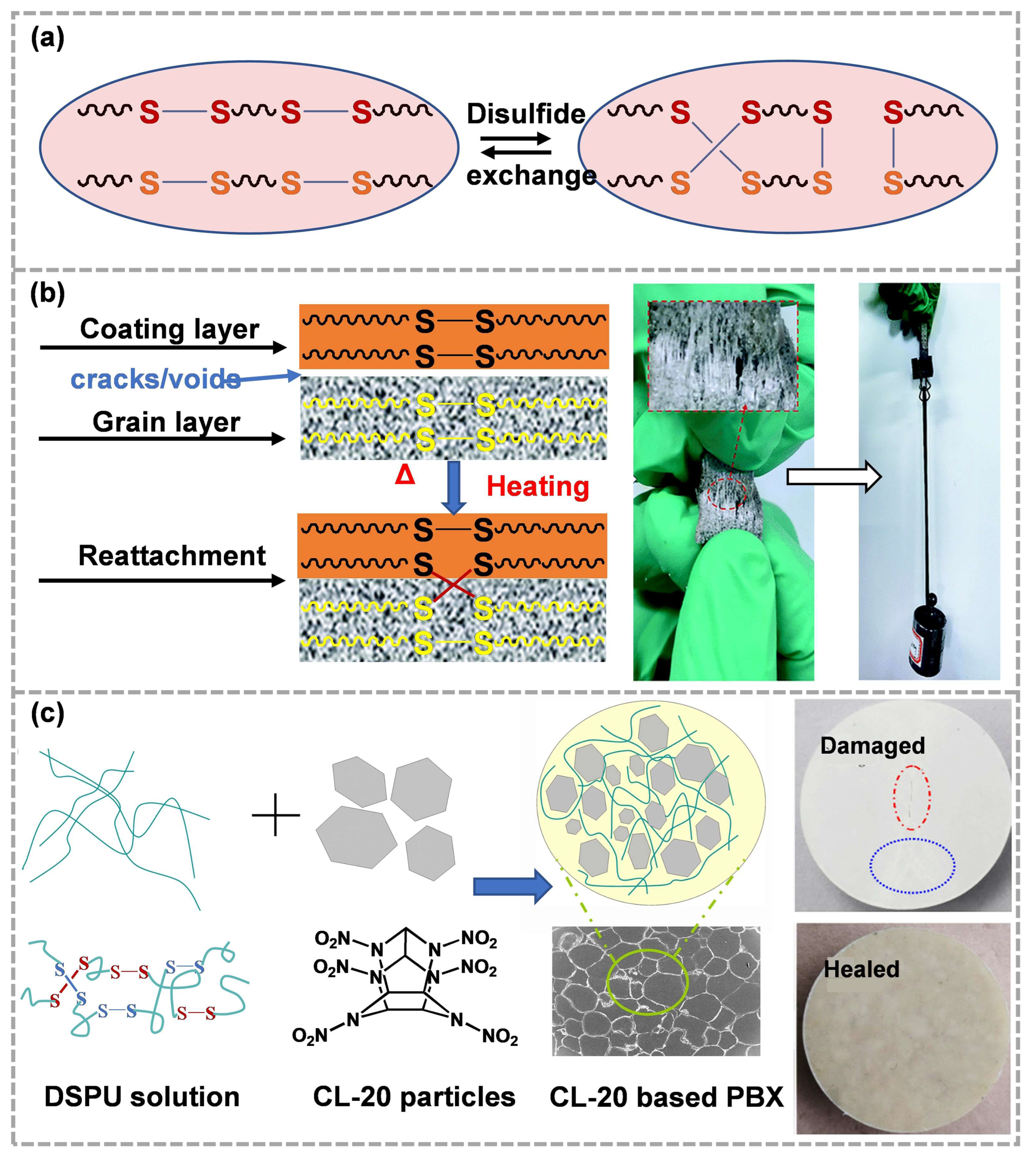
Figure 4. (a) Disulfide chain exchange (reproduced from Ref. [63] with permission; Copyright Copyright Springer Nature, 2020), (b) the self-healing of the debonding interface between the grain and coating layer(reproduced from Ref. [70] with permission; Copyright Royal Society of Chemistry, 2020), (c) the preparation and the damage self-healing of CL-20-based PBX using DSPU as binder(reproduced from Ref. [71] with permission; Copyright Elsevier, 2019).
An energetic binder is a kind of polymer with a large number of energetic groups on molecular chains [72][73][74]. Compared to traditional inert binders, energetic binders possess not only the basic performance of binders, but also the energy properties [75][76]. Among the most energetic groups, -N3 has a high positive formation enthalpy (+355.6 kJ mol−1). The introduction of -N3 into the polymer will not affect the original hydrocarbon ratio. Moreover, -N3 could perform thermal decomposition independently before the main chain, which not only increases the energy density of the binder, but also facilitates the decomposition of ECMs to some extent [77]. Glycidyl azide polymer (GAP) is a hydroxy terminated polymer with a large amount of -N3, which is usually obtained using modification and azidation of poly(epichlorohydrin) [78][79][80]. In order to further improve the energy level of ECMs, GAP, as an energetic polyether, has gradually attracted attention. Compared with the inert binders, the ECMs with GAP-based binders showed excellent combustion performance (Figure 5a) [81].
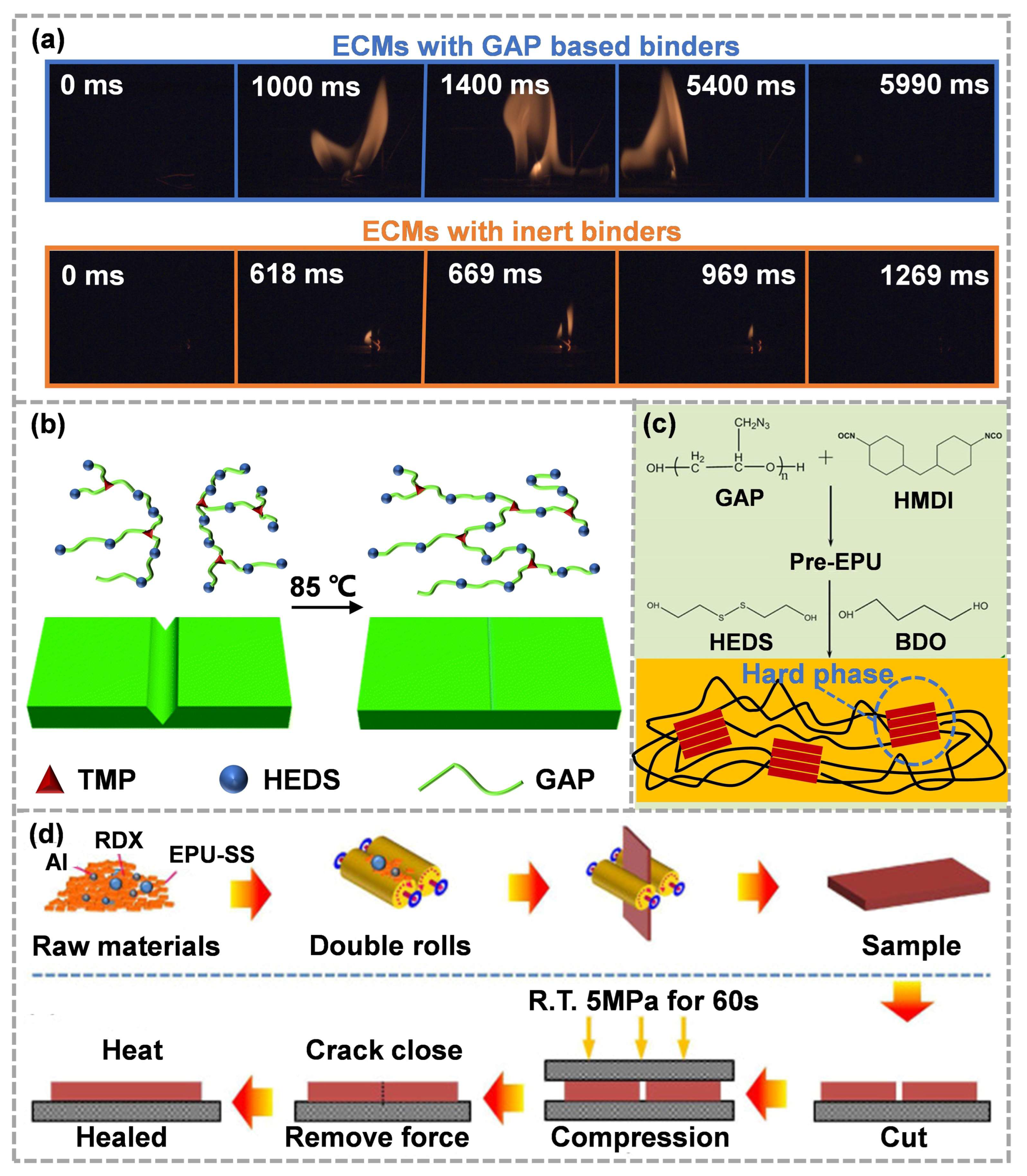
Figure 5. (a) Sequential open-combustion images of ECMs with GAP-based binders and ECMs with inert binders (reproduced from Ref. [81] with permission; Copyright Royal Society of Chemistry, 2021), (b) the self-healing process of GAPUV (reproduced from Ref. [82] with permission; Copyright Royal Society of Chemistry, 2021), (c) the synthesis process and microstructure of EPU-SS(reproduced from Ref. [83] with permission; Copyright American Chemical Society, 2021), (d) preparation and healing process of ECMs with EPU-SS as binders (reproduced from Ref. [84] with permission; Copyright Elsevier, 2022).
Hu et al. [82] utilized GAP, 2-hydroxyethyl disulfide (HEDS) and trimethylolpropane (TMP) as a soft segment, chain extender and cross-linking agent, respectively, to prepare a series of polyurethane vitrimers (GAPUVs). Using an optimization of the crosslinking density and composition of thermosetting GAPUVs, the mechanical properties were significantly improved. With the addition of dynamic disulfide bonds, GAPUVs showed obvious healing ability (Figure 5b) and reprocessing ability after mild heating. Furthermore, the scratch-healing efficiency of the ECMs with GAPUVs (binders) and aluminum powder could exceed 95%. Subsequently, Ding et al. [83] synthesized a self-healing energetic linear polyurethane elastomer (EPU-SS) based on disulfide bonds (Figure 5c). The elastomer was prepared using a two-step method and possessed high self-healing efficiency and mechanical properties, which were attributed to the carefully designed surface energy driving and dynamic hard domains. Then, based on the physical model of interface healing, the variation trend in surface tension, crack bottom radius and depth during the healing process was calculated, and the mechanism of interface healing was obtained. The polyurethane elastomer with low crosslink density could generate excess surface energy at the damage sites to drive the self-healing process, and the addition of a small number of disulfide bonds could further reduce the healing energy barrier. Overall, high filler loading will improve the hardness of polymer composites but will also hinder the process of interface healing. Therefore, the healing ability and mechanical strength of ultra-high-filling polymer composites are contradictory and difficult to optimize at the same time. Ding et al. [84] used EPU-SS as a binder to prepare ECMs with RDX and aluminum powder, which developed a crack-healing method (Figure 5d). The compressive stress at room temperature significantly increased the interface contact effect. Then, the adhesion effectively closed the crack, the surface energy driving promoted the movement of the polymer chains and the reversibility of disulfide bonds helped to rebuild a new polymer network at the interface, thus showing that the cracks could be repaired. In the future, it will be more practical if the reasonable configuration of mechanical properties and efficient self-healing ability can be realized to simplify the crack-healing process.
2.3. Dynamic Chemical Reactions of Other Covalent Bonds
Acyl semicarbazide (ASCZ) is a combination of urea and amide linked by an N–N bond, which can be easily formed using the addition reaction of isocyanate and hydrazide [24]. The ASCZ motif is dynamic, which can form an activated n-center transition state, promote proton transfer and, thus, reduce the dissociation energy barrier in ASCZ motifs [85]. Under high temperature, the ASCZ group can reversibly generate isocyanate and hydrazide (Figure 6a) [24], which shows thermal reversibility, providing a new direction for the molecular engineering design of high-performance dynamic polymers. However, similar to Diels–Alder bonds, the rapid dissociation of ASCZ groups needs to be carried out at ~120 °C.
As a major group element, selenium has similar chemical properties to sulfur. It is worth noting that the bond energy of the Se–Se bond (172 kJ mol−1) is lower than that of the S–S bond (240 kJ mol−1), which means that the selenium bond, as a dynamic covalent bond, can respond to more mild stimuli, thus stimulating new molecular engineering [86][87]. It has been proven that the dynamic exchange reaction of the selenide bond can take place under heating or visible light irradiation (Figure 6b) [88]. It is reported that the dynamic recombination of the selenide bonds could be effectively used to restore the integrity of the asphalt network at fracture (Figure 6c) [34], providing a new design strategy for ECMs with ultra-high filling. Compared to disulfide bonds, lower bond energy reduces the energy barrier of self-healing. It can be predicted that the dynamic exchange of selenium bonds at the crack/defect can realize the autonomous repair of composite materials. However, low bond energy is not conducive to mechanical properties. Great efforts are still needed to balance network dynamics and robustness.
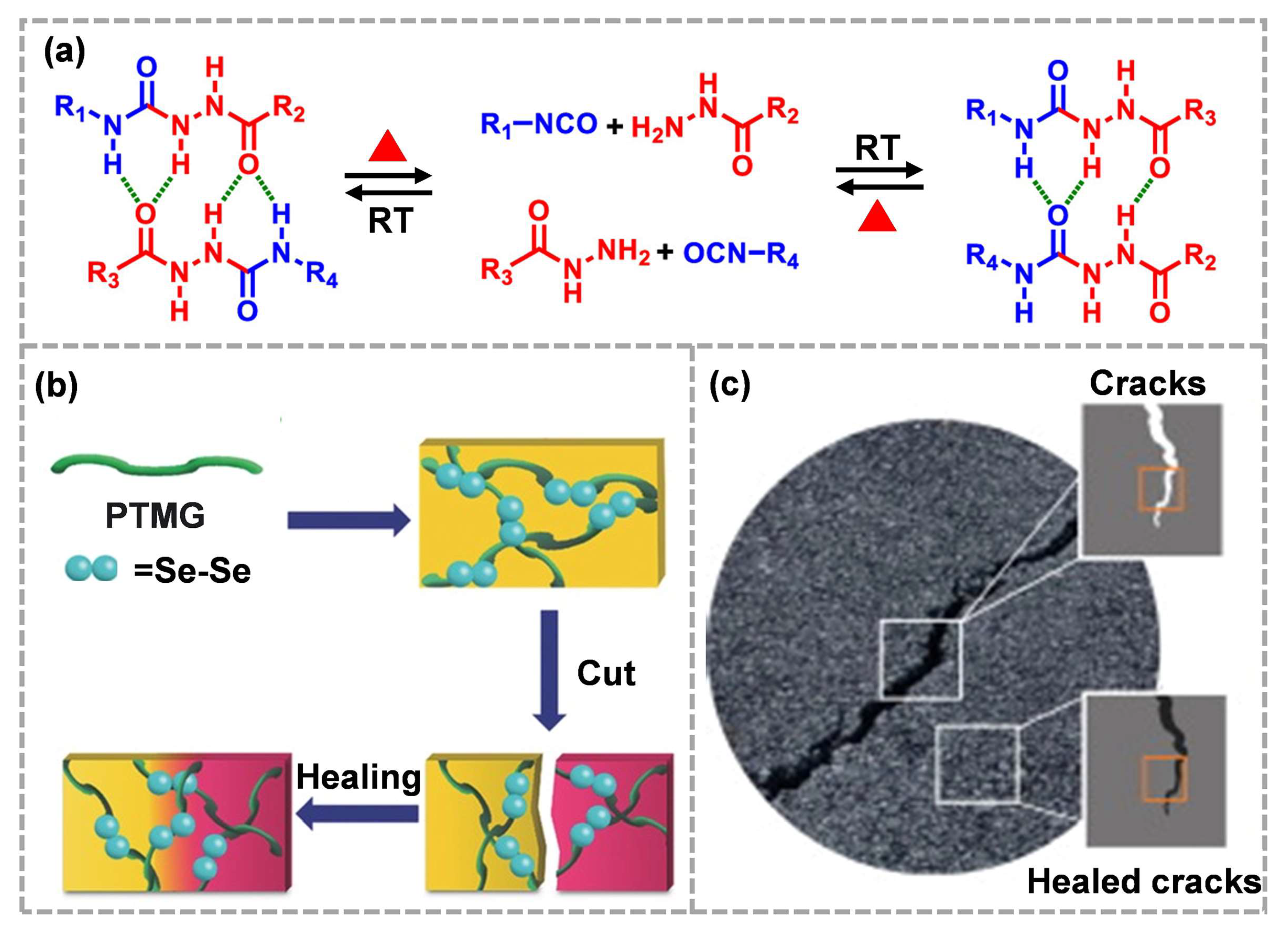
Figure 6. (a) Dynamic chemistry of ASCZ groups (reproduced from Ref. [24] with permission; Copyright American Chemical Society, 2020), (b) visible-light-induced self-healing process of diselenide-containing polymer (reproduced from Ref. [88] with permission; Copyright Wiley, 2015), (c) crack healing of composites using diselenide-containing polymers as binders (reproduced from Ref. [34] with permission; Copyright Elsevier, 2021).
This entry is adapted from the peer-reviewed paper 10.3390/molecules28010428
References
- Chen, Y.; Liu, Y.F.; Shi, L.; Yang, W.; Yao, W.S. Study on the Synthesis and Interfacial Interaction Performance of Novel Dodecylamine-Based Bonding Agents Used for Composite Solid Propellants. Propellants Explos. Pyrotech. 2015, 40, 50–59.
- Zhen, F.; Zhou, X.Y.; Zou, M.S.; Meng, L.C.; Yang, R.J.; Wang, L.Q.; Huang, F.L.; Li, J.M. Investigation of the agglomeration reduction mechanism of the aluminized HTPB propellant containing ferric perfluorooctanoate . RSC Adv. 2019, 9, 19031–19038.
- Yang, K.; Wu, Y.Q.; Duan, H.Z.; Huang, F.L. Sensitization and desensitization of PBXs stemming from microcrack and microvoid in responses to pressure-time loading. Appl. Phys. Lett. 2021, 119, 014102.
- Zhu, M.S.Q.; Liu, J.; Gan, L.H.; Long, M.N. Research progress in bio-based self-healing materials. Eur. Polym. J. 2020, 129, 109651.
- Xu, J.H.; Ding, C.D.; Chen, P.; Tan, L.H.; Chen, C.B.; Fu, J.J. Intrinsic self-healing polymers for advanced lithium-based batteries: Advances and strategies. Appl. Phys. Rev. 2020, 7, 031304.
- Zhang, L.Z.; Liu, Z.H.; Wu, X.L.; Guan, Q.B.; Chen, S.; Sun, L.J.; Guo, Y.F.; Wang, S.L.; Song, J.C.; Jeffries, E.M.; et al. A Highly Efficient Self-Healing Elastomer with Unprecedented Mechanical Properties. Adv. Mater. 2019, 31, 1901402.
- Urban, M.W.; Davydovich, D.; Yang, Y.; Demir, T.; Zhang, Y.Z.; Casabianca, L. Key-and-lock commodity self-healing copolymers. Science 2018, 362, 220–225.
- Rottger, M.; Domenech, T.; Van der Weegen, R.; Nicolay, A.B.R.; Leibler, L. High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis. Science 2017, 356, 62–65.
- Xu, C.Y.; Chen, Z.; Wang, C.X.; Chen, K.L. Fabrication of Dual Self-Healing Multifunctional Coating Based on Multicompartment Microcapsules. ACS Appl. Mater. Interfaces 2021, 13, 59298–59309.
- Li, F.R.; Jiao, S.Z.; Sun, Z.C.; Liu, Y.Y.; Zhang, Q.Q.; Wen, J.Y.; Zhou, Y. Self-repairing microcapsules with aqueous solutions as core materials for conductive applications. Green Chem. 2021, 23, 927–934.
- Li, Z.K.; Li, K.K.; Li, X.; Feng, Y.Y.; Li, H.Y.; Wang, H.Y. Preparation of linseed oil-loaded porous glass bubble/wax microcapsules for corrosion- and wear-resistant difunctional coatings. Chem. Eng. J. 2022, 437, 135403.
- Postiglione, G.; Alberini, M.; Leigh, S.; Levi, M.; Turri, S. Effect of 3D-Printed Microvascular Network Design on the Self-Healing Behavior of Cross-Linked Polymers. ACS Appl. Mater. Interfaces 2017, 9, 14371–14378.
- Li, P.; Liu, Y.; Zou, T.; Huang, J.Y. Optimal design of microvascular networks based on non-dominated sorting genetic algorithm II and fluid simulation. Adv. Mech. Eng. 2017, 9, 1–9.
- Kato, Y.; Minakuchi, S.; Ogihara, S.; Takeda, N. Self-healing composites structure using multiple through-thickness microvascular channels. Adv. Compos. Mater. 2021, 30, 1–18.
- Lee, M.W.; An, S.; Yoon, S.S.; Yarin, A.L. Advances in self-healing materials based on vascular networks with mechanical self-repair characteristics. Adv. Colloid Interface Sci. 2018, 252, 21–37.
- Wen, N.; Song, T.T.; Ji, Z.H.; Jiang, D.W.; Wu, Z.J.; Wang, Y.; Guo, Z.H. Recent advancements in self-healing materials: Mechanicals, performances and features. React. Funct. Polym. 2021, 168, 105041.
- Lv, Z.; Yao, J.B.; Cui, G.J.; Chen, H.S. Geometrical probability of a capsule hitting irregular crack networks: Application to capsule-based self-healing materials. Appl. Math. Model. 2022, 101, 406–419.
- Li, Y.; Jiang, B.; Huang, Y.D. Interfacial self-healing performance of carbon fiber/epoxy based on postsynthetic modification of metal-organic frameworks. Compos. Sci. Technol. 2022, 227, 109564.
- Orozco, F.; Kaveh, M.; Santosa, D.S.; Lima, G.M.R.; Gomes, D.R.; Pei, Y.T.; Araya-Hermosilla, R.; Moreno-Villoslada, I.; Picchioni, F.; Bose, R.K. Electroactive Self-Healing Shape Memory Polymer Composites Based on Diels-Alder Chemistry. ACS Appl. Polym. Mater. 2021, 3, 6147–6156.
- Zhou, Q.; Sang, Z.; Rajagopalan, K.K.; Sliozberg, Y.; Gardea, F.; Sukhishvili, S.A. Thermodynamics and Stereochemistry of Diels-Alder Polymer Networks: Role of Crosslinker Flexibility and Crosslinking Density. Macromolecules 2021, 54, 10510–10519.
- Chang, K.; Jia, H.; Gu, S.Y. A transparent, highly stretchable, self-healing polyurethane based on disulfide bonds. Eur. Polym. J. 2019, 112, 822–831.
- Liu, M.C.; Zhong, J.; Li, Z.J.; Rong, J.C.; Yang, K.; Zhou, J.Y.; Shen, L.; Gao, F.; Huang, X.L.; He, H.F. A high stiffness and self-healable polyurethane based on disulfide bonds and hydrogen bonding. Eur. Polym. J. 2020, 124, 109475.
- Zheng, T.; Zhou, Q.; Yang, T.; Zhao, Y.; Fan, B.; Bo, J.; Fan, L.S.; Peng, R.F. Disulfide bond containing self-healing fullerene derivatized polyurethane as additive for achieving efficient and stable perovskite solar cells. Carbon 2022, 196, 213–219.
- Fu, D.H.; Pu, W.L.; Escorihuela, J.; Wang, X.R.; Wang, Z.H.; Chen, S.Y.; Sun, S.J.; Wang, S.; Zuilhof, H.; Xia, H.S. Acylsemicarbazide Moieties with Dynamic Reversibility and Multiple Hydrogen Bonding for Transparent, High Modulus, and Malleable Polymers. Macromolecules 2020, 53, 7914–7924.
- Hua, J.C.; Liu, C.; Fei, B.; Liu, Z.F. Self-Healable and Super-Tough Double-Network Hydrogel Fibers from Dynamic Acylhydrazone Bonding and Supramolecular Interactions. Gels 2022, 8, 101.
- Ren, J.Y.; Dong, X.B.; Duan, Y.J.; Lin, L.; Xu, X.W.; Shi, J.C.; Jia, R.P.; Wu, D.D.; He, X.Y. Synthesis and self-healing investigation of waterborne polyurethane based on reversible covalent bond. J. Appl. Polym. Sci. 2022, 139, 52144.
- An, H.; Yang, Y.; Zhou, Z.W.; Bo, Y.Y.; Wang, Y.; He, Y.N.; Wang, D.; Qin, J.L. Pectin-based injectable and biodegradable self-healing hydrogels for enhanced synergistic anticancer therapy. Acta. Biomater. 2021, 131, 149–161.
- Liu, Y.; Lin, S.H.; Chuang, W.T.; Dai, N.T.; Hsu, S.H. Biomimetic Strain-Stiffening in Chitosan Self-Healing Hydrogels. ACS Appl. Mater. Interfaces 2022, 14, 16032–16046.
- Chang, Y.; Sun, J.L.; Dong, L.; Jiao, F.H.; Chang, S.L.; Wang, Y.; Liao, J.; Shang, Y.Y.; Wu, W.W.; Qi, Y.; et al. Self-powered multi-color display based on stretchable self-healing alternating current electroluminescent devices. Nano Energy 2022, 95, 107061.
- Gu, W.D.; Li, F.; Liu, T.; Gong, S.S.; Gao, Q.; Li, J.Z.; Fang, Z. Recyclable, Self-Healing Solid Polymer Electrolytes by Soy Protein-Based Dynamic Network. Adv. Sci. 2022, 9, 2103623.
- Ji, F.; Li, J.H.; Zhang, G.P.; Lan, W.J.; Sun, R.; Wong, C.P. Alkaline monomer for mechanical enhanced and self-healing hydrogels based on dynamic borate ester bonds. Polymer 2019, 184, 121882.
- Ma, J.Z.; Yang, Y.Z.; Valenzuela, C.; Zhang, X.; Wang, L.; Feng, W. Mechanochromic, Shape-Programmable and Self-Healable Cholesteric Liquid Crystal Elastomers Enabled by Dynamic Covalent Boronic Ester Bonds. Angew. Chem. Int. Ed. 2022, 61, e202116219.
- Li, S.B.; Zuo, C.; Zhang, Y.; Wang, J.R.; Gan, H.H.; Li, S.Q.; Yu, L.P.; Zhou, B.H.; Xue, Z.G. Covalently cross-linked polymer stabilized electrolytes with self-healing performance via boronic ester bonds. Polym. Chem. 2020, 11, 5893–5902.
- Lyu, L.; Li, D.; Chen, Y.X.; Tian, Y.F.; Pei, J.Z. Dynamic chemistry based self-healing of asphalt modified by diselenide-crosslinked polyurethane elastomer. Constr. Build. Mater. 2021, 293, 123480.
- Liu, X.H.; Song, X.; Chen, B.F.; Liu, J.M.; Feng, Z.Q.; Zhang, W.C.; Zeng, J.J.; Liang, L.Y. Self-healing and shape-memory epoxy thermosets based on dynamic diselenide bonds. React. Funct. Polym. 2022, 170, 105121.
- Irigoyen, M.; Fernandez, A.; Ruiz, A.; Ruiperez, F.; Matxain, J.M. Diselenide Bonds as an Alternative to Outperform the Efficiency of Disulfides in Self-Healing Materials. J. Org. Chem. 2019, 84, 4200–4210.
- Song, P.A.; Wang, H. High-Performance Polymeric Materials through Hydrogen-Bond Cross-Linking. Adv. Mater. 2020, 32, 1901244.
- Kim, S.M.; Jeon, H.; Shin, S.H.; Park, S.A.; Jegal, J.; Hwang, S.Y.; Oh, D.X.; Park, J. Superior Toughness and Fast Self-Healing at Room Temperature Engineered by Transparent Elastomers. Adv. Mater. 2018, 30, 1705145.
- Li, T.; Zheng, T.Z.; Guo, Z.X.; Xu, J.; Guo, B.H. A Well-defined Hierarchical Hydrogen Bonding Strategy to Polyureas with Simultaneously Improved Strength and Toughness. Chin. J. Polym. Sci. 2019, 37, 1257–1266.
- Gong, Z.; Huang, J.R.; Cao, L.M.; Xu, C.H.; Chen, Y.K. Self-healing epoxidized natural rubber with ionic/coordination crosslinks. Mater. Chem. Phys. 2022, 285, 126063.
- Charlet, A.; Lutz-Bueno, V.; Mezzenga, R.; Amstad, E. Shape retaining self-healing metal-coordinated hydrogels. Nanoscale 2021, 13, 4073–4084.
- Pignanelli, J.; Qian, Z.Y.; Gu, X.D.; Ahamed, M.J.; Rondeau-Gagne, S. Modulating the thermomechanical properties and self-healing efficiency of siloxane-based soft polymers through metal-ligand coordination. New J. Chem. 2020, 44, 8977–8985.
- Xuan, H.Y.; Ren, J.Y.; Zhang, J.H.; Ge, L.Q. Novel highly-flexible, acid-resistant and self-healing host-guest transparent multilayer films. Appl. Surf. Sci. 2017, 411, 303–314.
- Hu, Z.; Zhang, D.Y.; Lu, F.; Yuan, W.H.; Xu, X.R.; Zhang, Q.; Liu, H.; Shao, Q.; Guo, Z.H.; Huang, Y.D. Multistimuli-Responsive Intrinsic Self-Healing Epoxy Resin Constructed by Host-Guest Interactions. Macromolecules 2018, 51, 5294–5303.
- Sugane, K.; Maruoka, Y.; Shibata, M. Self-healing epoxy networks based on cyclodextrin-adamantane host-guest interactions. J. Polym. Res. 2021, 28, 423.
- Wang, C.; Fadeev, M.; Vazquez-Gonzalez, M.; Willner, I. Stimuli-Responsive Donor-Acceptor and DNA-Crosslinked Hydrogels: Application as Shape-Memory and Self-Healing Materials. Adv. Funct. Mater. 2018, 28, 1803111.
- Das, A.; Ghosh, S. Supramolecular Assemblies by Charge-Transfer Interactions between Donor and Acceptor Chromophores. Angew. Chem. Int. Ed. 2014, 53, 2038–2054.
- Ying, W.B.; Wang, G.Y.; Kong, Z.Y.; Yao, C.K.; Wang, Y.B.; Hu, H.; Li, F.L.; Chen, C.; Tian, Y.; Zhang, J.W.; et al. A Biologically Muscle-Inspired Polyurethane with Super-Tough, Thermal Reparable and Self-Healing Capabilities for Stretchable Electronics. Adv. Funct. Mater. 2021, 31, 2009869.
- Tamate, R.; Watanabe, M. Recent progress in self-healable ion gels. Sci. Technol. Adv. Mater. 2020, 21, 388–401.
- Tamate, R.; Hashimoto, K.; Ueki, T.; Watanabe, M. Block copolymer self-assembly in ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 25123–25139.
- Zhang, Q.; Liu, L.B.; Pan, C.G.; Li, D. Review of recent achievements in self-healing conductive materials and their applications. J. Mater. Sci. 2018, 53, 27–46.
- Thangavel, G.; Tan, M.W.M.; Lee, P.S. Advances in self-healing supramolecular soft materials and nanocomposites. Nano Converg. 2019, 6, 29.
- Yang, L.; Lu, X.L.; Wang, Z.H.; Xia, H.S. Diels-Alder dynamic crosslinked polyurethane/polydopamine composites with NIR triggered self-healing function. Polym. Chem. 2018, 9, 2166–2172.
- Zheng, K.W.; Tian, Y.Z.; Fan, M.J.; Zhang, J.Y.; Cheng, J. Recyclable, shape-memory, and self-healing soy oil-based polyurethane crosslinked by a thermoreversible Diels-Alder reaction. J. Appl. Polym. Sci. 2018, 135, 46049.
- Mondal, P.; Behera, P.K.; Voit, B.; Bohme, F.; Singha, N.K. Tailor-Made Functional Polymethacrylates with Dual Characteristics of Self-Healing and Shape-Memory Based on Dynamic Covalent Chemistry. Macromol. Mater. Eng. 2020, 305, 2000142.
- Cai, C.T.; Zhang, Y.; Li, M.; Chen, Y.; Zhang, R.C.; Wang, X.L.; Wu, Q.; Chen, T.H.; Sun, P.C. Multiple-responsive shape memory polyacrylonitrile/graphene nanocomposites with rapid self-healing and recycling properties. RSC Adv. 2018, 8, 1225–1231.
- Liang, C.Y.; Li, J.; Xia, M.; Li, G.P.; Luo, Y.J. Performance and Kinetics Study of Self-Repairing Hydroxyl-Terminated Polybutadiene Binders Based on the Diels-Alder Reaction. Polymers 2017, 9, 200.
- Xia, M.; Zhang, Y.J.; Na, Q.; Guo, T.; Zhang, M.H.; Qi, Z.Y.; Liu, N.N.; Yang, F.Z.; Luo, Y.J.; Yang, W. Preparation and characterization of self-healing furan-terminated polybutadiene (FTPB) based on Diels-Alder reaction. RSC Adv. 2021, 11, 32369–32375.
- Li, Y.B.; Yang, Z.J.; Zhang, J.H.; Ding, L.; Pan, L.P.; Huang, C.; Zheng, X.; Zeng, C.C.; Lin, C.M. Novel polyurethane with high self-healing efficiency for functional energetic composites. Polym. Test. 2019, 76, 82–89.
- Banerjee, S.L.; Bhattacharya, K.; Samanta, S.; Singha, N.K. Self-Healable Antifouling Zwitterionic Hydrogel Based on Synergistic Phototriggered Dynamic Disulfide Metathesis Reaction and Ionic Interaction. ACS Appl. Mater. Interfaces 2018, 10, 27391–27406.
- Kamada, J.; Koynov, K.; Corten, C.; Juhari, A.; Yoon, J.A.; Urban, M.W.; Balazs, A.C.; Matyjaszewski, K. Redox Responsive Behavior of Thiol/Disulfide-Functionalized Star Polymers Synthesized via Atom Transfer Radical Polymerization. Macromolecules 2010, 43, 4133–4139.
- Wang, Z.J.; Tian, H.M.; He, Q.G.; Cai, S.Q. Reprogrammable, Reprocessible, and Self-Healable Liquid Crystal Elastomer with Exchangeable Disulfide Bonds. ACS Appl. Mater. Interfaces 2017, 9, 33119–33128.
- Wang, S.Y.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583.
- Guo, H.S.; Han, Y.; Zhao, W.Q.; Yang, J.; Zhang, L. Universally autonomous self-healing elastomer with high stretchability. Nat. Commun. 2020, 11, 2037.
- Chaturvedi, S.; Dave, P.N. Solid propellants: AP/HTPB composite propellants. Arab. J. Chem. 2019, 12, 2061–2068.
- Kalman, J.; Essel, J. Influence of Particle Size on the Combustion of CL-20/HTPB Propellants. Propellants Explos. Pyrotech. 2017, 42, 1261–1267.
- Gui, D.Y.; Zong, Y.Y.; Ding, S.; Li, C.H.; Zhang, Q.L.; Wang, M.L.; Liu, J.H.; Chi, X.H.; Ma, X.G.; Pang, A.M. In-situ Characterization and Cure Kinetics in NEPE Propellant/HTPB Liner Interface by Microscopic FT-IR. Propellants Explos. Pyrotech. 2017, 42, 410–416.
- Zhou, Q.C.; Ju, Y.T.; Wei, Z.; Han, B.; Zhou, C.S. Cohesive Zone Modeling of Propellant and Insulation Interface Debonding. J. Adhes. 2014, 90, 230–251.
- Han, B.; Ju, Y.T.; Zhou, C.S. Simulation of crack propagation in HTPB propellant using cohesive zone model. Eng. Fail. Anal. 2012, 26, 304–317.
- Tu, J.; Xu, H.; Liang, L.; Li, P.Y.; Guo, X.D. Preparation of high self-healing efficient crosslink HTPB adhesive for improving debonding of propellant interface. New J. Chem. 2020, 44, 19184–19191.
- Li, Y.B.; Yang, Z.J.; Ding, L.; Pan, L.P.; Zhang, J.H.; Zheng, X.; Lin, C.M. Feasible self-healing CL-20 based PBX: Employing a novel polyurethane-urea containing disulfide bonds as polymer binder. React. Funct. Polym. 2019, 144, 104342.
- Wu, Y.G.; Luo, Y.J.; Ge, Z. Properties and Application of a Novel Type of Glycidyl Azide Polymer (GAP)-Modified Nitrocellulose Powders. Propellants Explos. Pyrotech. 2015, 40, 67–73.
- Xu, R.Q.; Li, Z.M.; Chen, Y.H.; Wang, Y.L.; Zhao, B.D. Synthesis, characterization, and properties of 1,2,8,9-tetraazido-4,6-dioxol-nonane: A promising multi-azido ether energetic plasticizer for glycidyl azide polymer. Dalton Trans. 2020, 49, 9016–9023.
- Lysien, K.; Stolarczyk, A.; Jarosz, T. Solid Propellant Formulations: A Review of Recent Progress and Utilized Components. Materials 2021, 14, 6657.
- Chen, L.; Mao, X.D.; Li, Q.; Cao, X.; Zhang, J.W.; Wang, Y.B.; Liu, J.; He, W.D. One-step, safe and efficient preparation strategy of nitrate glycerol ether cellulose-based energetic composites with application potential in propellants. Compos. Commun. 2021, 28, 100956.
- Mohan, Y.M.; Mani, Y.; Raju, K.M. Synthesis of azido polymers as potential energetic propellant binders. Des. Monomers Polym. 2006, 9, 201–236.
- Sikder, A.K.; Reddy, S. Review on Energetic Thermoplastic Elastomers (ETPEs) for Military Science. Propellants Explos. Pyrotech. 2013, 38, 14–28.
- Boopathi, S.K.; Hadjichristidis, N.; Gnanou, Y.; Feng, X.S. Direct access to poly(glycidyl azide) and its copolymers through anionic (co-)polymerization of glycidyl azide. Nat. Commun. 2019, 10, 293.
- Wang, Z.; Zhang, T.F.; Zhang, Z.J.; Ge, Z.; Luo, Y.J. Effect of hard-segment content on rheological properties of glycidyl azide polyol-based energetic thermoplastic polyurethane elastomers. Polym. Bull. 2016, 73, 3095–3104.
- Zhang, Z.J.; Wang, G.; Wang, Z.; Zhang, Y.L.; Ge, Z.; Luo, Y.J. Synthesis and characterization of novel energetic thermoplastic elastomers based on glycidyl azide polymer (GAP) with bonding functions. Polym. Bull. 2015, 72, 1835–1847.
- Yang, J.; Zhang, G.P.; Wang, J.; Hao, Y.J.; Hao, G.Z.; Xiao, L.; Chen, J.Y.; Zhou, B.J.; Fu, J.J.; Jiang, W. Parthenocissus-inspired, strongly adhesive, efficiently self-healing polymers for energetic adhesive applications. J. Mater. Chem. A 2021, 9, 16076–16085.
- Hu, Y.F.; Tang, G.; Luo, Y.J.; Chi, S.M.; Li, X.Y. Glycidyl azide polymer-based polyurethane vitrimers with disulfide chain extenders. Polym. Chem. 2021, 12, 4072–4082.
- Ding, S.J.; Zhang, J.; Zhu, G.C.; Ren, X.; Zhou, L.; Luo, Y.J. Rationally Constructed Surface Energy and Dynamic Hard Domains Balance Mechanical Strength and Self-Healing Efficiency of Energetic Linear Polymer Materials. Langmuir 2021, 37, 8997–9008.
- Ding, S.J.; Zhu, G.C.; Zhao, S.; Wu, W.; Jin, P.; Jiao, Y.K.; Zhai, W.R.; Zhou, L.; Luo, Y.J. Simultaneously optimized healing efficiency and mechanical strength in polymer composites reinforced by ultrahigh loading fillers based on interfacial energy and dynamic disulfide bonds. Polymer 2022, 251, 124711.
- Wang, S.; Fu, D.H.; Wang, X.R.; Pu, W.L.; Martone, A.; Lu, X.L.; Lavorgna, M.; Wang, Z.H.; Amendola, E.; Xia, H.S. High performance dynamic covalent crosslinked polyacylsemicarbazide composites with self-healing and recycling capabilities. J. Mater. Chem. A 2021, 9, 4055–4065.
- Xia, J.H.; Li, T.Y.; Lu, C.J.; Xu, H.P. Selenium-Containing Polymers: Perspectives toward Diverse Applications in Both Adaptive and Biomedical Materials. Macromolecules 2018, 51, 7435–7455.
- Ji, S.B.; Xia, J.H.; Xu, H.P. Dynamic Chemistry of Selenium: Se-N and Se-Se Dynamic Covalent Bonds in Polymeric Systems. ACS Macro Lett. 2016, 5, 9–13.
- Ji, S.B.; Cao, W.; Yu, Y.; Xu, H.P. Visible-Light-Induced Self-Healing Diselenide-Containing Polyurethane Elastomer. Adv. Mater. 2015, 27, 7740–7745.
This entry is offline, you can click here to edit this entry!
