Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Arid environments are characterized by a higher evaporation than precipitation, as well as persistent water shortages, frequent droughts, high climate variability, and high wind velocity. These soil constraints can be ameliorated and the crop yields increased through case-specific optimization of irrigation and drainage management, enhancing the native beneficial soil microbes, and combinations of soil amendments, conditioners, and residue management.
- aridity
- calcareous
- drylands
- gypsic
- plant growth promoting bacteria
1. Introduction
Rapid population growth puts tremendous pressure on natural resources, and the demand for increased and high-quality food is the most important concern for fulfilling food and nutritional security today. People in low-income countries are highly prone to risk owing to unprecedented land degradation. The objective is thus to achieve a state of land degradation neutrality for sustainable agriculture [1]. To achieve land degradation neutrality, land must be maintained in such a way that it can support biological activities and produce enough food for human consumption [2]. In accordance with one of the United Nations’ Sustainable Development Goals (Target 15.3 for 2030), which specifies the need to fight desertification, it is important to rehabilitate degraded land and soil, particularly land affected by desertification and drought, in order to establish a land degradation-neutral world [1]. This indicates the need for developing and utilizing the land, especially previously overlooked areas. In this respect, the large soil resources of arid regions provide a potential agricultural habitat and comprise around 16% of the planet’s land surface [3]. Most developing nations with rapid population expansion are located in arid and semi-arid regions. However, since 1950, aridity has risen across the majority of the Earth’s surface, a trend that has been exacerbated by the ongoing effects of current global warming [4]. Because of limited natural water resources, arid areas are extremely susceptible to climate variability and extreme occurrences such as droughts and heatwaves, and as a result they experience rapid environmental and land degradation. Insidious land degradation, whether induced by natural forces or human mismanagement, has the potential to challenge the resilience of natural ecosystems, produce permanent alterations in their states, and in the worst situations, bring about permanent desertification [5]. Expanding and intensively using agricultural lands, improper irrigation methods, forest clearing, and overgrazing are all human activities that lead to desertification. These unsustainable practices place a heavy burden on the land by influencing its soil chemistry and hydrology in undesirable ways. The expected drier and warmer climate will have significant effects on biomass accumulation, decomposition, and C storage in a variety of ways, and eventually disturb the biogeochemical cycles of carbon (C), nitrogen (N), and phosphorus (P), resulting in a reduction in the provision of important services provided by arid ecosystems [6].
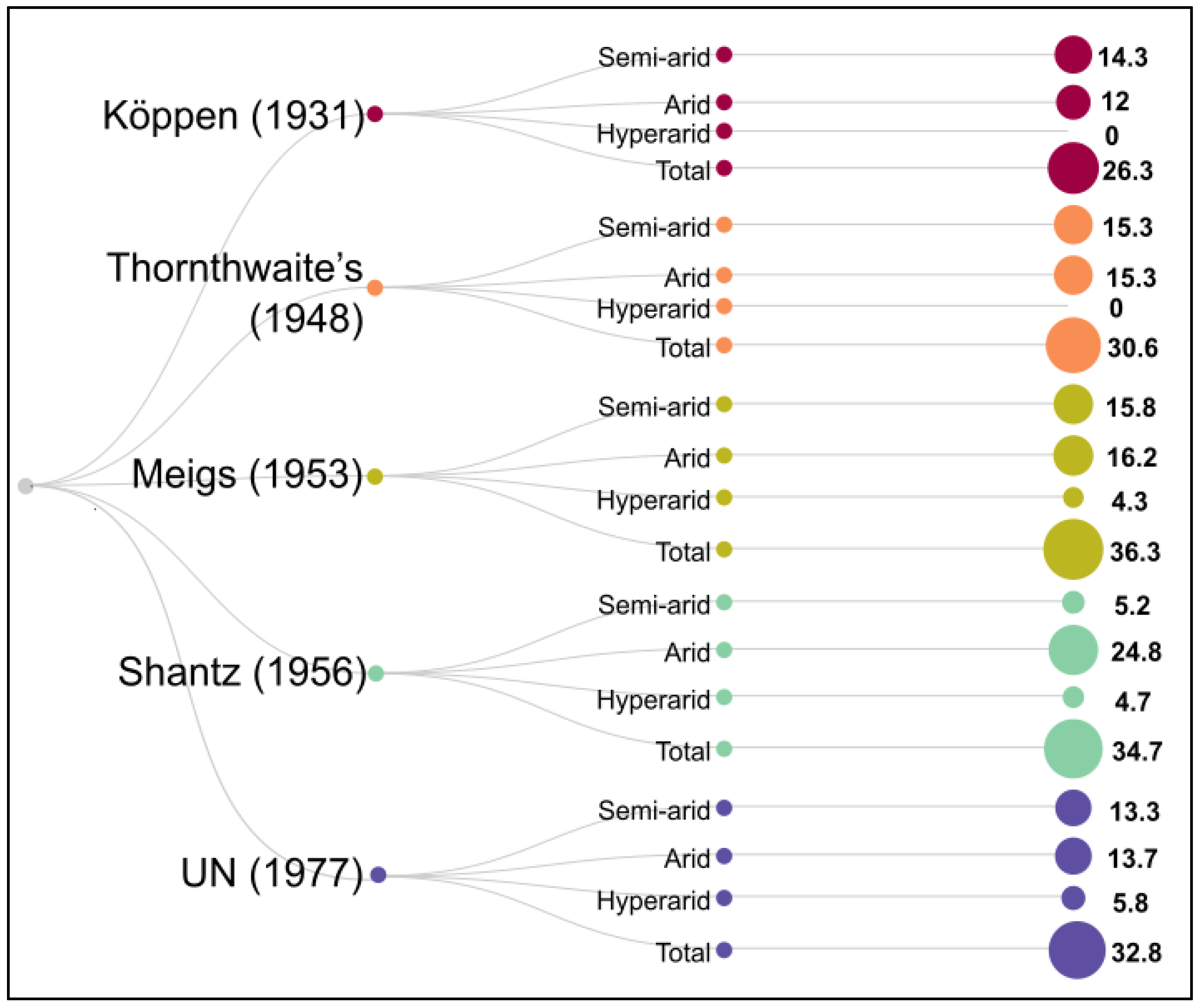
2. Concept and Distribution of Arid Zone
There are several classifications of arid zones, as shown in Figure 1. Meigs [7] classified arid regions based on whether they have hot or cold winters (Table 1). The Food and Agriculture Organization (FAO) defines arid zones as those having a length of growing period (LGP) of 0–179 days [8], while the United Nations Convention to Combat Desertification (UNCCD) uses the ratio of yearly precipitation to potential evapotranspiration (P/PET) as a criterion for its classifications. Based on the criteria established by UNCCD, arid regions have a P/PET ratio between 0.05 and 0.65. For the purposes of this research, the classification from UNCCD was used. The aridity index (AI) was used to categorise and define arid regions as hyper-arid (AI < 0.05), arid (AI = 0.05–0.20), semi-arid (AI = 0.20–0.50), or sub-humid zones (AI = 0.5–0.65) [9]. Arid regions account for 10.6% of the Earth’s land area, but semi-arid regions are much larger, covering 15.2% of the land area, and may be found on all seven continents. Finally, dry sub humid regions account for just 8.7% of the land area [10].

Table 1. The climates of an arid zone [7].
| Sl. No. | Arid Climate | Mean Temperature (°C) | Global Arid Lands (%) | |
|---|---|---|---|---|
| Coldest Month | Warmest Month | |||
| 1 | Cold winter | <0 | 10–30 | 24 |
| 2 | Cool winter | 0–10 | 10–30 | 15 |
| 3 | Mild winter | 10–20 | 10–30 | 18 |
| 4 | Hot | 10–30 | >30 | 43 |
More than a third of the world’s population lives in dryland regions, which cover 5.36 million km2 (41%) of the Earth’s land area [15]. There are extensive arid zones between 15° and 30° latitude in both the Northern and Southern hemispheres, including in North and South America, North Africa, the Sahelian region, Africa south of the Equator, the Near East, Asia, and the Pacific [16]. Africa possesses 37% of the world’s arid zones, making it the continent most at risk from land degradation and desertification, given that 66% of its territory is classified as desert or arid. Asia, home to 33% of the world’s arid zones, also experiences these consequences to a great extent.
3. Characteristics of an Arid Ecosystem and Its Soil Constraints
Arid environments are characterized by a higher evaporation than precipitation, as well as persistent water shortages, frequent droughts, high climate variability, and high wind velocity. When desertification occurs and previously non-arid areas become arid, a loss of biodiversity can also occur. The concept of aridity is based on the ratio of available water to the total amount of water used. Because of atmospheric stability, precipitation is often lower than evapotranspiration in arid regions, and frigid winters are typical. Dry, stable air masses that resist convective currents are a common source of aridity. The absence of storm systems, which cause convergence, generate unstable conditions, and supply the upward movement of air required for precipitation, can also lead to aridity. Most of the precipitation in hot deserts occurs in strong convectional showers that do not cover large areas, making widespread rains virtually unheard of in these regions. In low-latitude deserts, the skies are usually clear, allowing for plenty of sunshine. A low latitude desert’s annual temperature range is greater than that of any other tropical climate. Water is commonly lost as runoff in arid regions because the soils cannot absorb all of the rain that falls during heavy storms [17]. In other cases, when rain falls on dry land, much of the precipitation is lost to evaporation. As much as 90% of rainfall in arid settings evaporates back into the atmosphere, leaving only 10% for productive transpiration [18]. Low precipitation and high temperatures result in high evaporation in arid regions, which also leads to aridic and xeric soil moisture regimes.
3.1. Scarcity of Water
Water scarcity is a major limiting factor of agricultural production in arid locations. Because of low precipitation and high evapotranspiration, good quality water is in scarce supply in arid areas. This problem is aggravated by the current state of global climate change, which brings extreme weather events and prolonged dry seasons. Optimizing farming practices for production and water management improves soil, water, and product quality [19]. Because of the scarcity of water, the soils in arid regions have a poor natural primary production and low soil fertility. Arid soils typically have an alkaline pH and accumulate significant amounts of potassium (K), salt, calcium (Ca), and other minerals, which can be detrimental to plant growth. Furthermore, gypsic crusts are also formed in some arid soils that could support specialized species of plants. Therefore, low soil fertility has negatively affected plant growth and biodiversity in arid soils [20]. Because of the existence of subtle environmental conditions, which were not taken into account by the soil fertility assessment systems developed by researchers and scientists for temperate and humid areas, Hag Husein et al. [21] concluded that the conventional systems have a low potential for adoption in arid areas. Therefore, soil fertility assessment tools tailored to arid areas should be developed to ensure widespread adoption and actual application.
Management to Cope with Water Scarcity
There are two different aspects of arid regions, namely (i) arid regions with a persistent lack of precipitation (absolute deserts) and (ii) arid regions with erratic precipitation (periodic drought of unpredictable severity and duration). The selection of crops, their composition and rotation, and, especially, the quantitative needs of fields and water requirement, schedule of watering, and the combination of watering with atmospheric precipitation all depend on an accurate assessment of the hydrothermal conditions of the region. In general, during the growing season, non-saline soils should not have a relative humidity that falls below 65–70% of the field moisture capacity. When watering non-saline soils, only a deficit level of the field moisture capacity should be applied [22]. In fact, the high potential productivity of irrigated agriculture in arid lands has been realised, and admiring remarks by visitors from temperate lands may be found in the classical literature. In 1800, Napoleon’s savants conducted a survey that included a quantitative analysis of the productivity of traditional irrigated agriculture in Egypt. Based on the relative yields of the wheat crop, they concluded that Egypt’s agricultural output was more than double that of France [23].
In arid areas, it is challenging to meet the agriculture water demand solely by utilising conventional water sources. Fader et al. [24] reported that by implementing more efficient irrigation and conveyance systems, the Mediterranean region could save up to 35% of water used. Wastewater reuse or low-quality water could be a potential option in the region. Recovering and reusing large quantities of low-quality water for irrigation, such as that from urban and industrial wastewater treatment plants, has the potential to reduce the need for groundwater. However, there are potential drawbacks associated with their usage in irrigation, including pollutants and crop toxicity, soil quality decline, parasite transmission, and system flaws [25][26]. The toxicity, solubility, and concentration of the chemicals will determine the severity of the potential effects. The rate and frequency of wastewater application, the type of crop and the desired yields, the inherent soil properties and condition, the prevailing weather patterns, the farmers’ level of technological capabilities, and their socioeconomic standing are also significant. In the arid western United States, sites irrigated with recycled wastewater have shown 187% higher electrical conductivity and 481% higher sodium adsorption ratio (SAR) compared with the sites irrigated with fresh water [27]. Poor irrigation management and inadequate soil drainage systems are the primary causes of persistent soil salinity and/or sodicity issues caused by the use of saline irrigation water [28], which is more prevalent in arid areas. However, the proper choice of crops is necessary in this regard. For example, in the arid Mediterranean region, the yield of maize grown using drip irrigation is around 25% higher than that of maize grown with surface irrigation [29]. Using a drip irrigation system with salt water with an electrical conductivity of 12 dS/m, the maize produced yields that were comparable to those obtained using fresh water. Compared wih barley, which has a yield threshold of 8 dS/m, bread wheat (Triticum aestivum L.) is only moderately salt tolerant, with a threshold of 6 dS/m; durum wheat (Triticum durum Desf.) is even less salt tolerant than bread wheat [30]. When compared with other legume species in the arid Mediterranean region, faba beans score the highest in their ability to thrive in dry conditions due to their rapid growth, early flowering, and maturity, which allow them to avoid drying up and dying [31]. However, lentils (Lens culinaris Medicus) also have osmotic adjustment, can escape drought through being tolerant to a low temperature, have rapidly filling seed, and mature early, while chickpeas (Cicer arietinum L.) have deep roots, osmotic adjustment, and a generally high level of drought resistance and cold tolerance. One of the crops chosen to ensure food security in the 21st century is quinoa (Chenopodium quinoa Willdenow) [32]. The intrinsic low osmotic potential and the plant’s capacity for growth plasticity and tissue elasticity [33] allow quinoa to survive in dry environments [34]. As a result of the plant’s deep root system, reduced leaf area, vesicular bladders, small and thick-walled cells suited to losses of water without loss of turgor, and stomatal closure, the crop is protected against the detrimental effects of drought [34].
Moreover, there is an addition of excessive nitrogen through wastewater irrigation, causing eutrophication in arid areas [35]. Selecting crops that can take advantage of high concentrations of nutrients, such as fodder grass [36], or employing the method of crop rotation to permit the removal of any excess nutrients, could help to reduce the need for the excessive supply of nutrients, especially nitrogen. According to Hamilton et al. [35], the possibility of nitrate leaching into groundwater can be significantly lowered through careful adaptation of crop and plant production systems in alignment with local weather patterns and effluent characteristics. When it comes to reducing nitrate leaching, for instance in arid regions, high yielding crops with substantial concentrations of nitrogen in their biomass (such as leafy vegetable and fodder grass) are likely to be more beneficial than tree plantations [35][36].
A strong need exists for the development of regional decision tools to determine the most appropriate agricultural management strategies (i.e., crop choice, sowing time, management of soil cover, timings, and rates of fertilizer application, etc.) according to the amount of water held in the soil, especially given the inconsistency of rainfall in most arid areas. In order to boost biomass production and, as a consequence, both above- and below-ground inputs of C to the soil, it is necessary to increase the amount of plant-available water. This can be achieved by optimising the amount of precipitation collected, the amount of water retained by the soil, and the efficiency with which crops use available water. Capturing rainwater is highly dependent on the soil structure, as well as the presence and connectivity of macropores at the soil surface; however, improvement in the soil structure in the arid region is quite a challenging task, amidst less crop biomass and low organic input addition. Understanding the complex ecological processes associated with vegetation on soil moisture is vital for vegetation restoration in arid environments, even though the impacts of vegetation on soil moisture are multifarious. Vegetation growth and succession are influenced by soil moisture at the root zone [37], and vegetation in turn influences the soil capacity for storing, transferring, and evaporating water at the canopy level [38]. Canopy interception and stem flow are two ways in which vegetation re-distributes precipitation and hence alters post-rainfall infiltration processes [39][40].
Recent years have seen an increase in the usage of highly hydrophilic superabsorbent polymers (SAPs) in agriculture, where they are believed to function as a reservoir for both nutrients and water [41]. Some researchers have indicated that after being applied to farms, these SAPs can maintain soil moisture and store some nutrients for up to five years [42]. These polymers have been shown to enhance the physical properties of soil and especially soil aggregation, hence enhancing the quality and quantity of many agricultural products (Table 2) [43]. The benefits to the soil have been well documented, and include increased water penetration into the soil, decreased soil erosion, decreased soil bulk density [44], improved nutrient intake efficiency [45], reduced evaporation rate from the soil surface [46], better weathering, reduced leaching of soil nutrients, and increased activity and proliferation of mycorrhizal fungi and other soil microorganisms [47]. Jahan and Nassiri Mahallati [41] conducted a meta-analysis to understand whether the application of SAPs has been effective at enhancing the crop production in arid soils of Iran. It was found that the average seed yield for cereals increased by 15.2% after being treated with 83 kg ha−1 of SAPs in arid soils compared with untreated seeds.
Table 2. Effect of superabsorbent polymers (SAPs) on crop growth in water-limited environments.
| Sl. No. | Crop | Location | Treatments | Main Results | References |
|---|---|---|---|---|---|
| 1 | Forage oat (Avena sativa L.) | Northern China | Different fertilizer doses (standard, 300; medium, 225; and low, 150 kg ha−1) treatments with (60 kg ha−1) or without the application of a superabsorbent polymer | Aboveground biomass accumulation decreased by 14.8% under medium and 32.6% under low fertilizer levels, whereas the application of SAP increased it significantly by 39.7%. | Islam et al. [48] |
| 2 | Maize (Zea mays L.) | North Carolina, USA | Three K-based SAPs (Stockosorb 660, Hydrosource and SuperAB A200) at 0.15%, 0.30% and 0.45% w/w | Stockosorb 660 performed best in terms of rainwater saving (95% as compared with 59% in unamended soil), while Hydrosource was the most effective in terms of plant growth and biomass water productivity (1.17 ± 0.28 g mm−1 as compared to 0.41 ± 0.06 g mm−1 in control). | AbdAllah et al. [49] |
| 3 | Maize (Zea mays L.) | Iran | Control, 100% animal manure (AM) (40 t/ha), 100% SAP (200 kg/ha), 50% AM + 50% SAP, 35% AM + 65% SAP, 65% AM + 35% SAP |
Grain and biological yield increased by using animal manure and superabsorbent polymer together as maximum yield grain was obtained by using 65% animal manure and 35% SAP. | Khadam et al. [50] |
| 4 | Maize (Zea mays L.) | China | Carbohydrate-based SAP | Improved root length, shoot length, total biomass, germination potential and germination rate with no toxicity to plants. | Tao et al. [51] |
| 5 | Seidlitzia rosmarinus | Iran | SAP concentration gradient (0, 1, and 3 g dm−3 of soil) | SAP @ 1 g dm−3 of soil increased available water content up to 68.5% and decreased soil bulk density by 25.5% and soil infiltration rate by 21.5%. | Abrisham et al. [52] |
| 6 | - | Iran | Added the polymer Super AB, A-200 (Iran Polymer Institute), to dune sand in ratios of 0.3%, 0.6%, and 1% w/w | The plant available water content (PAW) increased from 0.005 for the untreated sand to 0.06, 0.20, and 0.28 g g−1, respectively, for the sand with the three polymer additions. | Benedjschafie et al. [53] |
| 7 | Apple (Malus domestica Borkhausen) | Iran | SAP at 200 g tree−1 | 15% increase in yield than control | Keivanfar et al. [54] |
| 8 | Onion (Allium cepa L.) | Egypt | 240:140:240 kg NPK ha−1 + 25 kg ha−1 SAP and control (only NPK) | 27.8% increase in yield than control | Soubeih et al. [55] |
| 9 | Tomato (Solanum lycopersicum L.) | Egypt | Irrigation at 100, 75, and 50% field capacity (FC) + cellulose/starch polymer at 2 g kg−1 soil | Yield increased by 20.9, 50.0, and 92.9% at 100, 75, and 50% FC, respectively, over 100% FC | Ahmed et al. [56] |
Plastic film mulching has been shown to be an invaluable tool for increasing crop yields and adapting farming practices in arid regions [57]. For example, wheat yield and water use efficiency on the arid Loess Plateau of Northwest China were much higher when plastic film mulching was used as opposed to straw mulching [58]. Plastic mulching is an excellent way to prevent soil moisture from being lost through evaporation and to maximize the use of scarce rainfall, which can help drought-stricken areas in arid regions [59]. However, some studies have found that using plastic mulch reduced the crop yields. Plastic mulching, for instance, altered the water and temperature conditions outside the range of crop adaptation in low-lying areas with an abundance of resources, leading to poorer yields [60]. Film mulching can also increase the root growth ability during the early growth stage, which led to an overabundance of soil moisture being used. Inadequate coupled with late-season precipitation and soil moisture led to an imbalance between vegetative and reproductive growth, which resulted in a lower crop production and water use efficiency [59]. However, Gao et al. [57] found that both crop yield and water use efficiency were improved in China’s arid regions when farmers began using transparent plastic and ridge row mulching.
3.2. Soil Organic and Inorganic Carbon
SOC/SOM is a primary component influencing both the composition and structure of soil. SOM also contributes to greater drought resilience in arid regions and higher crop yields. Because of climate constraints, the soils in arid regions have an inherently low stock of organic C (Table 3). However, they also have a lot of inorganic C, mostly in the form of soil carbonates (Table 3) [61]. On average, Lal [62] reported that dryland ecosystems sequestered between 0.1 and 0.2 Mg ha−1 year−1 of soil inorganic C. In addition to acting as a sink for atmospheric CO2, inorganic C in soil may also play a positive function in soil aggregation via the interaction of carbonates with SOM. Moreover, SOM controls the positive effect of carbonates in the soil structure [63]. Water stability of soil macroaggregates is highly associated with the carbonate content at low SOM values [64].
The importance of vegetative coverings in preventing soil erosion and maintaining soil organic C in arid areas has been well recognized. The presence of a sufficient protection of the soil surface is hampered by conventional management practices, including intensive tillage, feed needs for animal production, and excessive grazing [65][66][67]. Soil quality and long-term food security are threatened by the extractive nature of using crop residues as fodder for cattle and animal manure as a cooking fuel in emerging countries of Asia and Africa [68]. The loss of SOC in these nations must be prevented by increasing the quantity of crop residues generated. However, in some emerging regions, such as West Africa, fertilisation is necessary to stimulate sufficient biomass production due to the highly weathered nature of soils [69].
Appropriate crop rotations, which encourage a greater diversity of plants, typically result in an increase in above-ground biomass and a preference for a more diversified root system (i.e., below-ground C allocation), with varying effects on the soil organic C by root-derived products [70]. Soil C stock can be improved with the use of deep rooting plants [71]. The selection of species and cultivars with deeper and better root systems, as well as other measures for optimal use of the complete soil profile, are all important factors to consider when functioning with rain-fed arid agriculture.
Improvements in soil productivity, agricultural profitability, and environmental sustainability can be achieved through the rotation of controlled perennial grass or grass–legume mixtures (ley) with annual crops [72]. Adding a perennial grass–legume to a crop and livestock rotation can boost landscape diversity while maintaining or improving yields compared with less varied systems [73]. The fluctuation between soil organic C (Cs) and soil organic N (Ns) is a feature that is characteristic of crop–pasture rotating systems. Carbon and nitrogen levels drop during the annual cropping phase, but swiftly recover during the perennial pasture phase [74]. Perennial plant species contribute three to seven times more C and N to the litter pool than annual species through greater root production [75]; these roots are also placed deeper in the soil profile, which explains the Cs and Ns recovery during the pasture phase [76]. The majority of Ns improvement can be attributed to biological nitrogen fixation. The demand for N fertiliser for non-legume annual crops is reduced, as N stored during the grazing phase is gradually mineralized throughout the annual phase. This slow process of N mineralization is thought to enhance the synchronisation between N supply and N intake, hence decreasing N loss opportunities and allowing for greater productivity. Even when annual crops are maintained with no-till, the yield ceiling might drop and the yield gap can widen when pastures are removed from the rotation, as has been demonstrated for wheat [77].
Table 3. Elemental stocks (mean ± standard deviation) of the global hyper arid and arid soils in three different soil depths; modified from [78].
| Parameters (Pg) | Hyper arid | Arid |
|---|---|---|
| Organic Carbon | 11 ± 1 (D1), 22 ± 1 (D2), 31 ± 1 (D3) | 45 ± 3 (D1), 91 ± 3 (D2), 127 ± 3 (D3) |
| Inorganic Carbon | 20 ± 2 (D1), 65 ± 3 (D2), 127 ± 5 (D3) | 63 ± 2 (D1), 241 ± 5 (D2), 487 ± 9 (D3) |
| Total Nitrogen | 1.3 ± 0.1 (D1), 2.9 ± 0.1(D2), 4.5 ± 0.2 (D3) | 4.9 ± 0.2 (D1), 10.9 ± 0.2 (D2), 17.3 ± 0.3 (D3) |
D = soil depths (D1 = 0–30 cm; D2 = 0–100 cm; D3 = 0–200 cm).
3.3. Salinity
In arid regions, soil salinization has emerged as a serious environmental constraint threatening soil productivity, agricultural sustainability, and food security [79]. When water-soluble salts are retained in the soil, it becomes saline. It can occur naturally or as a result of poor anthropogenic activities, most often related to agricultural operations. Dry climates and low precipitations cause soil salinization because they prevent excess salts from being washed from the soil. High evaporation rates increase salts on the ground surface. Poor drainage or waterlogging prevents salts from being washed because of a lack of water transportation. Irrigation with salt-rich water increases the salt content in soil. The removal of deep-rooted vegetation elevates the water table. Saltwater intrusion into groundwater, coastal breezes transporting salty air masses inland, seawater submergence followed by salt evaporation, seawater submergence, and improper fertiliser use all contribute to soil salinization. At present, saline soils can be found in at least 100 different countries, with a total area of 932.2 Mha [79]. This includes particularly large areas in Pakistan, China, the United States, India, Argentina, the Sudan, and many nations in Central and Western Asia, as well as along the Mediterranean Coast [79]. Wichelns and Qadir et al. [80] estimated that the agricultural industry loses $27.3 million per year due to salinization in agricultural lands. According to Bridges and Oldeman [81], secondary salinization causes between 10 and 20 million hectares of irrigated land to become unproductive every year. This translates to 3 ha of arable land becoming unproductive every minute around the world. As the world’s population is predicted to grow to 9.8 billion by 2050 [82], soil salinization will continue to be a major barrier to food production and the satisfaction of people’s hunger.
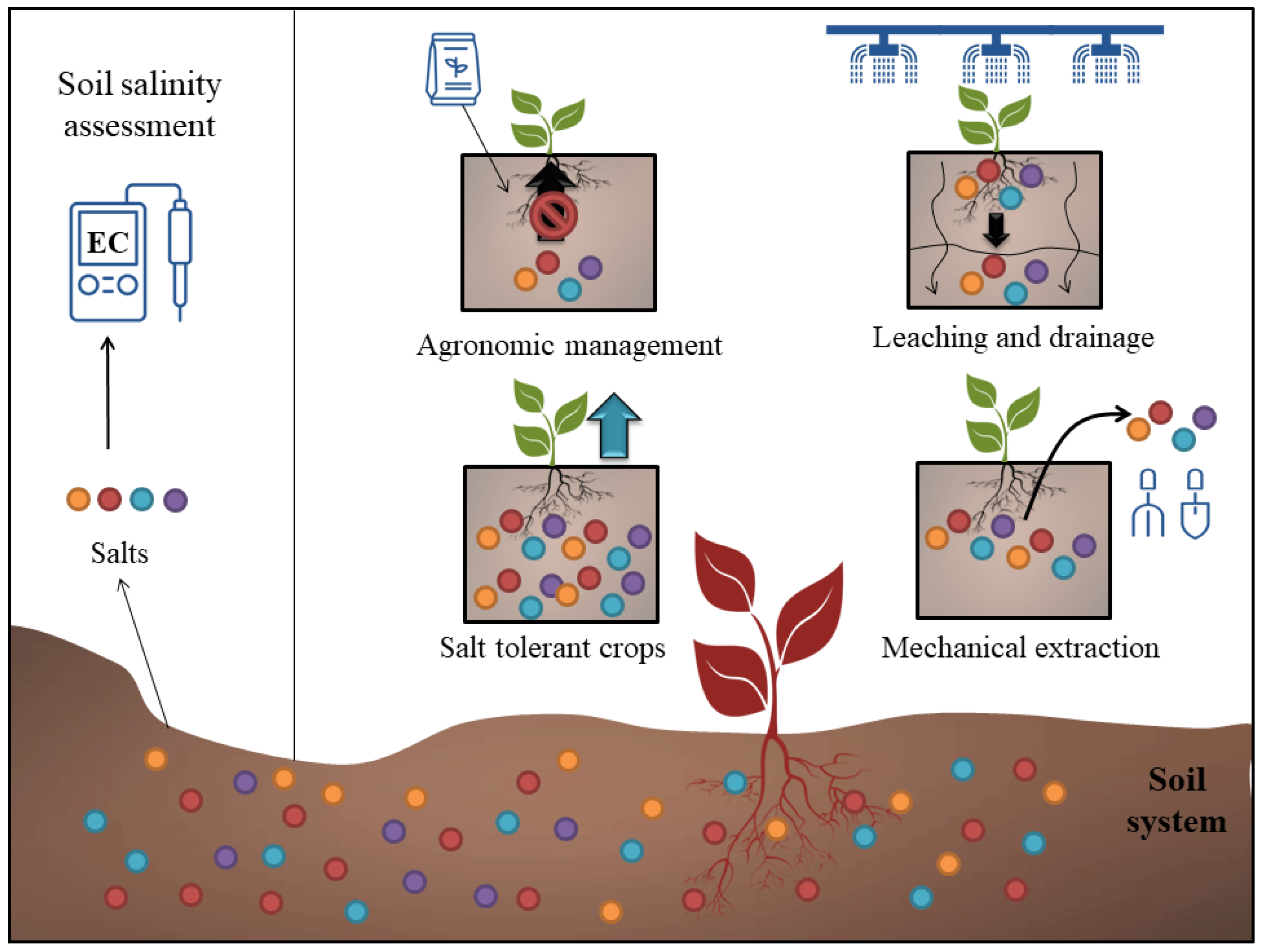
As salinization can have a significant economic impact on crops, farmers that face this issue often switch to a different cropping system. The land is either abandoned or attempts are made to mitigate the effects of salinization [83]. There are four main approaches to reducing secondary salinization in agricultural land [84] (Figure 2). The first method integrates a variety of agronomic practices with the goal of reducing the detrimental effects of salinity on crop yield and quality. The second strategy aims to reduce the rate of salinization by enhancing drainage and making use of higher-quality irrigation water. The third strategy includes growing salt-resistant plants to remove excess salts. The mechanical extraction of salts such as scraping from soils is the fourth and final method (Table 4).

Figure 2. The main approaches for ameliorating saline soils in arid regions. Different colors depicts the presence of different forms of salt ions in the soil system.
One of the most important methods for preventing topsoil erosion due to soil salinization is the regulation of irrigation and fertiliser application. If farmers can still make a profit growing crops that can tolerate low to moderate levels of salt, then it may be worth considering growing salt-tolerant varieties. So far, biosaline agriculture has been seen as a last choice for situations in which substantial levels of salts develop in the root zone of the soil. When plants are grown in groundwater and/or soil that is high in salt, this method is known as biosaline agriculture. While there is a rising interest in cultivating crops that can survive in highly salty environments, only a few of these crops are commercially viable [79]. By flushing salts from the top horizons into the lower soil layers, leaching can help minimise soil salinity. The idea is to drain the salts down below the root zone and to keep them dissolved. Under limited water supply of an inferior quality, farmers must choose between giving all irrigation water so as to plant the maximum area possible without applying a leaching fraction, thus assuming a decrease in crop yield per unit area, or reducing the cultivated area while allocating some water for leaching, thus boosting the crop yield per unit area in the long run [85]. In areas where leaching is done, improved drainage is necessary to remove the surplus of drainage water (which contains salts), hence minimising or eliminating the resulting evaporation of salts. A proper drainage system is thus required in order to make leaching work properly. In addition to implementing subsurface tile drainage [83], a number of researchers have advocated dry drainage (reserving a portion of the available land for the evaporation of excess water and transporting the accompanying salt as a partial remedy for groundwater salinization) [86] and bio-drainage (cultivating specific plant species whose primary water needs can be met by the canal seepage water or the capillary fringe immediately above it) [87].
Cultivating deep-rooted salt-tolerant plants can partially and temporarily restore salt-affected land. The replacement of deep-rooted perennials with shallow-rooted annuals has produced widespread salinization in Australia [88]. Replacing native permanent evergreen vegetation with annual crops and inactive fallows disrupts the hydrological equilibrium and increases the soil profile drainage. Salt is then leached deeper in the profile, raising water tables and delivering salt to the lower land. Modifying the original flora generates long-term effects that are difficult to understand, but the result is bare, erosion-prone soil and salinized streams. The over-optimization of irrigation systems contributes to the salinization problem; hence it is important to change farmers’ perspectives on the worth of water. Water pricing has improved, especially in arid regions where water is scarce [89]. Economically efficient irrigation will become the new irrigation management paradigm due to water scarcity and poor quality. Under the limited availability of irrigation water, reducing the cultivation area to distribute leaching water is more profitable. Economic efficiency demands decision-makers to openly weigh costs, revenues, and water opportunity costs. Carefully limiting fertigation permits water savings, decreases the direct and indirect effects of the fertilizers, lowers production costs, and delivers a higher net return for the farmer.
Straw mulching, which reduces soil water evaporation and modulates soil water and salt transport [90], is a potential solution for farmers seeking to control soil salinity. Straw mulching can significantly reduce salt levels in the top 40 cm of soil [91]. Straw mulching appears to lower the soil’s surface salt concentration and also controls the vertical distribution of salt, which in turn may lessen salt damage to crops, increase crop yields, and lessen the likelihood of soil salinization and erosion.
Under the changing climate scenario, the use of salt tolerant plants is trending as one of the management approaches of salt-affected soils [92]. Plants that can survive moderate salinity (glycophytes) are distinguished from those that have a adapted to highly salty soils (halophytes). However, most agricultural crops are glycophytes and can only tolerate low to moderate salinity. The term “halophyte” is only applied to a select few crops. Some crop varieties are more resistant to salinization than others, regardless of the species planted. This is because their phenology makes it possible for them to avoid salinity-inducing conditions. Thus, it is crucial to cultivate cultivars that can withstand high levels of salt throughout their lifecycle, without compromising production [93].
Soil microbiological diversity can be improved and the effects of salinity on plant growth can be reduced by exploitation and using beneficial microorganisms that are native to arid soils [94]. There are two main approaches in the management of soil microbial diversity in cultivated soils [95]. Firstly, an untargeted long-term approach includes a low-input agricultural system such as organic farming, conservation agriculture, and intercropping, increasing landscape diversity and agroforestry. The second approach is a targeted strategy including the use of biofertilizers, biostimulants, microbial consortium, biopesticides, etc. Improvements in soil quality are facilitated by cyanobacteria and biocrusts, which function as agents that alleviate soil salinity [96]. The “salt-out mechanism” is a known cyanobacterial reaction to an excessive salt concentration, and it involves the accumulation of suitable solutes within the cell that do not disrupt metabolic activities, as well as the active exporting of Na+ and Cl− [97]. Most cyanobacteria thrive in neutral to alkaline soils, with an optimal pH of 7.5 to 10, which means that arid soils can be inoculated with specific cyanobacteria strains for soil improvement and plant growth [97]. Water movement and subsequent crop root growth can be enhanced by cultivating perennial plants with deep roots that are tolerant of saline soil [98]. Reduced soil pH and enhanced CaCO3 dissolution from increased root respiration can also contribute to higher soil Ca levels. The potential exists for a decrease in exchangeable Na percentage and electrical conductivity in the root zone where these alterations improve profile leaching. Na leaching from the root zone can take a lot longer in rainfed systems, and the method is hampered in many places by a lack of knowledge on how to practically and economically include perennial species in cropping rotations [99]. Transgenic crops are another possible answer to the problem of salinized soils. No matter the strategy chosen, the actions proposed to halt the salinization of agricultural areas must always consider acceptability by the relevant stakeholders.
Table 4. A few common technologies advocated in ameliorating saline soils.
| Sl. No. | Amelioration Technologies | Advantages | References |
|---|---|---|---|
| 1 | Application of organic manure and other amendments | Ev, Ss | Srivastava et al. [100] |
| 2 | Mycorrhizal inoculations | Ad | Copeman et al. [101] |
| 3 | Bioremediation | Ad | Singh et al. [102] |
| 4 | Use of machinery but limited to light machinery | Dr, Ss | Iannetta et al. [103] |
| 5 | Desalination of irrigation water | Ir | Iannetta et al. [103] |
| 6 | Saline water irrigation at less sensitive maturity phase | Ad | Ali [104] |
| 7 | Salt tolerant plants | Ev, Ss and Sa | Qadir et al. [105] |
| 8 | Surface flushing | Sa | Qadir et al. [105] |
| 9 | Mechanical removal of salt crust | Sa | Qadir et al. [105] |
| 10 | Bio-priming with biological agents | Ad | Rawat et al. [106] |
| 11 | Mulching | Ev, Sa | Mao et al. [107] |
| 12 | Fertilization | Ad | Flores et al. [108] |
| 13 | Green manuring | Dr, Ss | Chatzigiannakis et al. [109] |
Ev = reduction in evaporation losses; Ss = improvement in soil aggregation; Ad = enhanced adaptation; Dr = drainage improvement; Sa = reduction in salt accumulation in plants; Ir = improving irrigation water quality.
3.4. Nutrient Imbalances
Jiao et al. [110] highlighted that even though arid soils are already C, N, and P limited, increasing aridity and drought can further reduce the availability of these nutrients. Soil C and N depletion due to aridity is a result of the decline in soil water availability and vegetation cover caused by aridity, both of which have direct or indirect effects on processes involving C and N, especially biological processes such as photosynthesis, atmospheric N fixation, and the activity of microorganisms and soil enzymes. The combination of higher temperatures and less vegetation cover also accelerates soil drying, leading to erosion that can wash away fine, nutrient-rich particles such as clay [111]. Although a low annual precipitation and high evaporation rate in arid sites may prevent available nutrients from being washed out of the soil profile, these processes can nonetheless lead to an aridity-related soil N loss. There is a significant correlation between N and photosynthesis; thus if N availability drops due to drought, plants will not be able to produce as much food and help offset CO2 emissions [112].
Salinity can limit N uptake in crop plants because an increase in the uptake of Cl− coincides with a decrease in the amount of NO3− in the shoots of many crops. Salinity also reduces rhizobia density and the colonisation of root hairs, which can hinder symbiotic N2-fixation. However, symbiosis develops the amount of photosynthate transferred to the nodule, which decreases due to the limited development of the host plant [113].
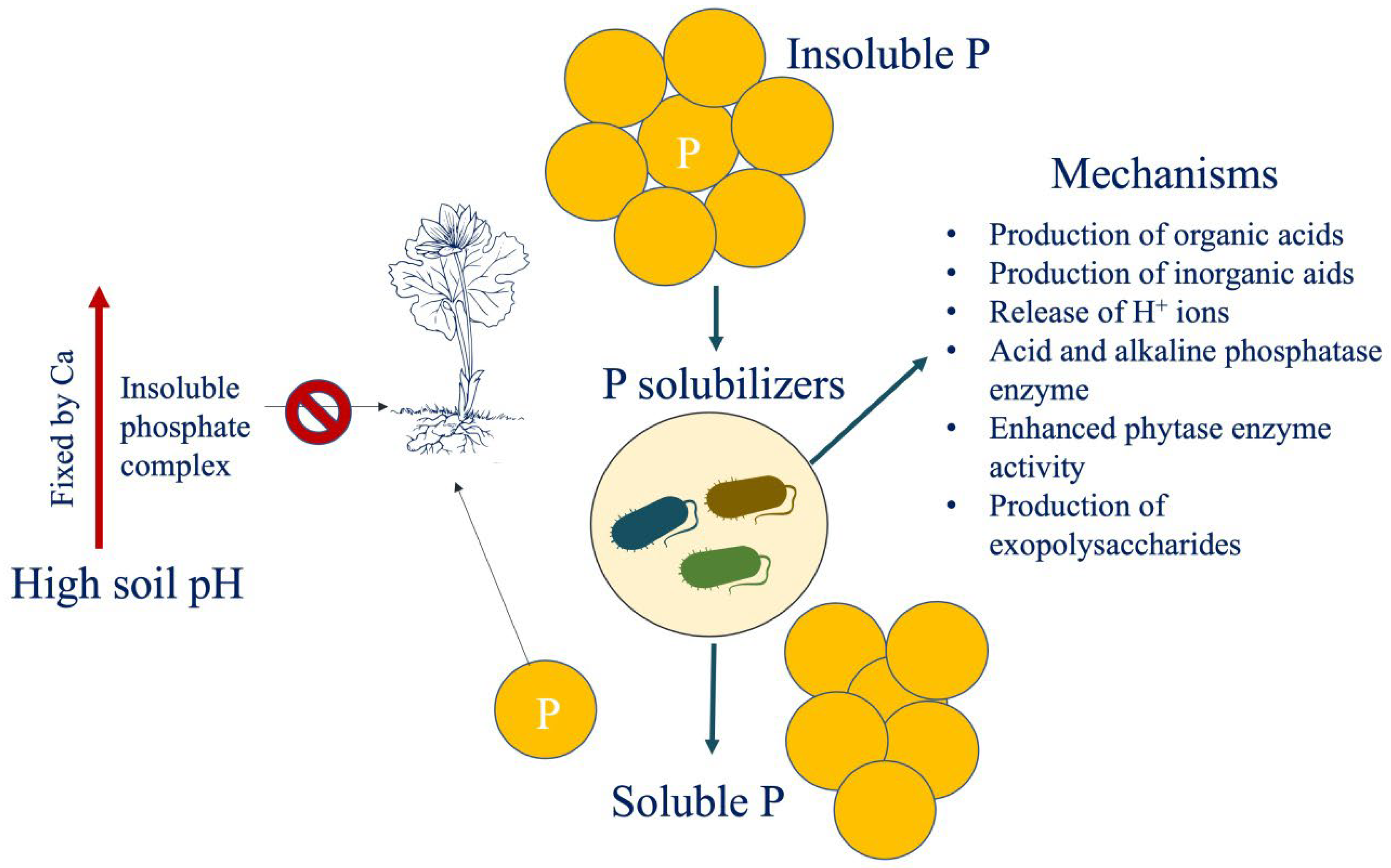
P, similar to N, is widely available in soil but has poor bioavailability. The average soil P level is estimated to be 0.05% (w/w), although only 0.1% of this is usable by plants. Thus, P-based fertilisers are commonly used; however, soon after application, a percentage equating to 75–90% of the supplied P precipitates with metal-cation complexes, rapidly becoming fixed in soil [114]. By increasing the availability of essential nutrients such as N, P, and iron (Fe), plant growth promoting rhizobacteria can boost plant growth. Here, phosphate-solubilizing bacteria could stand in for a long-term and eco-friendly method to increase plant-available P, especially in P-poor soils. The production of low molecular weight organic acids such as gluconic and citric acid, the release of protons, and the development of chelating compounds all contribute to the solubilization of inorganic P (Figure 3). On the other hand, the hydrolysis of phosphoric esters is catalysed by the enzymes phosphomonoesterase, phosphodiesterase, and phosphotriesterase, which are synthesised by micororganisms during the mineralization of organic P [115]. In general, microorganisms isolated from extreme soils (such as saline/alkaline soils and soil with nutrient deficiency) are better at solubilizing phosphate than those originating from soils characterised by moderate circumstances [116].

Figure 3. Implication of phosphorus (P) solubilizing microbes for improving phosphorus use efficiency in arid soils.
When compared with the global averages for both natural and agricultural soils, Moreno-Jiménez et al. [117] found that overall soil metallic micronutrient concentrations were lower in arid zones, especially for iron (Fe) and zinc (Zn). The provision of ecosystem services by arid areas may be jeopardised by the diminishing micronutrient availability associated with increasing aridity, in addition to other unfavourable effects such as water constraints. Low Zn contents were typical of arid soils, but Fe availability declined more rapidly in response to drying conditions. Species-specific techniques to boost iron uptake from the rhizosphere may be critical for plant survival under future scenarios of growing aridity and decreasing Fe and Zn availability. In arid zones, where the soil is typically coarse and underdeveloped, the low clay content may explain why other factors, such as organic C and pH, play more of a role in soil quality. Soil weathering and the formation of reactive sites on minerals may be slowed by arid circumstances, lowering their impact on metal availability [118]. Metal availability in arid soils is reduced indirectly by aridity, most likely as a result of a decrease in SOC and an increase in soil pH. The SOM stocks in arid regions are smaller than those in wetter regions because of the lower plant C inputs, and the soil pH is higher because of the greater accumulation of soluble salts and carbonates. Additional geochemical alterations in Fe speciation may be triggered by aridity. This is why Fe availability is more affected by drought than Zn. Iron forms that are more resistant and less mobile in soils are haematite (-Fe2O3) and goethite (-FeOOH), and water scarcity and high temperature may favour the transformation of ferrihydrite (Fe(OH)3) and goethite (-FeOOH) [119]. Zinc’s insoluble phases are primarily Zn–Al layered double hydroxides, which have a narrower range of exchangeable stable phases in soils compared with iron [120].
3.5. Sandy Soils
Soil texture and aggregation greatly impact C storage, with silt and clay size fractions able to protect SOC from breakdown [121]. Aggregates are formed when organic matter adheres to inorganic materials such as silt and clay during decomposition [122]. There was no correlation between the total C and clay + silt concentration in the soil studied by Hassink [121]; however, there was a correlation between the clay + silt content and the amount of C stored in the 20 μm size fraction. In contrast with the high levels of soil C identified in the silt and clay size fractions, which were reported by Gabarrón-Galeote et al. [123] and Tiessen and Stewart [124], low levels of soil C were discovered in the sand size fraction. According to the World Resource Base (WRB), Arenosols are soils that contain more than 700 g sand kg−1 (0.05–2.0 mm) and less than 150 g clay kg−1 (<0.002 mm). Sandy soils are found everywhere on the planet [125]. As their name implies, Arenosols are typically found in arid or semi-arid climates [126]. Arenosols, which lack substantial soil horizonation, are characterised by their poor development. Possible environments for their formation include quartz-rich rock weathering products, desert and beach sand deposits, and residual sands. Hot, dry weather typically results in a slower rate of chemical weathering. As a result of dramatic diurnal and seasonal temperature fluctuations, physical weathering predominates. Sandy soils have a high permeability to water, a low water-holding capacity, and a low capacity to retain or exchange nutrients [126]. As sandy soil contains relatively little in the form of silt and clay, it often has a poorly established soil structure and is highly vulnerable to wind erosion [127]. Despite the widespread perception that sandy soils have low levels of organic C, some studies have found that the SOC levels in the top layer of sandy soils can reach as high as 50 g kg−1, and that the SOC levels in the sub layer can reach as high as 20 g kg−1 [128].However, this C is vulnerable to loss due to several factors of desertification. Low nutrient levels in sandy soils make it imperative to supplement those soils with outside sources of nutrients in order to achieve optimal crop yields. Lot of the world’s sandy soils are located in the driest areas, which mean that not only is irrigation necessary, but the leaching of fertilisers and biocides is a real possibility [129]. Using Pedon databases, data from the literature, and results from in-depth case studies, Yosh and Hartemink [125] investigated SOC levels in sandy soils around the world. Sandy soils in the temperate and cold zones had the highest average levels of organic C in the soil (at 19 g kg−1), whereas arid zone soils had the lowest levels (at 5 g kg−1 concentrations of SOC were the highest in Spodosols and the lowest in Aridisols). Sandy soils have a higher SOC after being fertilised; however they are hydrophobic when their SOC and pH are increased. In addition to the effects of burning, drying, and an abundance of organic matter, water repellency can result when soil particles are coated by hydrophobic organic compounds such as fungal mycelia/hyphae, humic acids, and plant litter [130]. Fertilizer and manure applications increased the SOC on sandy soils that had previously supported winter wheat and summer maize or soybeans. SOC increased by as much as 3 g kg−1 when fertilised alone, and by an even greater amount when manure was added to sandy soils [131][132]. Sandy soil in forest and grassland areas had high SOC levels (90 and 187 g kg−1, respectively), while those in agricultural and shrub areas had low SOC levels (38 and 36 g kg−1, respectively) [125].
To a large extent, cyanobacterial inoculants and their bio-essudates are capable of stabilizing sandy soils (those composed of 80% sand or more). The spread of cyanobacterial filaments is inhibited by the existence of macro-pores in sandy soils; thus, the inoculant must develop more intricately to create biocrusts [133]. When biocrusts are generated on sandy surfaces, the sand becomes entangled in a web of filaments with several voids between them. Cyanobacteria species differ in their drought tolerance, but the soil’s physicochemical make-up may have a contribution as well [134]. When considering both net and gross photosynthesis, cyanobacteria prefer moisture levels between 40% and 80% of their field capacity [135]. Isolated from Israel’s Negev Desert, Leptolyngbya ohadii is capable of forming biocrusts on a hyperarid sandy substrate with a water supply comparable to that seen in the region annually [136]. Some species may be able to survive only on water obtained from their native environment.
The potential for natural N2-fixation in agroecosystem biocrusts is of special interest because of the fertiliser needs of perennial crops. Nitrogen (N) and phosphorus (P) fertilisation applications may cause biocrust N2-fixation rates in agroecosystems to differ from those in natural ecosystems. For example, both multi-species lab cultures and field samples of biocrusts have revealed that fertiliser N can reduce N2 fixation by biocrusts. For instance, in arid biocrusts, N additions that mimic atmospheric deposition drastically decreased N2-fixation rates. Moreover, N2 fixation in agroecosystem biocrust cell cultures was cut by 80% after being exposed to 55 lbs N acre−1 for 25 days [137].
3.6. Soil Loss through Erosion
The arid ecosystem is also characterized by soil loss through erosion or large areas of degraded soils (Table 5). In most arid agroecosystems, the prospect of expanding irrigation is extremely low; hence, it is necessary to find alternative methods of optimising land usage [138]. Because of the lack of substantial soil cover, poor soil structural stability accompanied with a restricted amount of SOC, and a high human pressure, arid regions are particularly vulnerable to soil erosion. Vegetative cover has been known to play a significant role in preventing soil erosion and preserving SOM for a long time in arid areas. Although adequate soil surface protection is needed in these regions, conventional management practices such as intensive tillage, excessive grazing, high feedstock demand for bioenergy, and feed needs for livestock production all function against the availability of a high SOC content in arid soils [139]. Engineering, chemical, and biological solutions are currently used to reduce soil erosion. Reducing soil erosion through engineering techniques such as checkerboards made of organic materials such as straw or tree branches, and chemical procedures such as the use of organic or inorganic substances are both viable options [140]. Inoculation-based strategies for manipulating the soil microbial community are also gaining interest as a promising biotechnology strategy [141]. Several studies have shown that inoculating soil with cyanobacteria can improve its quality: to stabilise and fertilise Mediterranean desert soils, the fixation of moving sand in Chinese arid lands, stabilisation of dried-up sand lakebeds, management of run-off on damaged soils in North West Iran, and rehabilitation of post-mining soils in South Australia [141][142]. Soil erosion can be greatly reduced by employing conservation tillage (no-tillage) practices by leaving crop residues on the field [143]. In the vast arid regions of Northern China, for example, the government is encouraging the use of conservation tillage measures due to their potential for decreasing soil deterioration [144]. New technologies can help make no-till farming work better in dry areas. For instance, the use of stripper-headers, which are implement attachments, has considerable promise in lowering soil erosion hazards associated with no-tillage in field crop production. By minimising fuel use during harvest [145], this technological advancement helps farmers save money while also reducing their impact on the environment by cutting CO2 emissions. When there are taller vertical crop stalks, the wind speed slows down. This makes it less likely that soil C will be blown away by wind and less likely that water will evaporate. For a long time, it has been understood that leaving crop residues on the soil surface after using conservation tillage practices was a great way to reduce soil erosion [143]. Unfortunately, the benefits of no-till farming have not been well verified in all of the world’s arid agricultural regions [146]. However, in arid regions, farmers are not likely to adopt conservation agricultural practices because of the high level of competition from alternative residue uses [147].
Table 5. Extent of soil degradation in arid soils of different continents; modified from [148].
| Sl. No. | Continent | Total Area of Degraded Soil (Million Hectares) |
|---|---|---|
| 1 | Asia | 150.7 |
| 2 | Africa | 172.5 |
| 3 | Australia | 48.9 |
| 4 | Europe | 4.8 |
| 5 | South America | 7.5 |
| 6 | North America | 7.9 |
Biocrusts have a significant impact on soil stabilisation and are a potential solution for reducing wind and water erosion and promoting plant establishment and succession [149]. With the growth of the biocrust, a rise in hydrophobicity is anticipated. Hydrophobicity is a measure of the accumulation of organic matter and exopolysaccharides (EPS) [150], and is correlated with biocrust maturation and the types of organisms in the biocrust [151]. According to Lázaro and Mora [152], an increase in hydrophobicity is correlated with a decrease in water infiltration and an increase in runoff. However, hydrophobicity also influences water retention in biocrusts and makes it accessible to microbial communities; hence, it might be considered as a favourable consequence of inoculation, particularly on sandy soils [137].
3.7. Hardsetting and Hardpan
Soils that dry into a solid, unstructured mass that is difficult or impossible to work until the profile is rewetted are considered hardsetting soils. More than 110 million hectares of agricultural land are affected by hardsetting, particularly in arid tropical, semi-arid, and Mediterranean regions [153]. Hardsetting soils cause a range of problems in agriculture, including a smaller period in which soil may be tilled and more physical barriers to healthy root growth. Soil degradation processes such as erosion, compaction, and crusting are often linked to hardsetting [153]. The low yield and high expense of farming in these soils is a common source of dissatisfaction for farmers. Hardsetting soil is unstable because its structure collapses as it becomes wet, and then shrinks further when it dries. Consequently, the soil layer becomes “massive”, meaning it has very few or no cracks and a very small amount of pore space [154]. This “massive” construction typically has limited water-holding capacity, high soil strength, and poor infiltration. Soil aggregates with a high exchangeable sodium percentage (ESP) can disperse, leading to hardsetting. Horizons with a high density or cemented soil particles are formed when traffic or soil genetic features generate hard layers [155]; these horizons have high penetration resistances that impede root development and reduce water and air circulation. Poor root development limits the capacity of plants to absorb water and nutrients. If water flow is slowed, rain and irrigation cannot infiltrate into the soil and be used later. Reduced airflow prevents plants and microorganisms from getting the oxygen they need to thrive. Crop yields are lowered as a result of these constraints.
Tillage is the most common method for breaking hard layers, but water/crop management and organic manure and soil amendments application are other viable options. Commonly used to address hardpan problems, tillage physically breaks up hard layers. Shanks can be used to tackle harder layers (>15 cm) at depth. The problem with tillage is that the reduction of penetration resistance is transient [156]. This means that ploughing must be repeated at set intervals, generally seasonally or annually, in order to maintain the desired effect. Researchers and farmers have been working to boost soil organic matter for decades [157]. This boosts yield, improves fertility, and weakens hard layers (particularly at the surface) [158]. As the availability of crop residues is one the main challenges in arid zones, the organic matter used to weaken hard layers can come from sources other than crop waste. In addition, it can be added through root growth, which is particularly useful for subsurface hard layers, through the so-called “biodrilling effect” [159]. Growing cover crops between growing seasons allows their roots to penetrate the hard layers, softening the soil. Irrigating the soil with drip tubes buried just above the hard layer has also shown some success in softening the hard layers. The hard layer is kept pliable and the crop’s needs are satisfied through irrigation.
3.8. Gypic Soils
Gypsic soil makes up a small proportion of the world’s total soil area and is most common in sparsely populated, arid, or semi-arid environments [160]. Gypsum presence in agricultural soils provides possible concerns with dissolving pipework, particularly in response to irrigation, in areas of more extensive management. In addition, water and nutrient loss are common in these soils. In order to meet the food demands of a growing population, farmers will have to start cultivating more marginal soil landscapes, such as those found in gypsic soils. Understanding the land use potential and function of this unique substrate is necessary for increasing productivity on gypsic soils currently under agriculture or rangeland management. There are approximately 60,000 km2 of soil with petrogypsic layers and 2.01 million km2 of soil with gypsic horizons, according to estimates by Eswaran and Zi-Tong [161].
Soils in arid and semi-arid regions have been reported to have gypsum at higher concentrations than any other sulphate mineral. Herrero et al. [162] discovered that under aridic soil moisture regimes, micro-crystalline gypsum (a weathering product of rock gypsum) can be transported as a result of mass movement and runoff, followed by dissolution and reprecipitation or leaching. The formation of gypsic soils is mostly governed by the parent materials in regions with limited precipitation, minimal leaching, and shallow wetting depths. The sources of pedogenic gypsum in gypsic soils are directly associated with gypsum-rich parent materials, such as rock gypsum, alluvial sediments weathered from rock gypsum and transported in runoff or mass movement, gypsum-rich lacustrine deposits, eolian deposits derived from weathered gypsum crystals, and gypsum-rich coastal precipitates. In addition to parent materials, the climate plays a significant role in the production of gypsiferous and gypsic soils in aridic, xeric, and ustic soil moisture regimes. As a semi-soluble mineral, gypsum is easily leached under humid soil moisture regimes; hence, gypsum-rich soils are uncommon in humid climates. The equilibrium between leaching and evaporation favours the build-up and permanence of gypsum in aridic or xeric soils.
Traditional rangeland management practices have been the typical for gypsic soils in arid environments, which is why these types of landscapes are typically associated with sparse human populations. However, gypsic soils in dry places have been successfully farmed for barley and wheat, with fallow years in between, for hundreds of years [163]. Gypsic soils have a low water-holding capacity; thus, crop yields are highly dependent on adequate and timely precipitation [164]. However, farming becomes unprofitable and leads to low-input agriculture when precipitation is unpredictable. Soils are easily eroded by runoff when a gypsic horizon occurs at the land’s surface because of their low cohesiveness in wet conditions. If irrigation water is available, agriculture can be performed more intensively in arid regions, but only if the plants can tolerate the toxicity and osmotic stress of brackish water. While Van Alphen and de los Ros [165] detailed the irrigation of gypsic soils in a variety of countries and crop types, Laya et al. [166] evaluated the suitability of gypsum-rich soils for irrigated alfalfa. However, when the gypsum-rich horizon is thick or the underlying rocks have a high gypsum content, subsidence sinkholes and collapse can occur as a result of surface irrigation. Because of the low water-holding capacity of gypsic soils, these issues are exacerbated by the frequent irrigation required in arid regions for water-demanding crops. Because of their limited cation exchange capacity and complex interactions with several plant nutrients, gypsum-rich soils can be a barrier to the use of synthetic fertilisers and other chemical inputs in agriculture [167]. Drip irrigation systems, plastic materials to slow evaporation, and other methods of water transfer can help farmers deal with these problems.
3.9. Calcareous Soils
A soil that is high in calcium carbonates or calcium and magnesium carbonates is referred to as calcareous, calcisols, or calcids. Because of low levels of leaching, calcareous soils are found naturally in arid and semi-arid locations [168]. If the parent material is young and has undergone minimal weathering (such as limestone, shells, or calcareous glacial tills), it can also be found in humid and semi-arid regions [169]. Calcareous soils form in low-rainfall areas and require irrigation to be cultivated [170]. Consequently, the accessibility of water for irrigation in arid areas is one of the primary productivity constraints. When farming on calcareous soils, it is essential that the irrigation water be of a high quality to ensure long-term viability. Almost all irrigation waters have dissolved inorganic salts. The accumulation of these salts within the soil profile can alter the soil structure, reduce water permeability, and severely harm plant growth. The crusting of the surface is also another fundamental issue. Soils such as these have a low organic matter content of less than 0.4% and are typically substantially lower than that. These soils appear to be in good physical condition before being irrigated for the first time, but once water is introduced, significant chemical changes take place. Crust development, which is influenced by factors such as texture and salt dominance, is enhanced by the dissolution of carbonates to bicarbonates, and the subsequent precipitation of the latter upon drying. Crusting of the surface might hinder infiltration and soil aeration, which can in turn prevent seedlings from germinating.
Root growth and water-movement characteristics may be hampered by cementing conditions in the subsurface layers. Soils that are predominantly high in calcium carbonate are typically deficient in available N. Micronutrients such as zinc and iron may be less accessible in high-pH environments because of the development of insoluble calcium phosphates such as apatite (lime induced chlorosis). Calcium deficiency may also cause K and magnesium deficiencies. CaCO3 alters the chemistry and availability of many macro and micro-elements. N fertilisers should be incorporated into calcareous soils to reduce the possibility of ammonium-N volatilization [168].
The availability of P and molybdenum is decreased by carbonates because of their high calcium and magnesium content. Low solubility at alkaline pH levels makes iron, boron, zinc, and manganese deficits frequent in high CaCO3 soils. The inoculation of calcareous soils with Azotobacter or Bradyrhizobium significantly boosted N uptake by plants and all of the assessed yield components for maize and cowpea compared with the uninoculated crops [171]. Fertilizer N loss in calcareous soils can be greatly reduced with the addition of zeolite or additional organic compounds such as cellulose [172]. Soybean production was improved in calcareous soils after foliar spraying of super-phosphate supernatant inoculated with Rhizobium [173]. When applied together, farmyard manure and super phosphate boosted sesame growth and yield more than either fertiliser used alone [174]. According to El-Dsouky and Attia [175], when peanuts were grown in sandy calcareous soil and then fertilised with phosphate solubilizing bacteria, organic manure, and phosphate, the inoculated plants produced the highest yields of shoots and pods as well as the highest levels of N, P, and K in their shoots. Sulfur compounds are added to soils for a number of reasons, including the provision of sulphur as a nutrient and as an acidifier for calcareous soils. These chemicals are acidifiers that can reduce the soil pH and increase its nutrient availability by neutralising CaCO3. The soil CaCO3 content determines the acidifier application rates necessary to stimulate a plant response.
3.10. Soil Biodiversity
As Vásquez-Dean et al. [176] demonstrated microbial structure appears to be highly controlled by mean annual precipitation and mean annual temperature and not by pH in arid soils, with Actinobacteria being more abundant in arid soils than Proteobacteria, Cyanobacteria, and Planctomycetes. Ren et al. [177] performed a meta-analysis and found that decreased rainfall significantly reduced the total soil microbial biomass. Percentage reductions (size) in rainfall were mostly responsible for explaining the directions and magnitudes, rather than the reductions in rainfall duration. A possible explanation is that substrate and microbial collisions are regulated by the availability of water under various rainfall reduction protocols [178][179]. In addition, the ability of microbes to access substrates, which significantly constrains the growth of soil microorganisms, may be affected by large-size rainfall reductions compared with low-size reductions [180]. To accurately estimate and project soil C dynamics, this meta-analysis stressed the significance of taking into account the magnitude of rainfall reductions and aridity levels.
Common in arid and semi-arid environments, where plant cover is typically sparse, biological soil crusts or biocrusts are complex communities of microbes found on soil surfaces [181]. Biocrusts have become an important and developing study focus in recent decades [182] due to their ecological and microbiological properties, linked with their ubiquitous occurrence in arid conditions. These biocrusts address several research subjects, such as stress survival, ecological assembly in harsh environments, and biogeochemical influence on their surrounding soils. The bacterial and fungal communities of biocrusts are specific to each crust type and distinct from those of the surrounding soil. Below-crust soils may be less subjected to abiotic stresses such as UV and moisture, but biocrusts are [183]. Strong selection of those organisms that can survive extreme arid environments with high UV indices might explain the reduced microbial diversity within the crusts in arid soils.
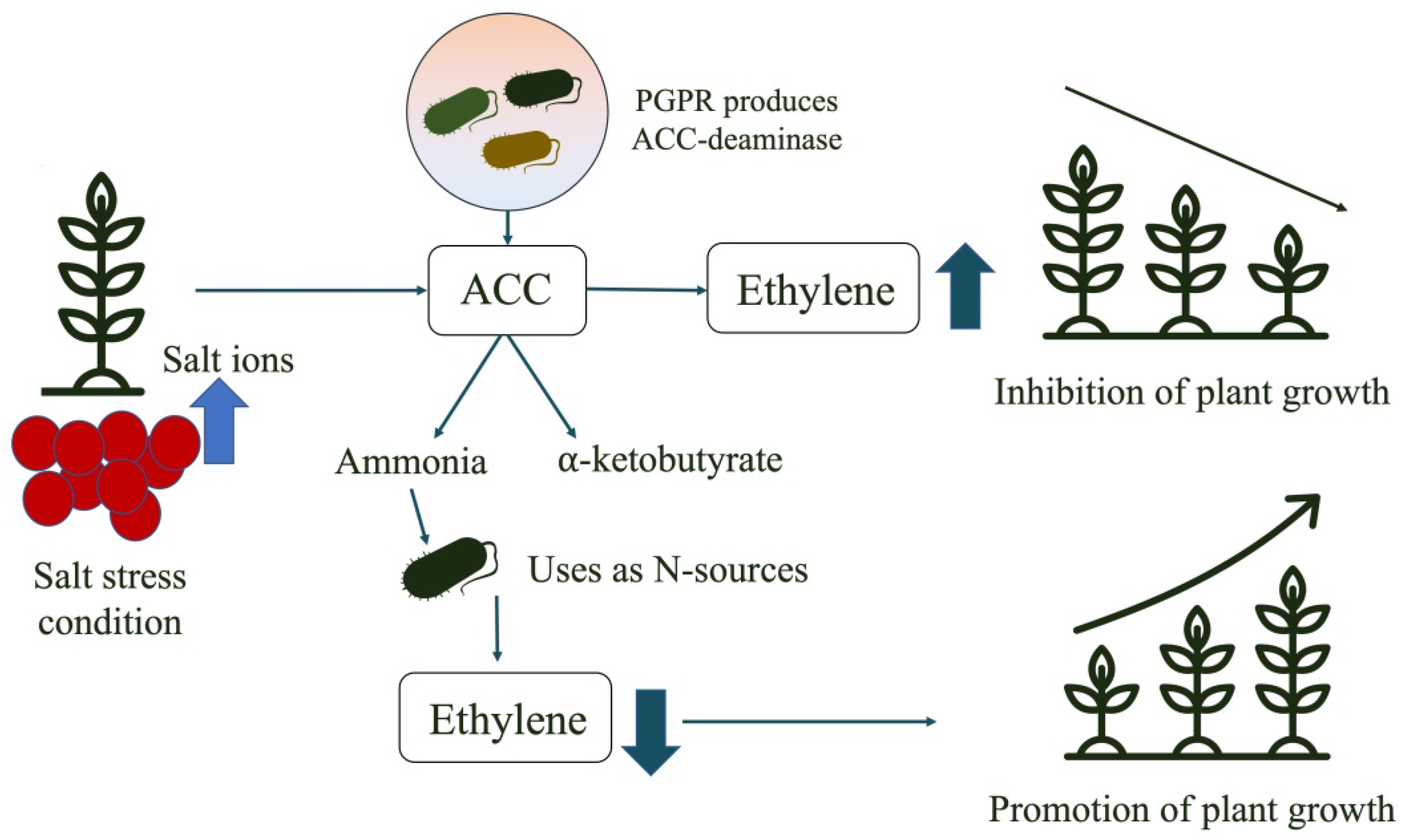
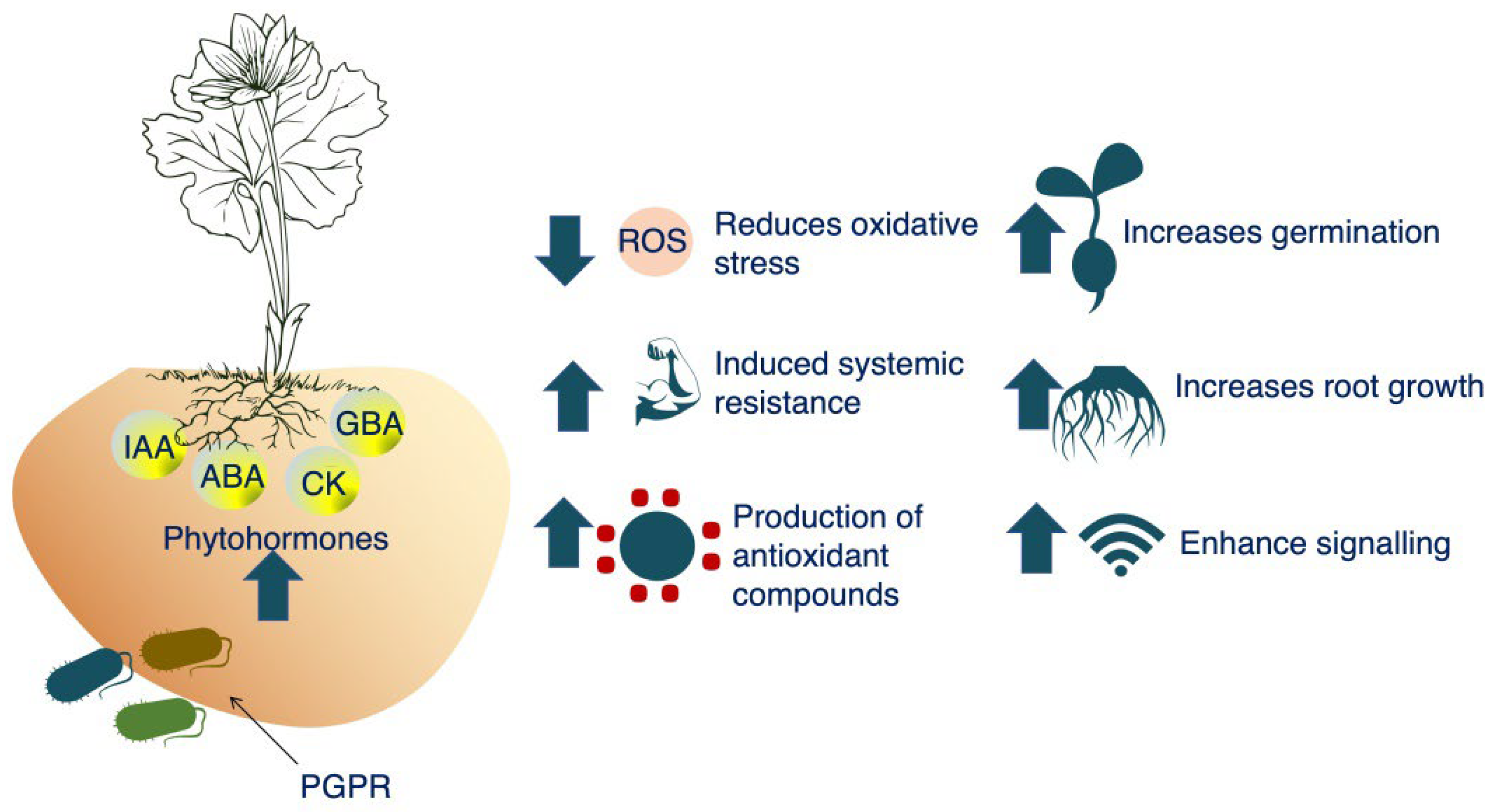
Rhizobacteria have the ability to synthesise phytohormones and regulate the endogenous concentrations of these compounds in plant tissues. The importance of phytohormones in plant development and growth is well-established [184]. Under stressful conditions, such as those found in arid soils, some plant growth promoting rhizobacteria produce ACC (1-aminocyclopropane-1-carboxylic acid (ACC) deaminase (Figure 4). By reducing the growth-inhibiting effects of ethylene, ACC deaminase makes it possible for IAA to stimulate plant growth. Therefore, plants harbouring bacteria able to synthesise ACC deaminase are more resilient under stressful conditions, and their growth is comparable to plants grown under normal conditions [185]. Most of the plant-growth-promoting bacteria (PGPB) strains produce phytohormones that help in the development of both root and shoots of the plants under stressed conditions of arid soils (Figure 5).

Figure 4. Plant growth promotion in arid soils through ACC-deaminase producing bacteria (ACC:1-aminocyclopropane-1-carboxylic acid).

Figure 5. Production of PGPB-mediated phytohormornes (IAA: indole-3-acetic acid; ABA: abscisic acid; CK: cytokininis; GBA: gibberellic acid) to boost plant health under the stress conditions of arid soils.
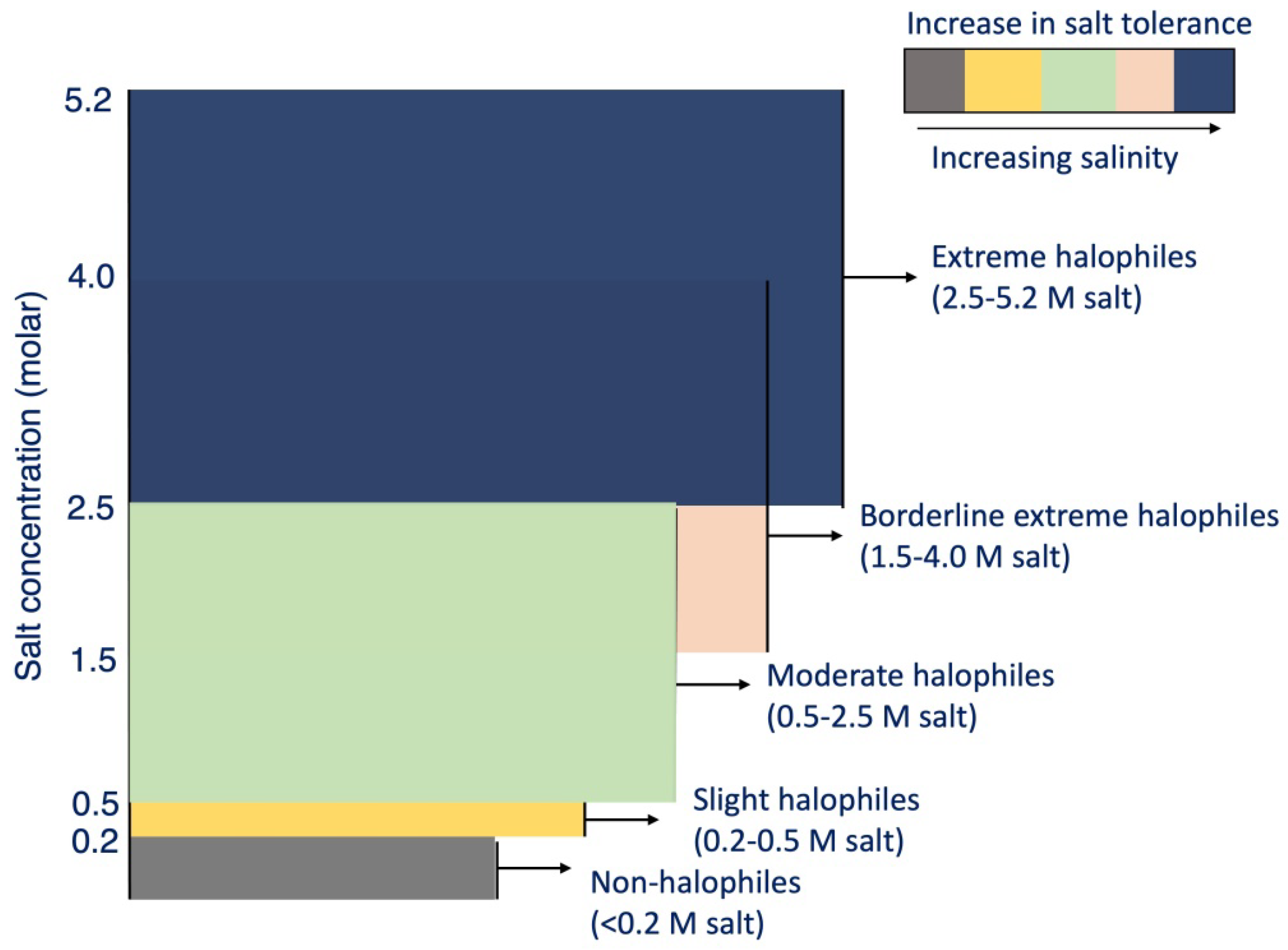
PGPB from the genera Pseudomonas, Azospirillum, and Rhizobium are known to create a close link with plant roots by producing an extracellular polysaccharide (EPS) layer that is essential in biofilm formation [186]. In addition, bacterial EPS production is increased in environments of stress, such as drought or salinity [187]. Plants are better able to withstand drought and an excess of salt in the soil after being inoculated with PGPB, which can synthesise EPS [188]. In order to keep micro and macro aggregates stable, it is essential that bacteria create EPS in the soil and all around the roots. It also helps produce a site where the soil microbial process occurs, which ultimately results in an improved flow of nutrients, water, and ions to plant roots, even in drought [189]. Increased nutrient uptake in plants is one benefit of employing PGPB. Inoculating Solanum lycopersicum with Achromobacter piechaudii and subjecting it to high salinity conditions boosts the plant’s ability to utilise water effectively while also enhancing its ability to take up P and K [190]. Wheat grown under extreme salt stress had its N, P, and K concentrations improved considerably after being inoculated with Bacillus aquimaris [191]. These findings emphasise the non-symbiotic capabilities of PGPB to solubilize insoluble P and fix atmospheric N. Similarly, PGPB can release siderophores to remove Fe, making ferri-siderophore easily accessible to plants [192]. Based on the tolerance level of soil microorganisms to salt concentrations, five types of halophiles (where their main characteristic is their salinity requirement) are known, which can be further exploited to produce bio-inoculants for promoting plant growth in arid soils (Figure 6).

Figure 6. Classes of halophiles microbiota found in saline soils.
This entry is adapted from the peer-reviewed paper 10.3390/agronomy13010220
References
- Pacheco, F.A.L.; Sanches Fernandes, L.F.; Valle Junior, R.F.; Valera, C.A.; Pissarra, T.C.T. Land Degradation: Multiple Environmental Consequences and Routes to Neutrality. Curr. Opin. Environ. Sci. Health 2018, 5, 79–86.
- Akhtar-Schuster, M.; Stringer, L.C.; Erlewein, A.; Metternicht, G.; Minelli, S.; Safriel, U.; Sommer, S. Unpacking the Concept of Land Degradation Neutrality and Addressing Its Operation through the Rio Conventions. J. Environ. Manage. 2017, 195, 4–15.
- Husein, H.H.; Mousa, M.; Sahwan, W.; Bäumler, R.; Lucke, B. Spatial Distribution of Soil Organic Matter and Soil Organic Carbon Stocks in Semi-Arid Area of Northeastern Syria. Nat. Resour. 2019, 10, 415–432.
- Trenberth, K.E.; Dai, A.; van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global Warming and Changes in Drought. Nat. Clim. Chang. 2014, 4, 17–22.
- Dai, A. Increasing Drought under Global Warming in Observations and Models. Nat. Clim. Chang. 2013, 3, 52–58.
- Yuan, Z.Y.; Chen, H.Y.H. Decoupling of Nitrogen and Phosphorus in Terrestrial Plants Associated with Global Changes. Nat. Clim. Chang. 2015, 5, 465–469.
- Meigs, P. World Distribution of Arid and Semi-Arid Homoclimates. In Reviews of Research on Arid Zone Hydrology; UNESCO, Ed.; UNESCO: Paris, France, 1953; pp. 203–210.
- FAO World Soil Resource Report: Land Resource Potential and Constraints at Regional and Country Levels. Available online: https://www.fao.org/documents/card/ru/c/15bf7a55-18ba-4c0b-95a3-fb63cb4849cb/ (accessed on 17 November 2022).
- Rossi, F. Beneficial Biofilms for Land Rehabilitation and Fertilization. FEMS Microbiol. Lett. 2020, 367, fnaa184.
- UN Decade for Deserts and the Fight against Desertification. Available online: http://www.un.org/en/events/desertification_decade/whynow.shtml (accessed on 17 November 2022).
- Köppen, W. Die Klimate Der Erde. W. de Gruyter: Berlin, Germany, 1931.
- Thornthwaite, C.W. An Approach toward a Rational Classification of Climate. Soil Sci. 1948, 66, 77.
- Shantz, H.L. History and Problems of Arid Lands Development. In The Future of Arid Lands; White, G.F., Ed.; American Association for the Advancement of Science: Washington, DC, USA, 1956; pp. 3–25.
- UN Map of the World Distribution of Arid Soil. Available online: https://catalogue.unccd.int/1060_1977_unesco_mab_technicalnotes_arid_lands_map.pdf (accessed on 17 November 2022).
- Mortimore, M.; Anderson, S.; Cotula, L.; Davies, J.; Faccer, K.; Hesse, C.; Morton, J.; Nyangena, W.; Skinner, J.; Wolfangel, C. Dryland Opportunities. A New Paradigm for People, Ecosystems and Development. Available online: https://www.cbd.int/doc/case-studies/inc/cs-inc-iucn-dryland-en.pdf (accessed on 11 October 2022).
- United Nations Environment Management Group Global Drylands: A UN System-Wide Response. Available online: https://www.unccd.int/sites/default/files/sessions/documents/ICCD_CRIC9_1/CRP1eng.pdf (accessed on 11 October 2022).
- Brooks, K.N.; Ffolliott, P.F.; Magner, J.A. Hydrology and the Management of Watersheds: Brooks/Hydrology and the Management of Watersheds, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; ISBN 9780470963050.
- Molden, D.; Oweis, T.Y.O. Pathways for Increasing Agricultural Water Productivity. In Water for Food, Water for Life; Molden, D., Ed.; Earthscan, Lsondon and International Water Management Institute: Colombo, Sri Lanka, 2007; pp. 279–310.
- Morison, J.I.L.; Baker, N.R.; Mullineaux, P.M.; Davies, W.J. Improving Water Use in Crop Production. Adv. Water Res. 2007, 34, 272–281.
- Dlamini, P.; Chivenge, P.; Manson, A.; Chaplot, V. Land Degradation Impact on Soil Organic Carbon and Nitrogen Stocks of Sub-Tropical Humid Grasslands in South Africa. Geoderma 2014, 235–236, 372–381.
- Hag Husein, H.; Lucke, B.; Bäumler, R.; Sahwan, W. A Contribution to Soil Fertility Assessment for Arid and Semi-Arid Lands. Soil Syst. 2021, 5, 42.
- Kovda, V.A. Arid land irrigation and soil fertility: Problems of salinity, alkalinity, compaction. In Arid Land Irrigation in Developing Countries; Elsevier: Amsterdam, The Netherlands, 1977; pp. 211–235. ISBN 978-0-08-021588-4.
- Girard, M.P.S. Memoire Sur l’agriculture, l’industrie et Le Commerce de l’Egypte. In Description de VEgypte, Etat Moderne, Vol. II, Part 1; Imperial Printers: Paris, France, 1812; pp. 491–714.
- Fader, M.; Shi, S.; Bloh, W.V.; Bondeau, A.; Cramer, W. Mediterranean irrigation under climate change: More efficient irrigation needed to compensate for increases in irrigation water requirements. Hydrol. Earth Syst. Sci. 2016, 20, 953–973.
- Rattan, R.K.; Datta, S.P.; Chhonkar, P.K.; Suribabu, K.; Singh, A.K. Long-Term Impact of Irrigation with Sewage Effluents on Heavy Metal Content in Soils, Crops and Groundwater—A Case Study. Agric. Ecosyst. Environ. 2005, 109, 310–322.
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Changes in Soil Quality Indicators under Long-Term Sewage Irrigation in a Sub-Tropical Environment. Environ. Geol. 2009, 56, 1237–1243.
- Qian, Y.L.; Mecham, B. Long-term Effects of Recycled Wastewater Irrigation on Soil Chemical Properties on Golf Course Fairways. Agron. J. 2005, 97, 717–721.
- Carr, G. Water Reuse for Irrigated Agriculture in Jordan: Soil Sustainability, Perceptions and Management. In Water, Life and Civilisation; Mithen, S., Black, E., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 415–428. ISBN 9780511975219.
- Yazar, A.; Sezen, S.M.; Gencel, B. Drip Irrigation of Corn in the Southeast Anatolia Project (GAP) Area in Turkey. Irrig. Drain. 2002, 51, 293–300.
- Mass, E.V.; Hoffman, G.J. Crop Salt Tolerance Current Assessment. Am. Soc. Civil Eng. Proc. J. Irrig. Drain. 1977, 103, 115–134.
- Siddique, K.H.M.; Loss, S.P.; Thomson, B.D. Cool Season Grain Legumes in Dryland Mediterranean Environments of Western Australia: Significance of Early Flowering. In Management of Agricultural Drought: Agronomic and Genetic Options; Saxena, N.P., Ed.; Science Publishers, Inc.: Enfield, CT, USA, 2003; pp. 151–162. ISBN 9781578081912.
- FAO. FAOSTAT data. In FAO Statistical Databases FAOSTAT; FAO: Rome, Italy, 2006; Available online: www.fao.org (accessed on 12 October 2022).
- Vacher, J.J. Responses of Two Main Andean Crops, Quinoa (Chenopodium quinoa Willd.) and Papa Amarga (Solanum Juzepczukii Buk.) to Drought on the Bolivian Altiplano: Significance of Local Adaptation. Agric. Ecosyst. Environ. 1998, 68, 99–108.
- Jensen, C.R.; Jacobsen, S.E.; Andersen, M.N.; Núñez, N.; Andersen, S.D.; Rasmussen, L.; Mogensen, V.O. Leaf Gas Exchange and Water Relation Characteristics of Field Quinoa (Chenopodium Quinoa Willd.) during Soil Drying. Eur. J. Agron. 2000, 13, 11–25.
- Hamilton, A.J.; Boland, A.M.; Stevens, D.; Kelly, J.; Radcliffe, J.; Ziehrl, A.; Dillon, P.; Paulin, B. Position of the Australian Horticultural Industry with Respect to the Use of Reclaimed Water. Agric. Water Manag. 2005, 71, 181–209.
- Simmons, R.; Qadir, M.; Drechsel, P. Farm-Based Measures for Reducing Human and Environmental Health Risks from Chemical Constituents in Wastewater. In Wastewater Irrigation and Health; Rechsel, P., Scott, C.A., Raschid-sally, L., Redwood, M., Bahri, A., Eds.; Routledge: London, UK, 2009; pp. 235–264. ISBN 9781849774666.
- Vivoni, E.R.; Rinehart, A.J.; Méndez-Barroso, L.A.; Aragón, C.A.; Bisht, G.; Cardenas, M.B.; Engle, E.; Forman, B.A.; Frisbee, M.D.; Gutiérrez-Jurado, H.A.; et al. Vegetation Controls on Soil Moisture Distribution in the Valles Caldera, New Mexico, during the North American Monsoon. Ecohydrology 2008, 1, 225–238.
- Zhang, X.; Zhao, W.; Liu, Y.; Fang, X.; Feng, Q. The Relationships between Grasslands and Soil Moisture on the Loess Plateau of China: A Review. Catena 2016, 145, 56–67.
- Yuan, C.; Gao, G.; Fu, B. Stemflow of a Xerophytic Shrub (Salix psammophila) in Northern China: Implication for Beneficial Branch Architecture to Produce Stemflow. J. Hydrol. (Amst.) 2016, 539, 577–588.
- Zhu, H.D.; Shi, Z.H.; Fang, N.F.; Wu, G.L.; Guo, Z.L.; Zhang, Y. Soil Moisture Response to Environmental Factors Following Precipitation Events in a Small Catchment. Catena 2014, 120, 73–80.
- Jahan, M.; Nassiri Mahallati, M. Can Superabsorbent Polymers Improve Plants Production in Arid Regions? Adv. Polym. Technol. 2020, 2020, 7124394.
- Trenkel, M.E. Improving Fertilizer Use Efficiency: Controlled-Release and Stabilized Fertilizers in Agriculture; International Fertilizer Industry Association (IFA): Paris, France, 1977; pp. 1–151. Available online: http://www.wnkgroup.com/Controlled-Release%20fertilizer%20in%20Agriculture.pdf (accessed on 17 November 2022).
- Islam, M.R.; Eneji, A.E.; Ren, C.; Li, J.; Hu, Y. Impact of Water-Saving Superabsorbent Polymer on Oat (Avena Spp.) Yield and Quality in an Arid Sandy Soil. Sci. Res. Essays 2011, 5, 720–728.
- Abedi-Koupai, J.; Sohrab, F.; Swarbrick, G. Evaluation of Hydrogel Application on Soil Water Retention Characteristics. J. Plant Nutr. 2008, 31, 317–331.
- Egrinya Eneji, A.; Islam, R.; An, P.; Amalu, U.C. Nitrate Retention and Physiological Adjustment of Maize to Soil Amendment with Superabsorbent Polymers. J. Clean. Prod. 2013, 52, 474–480.
- Setter, T.L.; Flannigan, B.A.; Melkonian, J. Loss of Kernel Set Due to Water Deficit and Shade in Maize: Carbohydrate Supplies, Abscisic Acid, and Cytokinins. Crop Sci. 2001, 41, 1530–1540.
- Prnyazpour, A.; Habib, D.; Roshan, B. What Is Super Absorbent? J. Agric. Nat. Resourc Eng. 2007, 4, 1–3.
- Islam, M.R.; Ren, C.; Zeng, Z.; Jia, P.; Eneji, E.; Hu, Y. Fertilizer Use Efficiency of Drought-Stressed Oat (Avena sativa L.) Following Soil Amendment with a Water-Saving Superabsorbent Polymer. Acta Agric. Scand. B Soil Plant Sci. 2011, 61, 721–729.
- AbdAllah, A.M.; Mashaheet, A.M.; Burkey, K.O. Super Absorbent Polymers Mitigate Drought Stress in Corn (Zea mays L.) Grown under Rainfed Conditions. Agric. Water Manag. 2021, 254, 106946.
- Khadem, S.A.; Galavi, M.; Ramrodi, M.; Mousavi, S.R.; Rousta, M.J.; Rezvani-moghadam, P. Effect of Animal Manure and Superabsorbent Polymer on Corn Leaf Relative Water Content, Cell Membrane Stability and Leaf Chlorophyll Content under Dry Condition. Aust. J. Crop Sci. 2010, 4, 642–647.
- Tao, J.; Zhang, W.; Liang, L.; Lei, Z. Effects of Eco-Friendly Carbohydrate-Based Superabsorbent Polymers on Seed Germination and Seedling Growth of Maize. R. Soc. Open Sci. 2018, 5, 171184.
- Abrisham, E.S.; Jafari, M.; Tavili, A.; Rabii, A.; Zare Chahoki, M.A.; Zare, S.; Egan, T.; Yazdanshenas, H.; Ghasemian, D.; Tahmoures, M. Effects of a Super Absorbent Polymer on Soil Properties and Plant Growth for Use in Land Reclamation. Arid Land Res. Manage. 2018, 32, 407–420.
- Banedjschafie, S.; Durner, W. Water Retention Properties of a Sandy Soil with Superabsorbent Polymers as Affected by Aging and Water Quality. J. Plant Nutr. Soil Sci. 2015, 178, 798–806.
- Keivanfar, S.; Ghazvini, R.F.; Ghasemnezhad, M.; Mousavi, A.; Khaledian, M.R. Effects of Regulated Deficit Irrigation and Superabsorbent Polymer on Fruit Yield and Quality of “Granny Smith” Apple. Agric. Conspec. Sci. 2019, 84, 383–389.
- Soubeih, K.A.A. Effect of Fertilizer Packages and Polymers on Onion Yield and Quality under Bahariya Oasis Conditions. Middle East J. Agric. Res. 2018, 7, 1769–1785.
- Ahmed, S.S.; Fahmy, A.H. Applications of Natural Polysaccharide Polymers to Overcome Water Scarcity on the Yield and Quality of Tomato Fruits. J. Soil Sci. Agric. Eng. 2019, 10, 199–208.
- Gao, H.; Yan, C.; Liu, Q.; Ding, W.; Chen, B.; Li, Z. Effects of Plastic Mulching and Plastic Residue on Agricultural Production: A Meta-Analysis. Sci. Total Environ. 2018, 651, 484–492.
- Wang, L.F.; Shangguan, Z.-P. Water-Use Efficiency of Dryland Wheat in Response to Mulching and Tillage Practices on the Loess Plateau. Sci. Rep. 2015, 5, 12225.
- Li, F.M.; Wang, J.; Xu, J.Z.; Xu, H.L. Productivity and Soil Response to Plastic Film Mulching Durations for Spring Wheat on Entisols in the Semiarid Loess Plateau of China. Soil Tillage Res. 2004, 78, 9–20.
- Zhu, J.; Ai, X.R.; Yi, Y.M.; Wang, B.Q. Effect of Plastic Film Mulching at Different Altitudinal Gradient and Different Fertility Levels on Potatoes. J. Hubei Univ. Nat. (Nat. Sci. Ed.) 2012, 30, 330–334.
- Naorem, A.; Jayaraman, S.; Dalal, R.C.; Patra, A.; Rao, C.S.; Lal, R. Soil Inorganic Carbon as a Potential Sink in Carbon Storage in Dryland Soils—A Review. Agriculture 2022, 12, 1256.
- Lal, R. Carbon Sequestration in Dryland Ecosystems. Environ. Manag. 2004, 33, 528–544.
- Bronick, C.J.; Lal, R. Soil Structure and Management: A Review. Geoderma 2005, 124, 3–22.
- Boix-Fayos, C.; Calvo-Cases, A.; Imeson, A.C.; Soriano-Soto, M.D. Influence of Soil Properties on the Aggregation of Some Mediterranean Soils and the Use of Aggregate Size and Stability as Land Degradation Indicators. Catena 2001, 44, 47–67.
- Álvaro-Fuentes, J.; Arrúe, J.L.; Gracia, R.; López, M.V. Tillage and Cropping Intensification Effects on Soil Aggregation: Temporal Dynamics and Controlling Factors under Semiarid Conditions. Geoderma 2008, 145, 390–396.
- López, M.V.; Moret, D.; Gracia, R.; Arrúe, J.L. Tillage Effects on Barley Residue Cover during Fallow in Semiarid Aragon. Soil Tillage Res. 2003, 72, 53–64.
- Hoffmann, C.; Funk, R.; Li, Y.; Sommer, M. Effect of Grazing on Wind Driven Carbon and Nitrogen Ratios in the Grasslands of Inner Mongolia. Catena 2008, 75, 182–190.
- Lal, R. Enhancing Crop Yields in the Developing Countries through Restoration of the Soil Organic Carbon Pool in Agricultural Lands. Land Degrad. Dev. 2006, 17, 197–209.
- Bationo, A.; Wani, S.P.; Bielders, C.L.; Vlek, P.L.G.; Mokwunye, A.U. Crop Residue and Fertilizer Management to Improve Soil Organiccarbon Content, Soil Quality and Productivity in the Desert Marginsof West Africa. In Global Climate Change and Tropical Ecosystems. Advances in Soil Science; Ba, L.R.K.J., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 117–145. ISBN 9781566704854.
- West, T.O.; Post, W.M. Soil Organic Carbon Sequestration Rates by Tillage and Crop Rotation: A Global Data Analysis. Soil Sci. Soc. Am. J. 2002, 66, 1930–1946.
- Hoyle, F.C.; D’Antuono, M.; Overheu, T.; Murphy, D.V. Capacity for Increasing Soil Organic Carbon Stocks in Dryland Agricultural Systems. Soil Res. 2013, 51, 657.
- Wortmann, C.S.; Bilgo, A.; Kaizzi, C.K.; Liben, F.; Garba, M.; Maman, N.; Serme, I.; Stewart, Z.P. Perennial Grass Ley Rotations with Annual Crops in Tropical Africa: A Review. Agron. J. 2021, 113, 4510–4526.
- Davis, A.S.; Hill, J.D.; Chase, C.A.; Johanns, A.M.; Liebman, M. Increasing Cropping System Diversity Balances Productivity, Profitability and Environmental Health. PLoS ONE 2012, 7, e47149.
- Díaz-Zorita, M.; Duarte, G.A.; Grove, J.H. A Review of No-till Systems and Soil Management for Sustainable Crop Production in the Subhumid and Semiarid Pampas of Argentina. Soil Tillage Res. 2002, 65, 1–18.
- DuPont, S.T.; Beniston, J.; Glover, J.D.; Hodson, A.; Culman, S.W.; Lal, R.; Ferris, H. Root Traits and Soil Properties in Harvested Perennial Grassland, Annual Wheat, and Never-Tilled Annual Wheat. Plant Soil 2014, 381, 405–420.
- Monti, A.; Zatta, A. Root Distribution and Soil Moisture Retrieval in Perennial and Annual Energy Crops in Northern Italy. Agric. Ecosyst. Environ. 2009, 132, 252–259.
- Ernst, O.R.; Kemanian, A.R.; Mazzilli, S.R.; Cadenazzi, M.; Dogliotti, S. Depressed Attainable Wheat Yields under Continuous Annual No-till Agriculture Suggest Declining Soil Productivity. Field Crops Res. 2016, 186, 107–116.
- Plaza, C.; Zaccone, C.; Sawicka, K.; Méndez, A.M.; Tarquis, A.; Gascó, G.; Heuvelink, G.B.M.; Schuur, E.A.G.; Maestre, F.T. Soil Resources and Element Stocks in Drylands to Face Global Issues. Sci. Rep. 2018, 8, 13788.
- Cuevas, J.; Daliakopoulos, I.N.; del Moral, F.; Hueso, J.J.; Tsanis, I.K. A Review of Soil-Improving Cropping Systems for Soil Salinization. Agronomy 2019, 9, 295.
- Wichelns, D.; Qadir, M. Achieving Sustainable Irrigation Requires Effective Management of Salts, Soil Salinity, and Shallow Groundwater. Agric. Water Manag. 2015, 157, 31–38.
- Bridges, E.M.; Oldeman, L.R. Global Assessment of Human-Induced Soil Degradation. Arid Soil Res. Rehabil. 1999, 13, 319–325.
- Shabala, S.; Munns, R. Salinity Stress: Physiological Constraints and Adaptive Mechanisms. In Plant Stress Physiology; CABI: Wallingford, UK, 2017; pp. 24–63. ISBN 9781780647296.
- Kitamura, Y.; Yano, T.; Honna, T.; Yamamoto, S.; Inosako, K. Causes of Farmland Salinization and Remedial Measures in the Aral Sea Basin—Research on Water Management to Prevent Secondary Salinization in Rice-Based Cropping System in Arid Land. Agric. Water Manag. 2006, 85, 1–14.
- Qadir, M.; Schubert, S.; Noble, A.D.; Saqib, M.; Saifullah, S. Amelioration Strategies for Salinity-Induced Land Degradation. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2006, 1, 12.
- Matthews, N.; Grové, B.; Barnard, J.H.; Van Rensburg, L.D. Modelling the Economic Tradeoffs between Allocating Water for Crop Production or Leaching for Salinity Management. Water SA 2010, 36, 37–43.
- Konukcu, F.; Gowing, J.W.; Rose, D.A. Dry Drainage: A Sustainable Solution to Waterlogging and Salinity Problems in Irrigation Areas? Agric. Water Manag. 2006, 83, 1–12.
- Bhutta, M.N.; Chaudhary, M.R. Biological Control of Waterlogging. In Proceedings of the Eighth ICID International Drainage Workshop, New Delhi, India, 31 January–4 February 2000; pp. 33–45.
- Connor, D.J. Designing Cropping Systems for Efficient Use of Limited Water in Southern Australia. Eur. J. Agron. 2004, 21, 419–431.
- Alcon, F.; Tapsuwan, S.; Brouwer, R.; de Miguel, M.D. Adoption of Irrigation Water Policies to Guarantee Water Supply: A Choice Experiment. Environ. Sci. Policy 2014, 44, 226–236.
- Qiao, H.; Liu, X.; Li, W.; Huang, W.; Li, C.; Li, Z. Effect of Deep Straw Mulching on Soil Water and Salt Movement and Wheat Growth. Chin. J. Soil Sci. 2006, 37, 885–889.
- Pang, H.C.; Li, Y.Y.; Yang, J.S.; Liang, Y.S. Effect of Brackish Water Irrigation and Straw Mulching on Soil Salinity and Crop Yields under Monsoonal Climatic Conditions. Agric. Water Manag. 2010, 97, 1971–1977.
- Khan, M.A.; Ansari, R.; Ali, H.; Gul, B.; Nielsen, B.L. Panicum Turgidum, a Potentially Sustainable Cattle Feed Alternative to Maize for Saline Areas. Agric. Ecosyst. Environ. 2009, 129, 542–546.
- Vinod, K.K.; Krishnan, S.G.; Babu, N.N.; Nagarajan, M.; Singh, A.K. Improving Salt Tolerance in Rice: Looking beyond the Conventional. In Salt Stress in Plants; Springer New York: New York, NY, USA, 2013; pp. 219–260. ISBN 9781461461074.
- Daliakopoulos, I.N.; Apostolakis, A.; Wagner, K.; Deligianni, A.; Koutskoudis, D.; Stamatakis, A.; Tsanis, I.K. Effectiveness of Trichoderma Harzianum in Soil and Yield Conservation of Tomato Crops under Saline Irrigation. Catena 2019, 175, 144–153.
- Bertola, M.; Ferrarini, A.; Visioli, G. Improvement of Soil Microbial Diversity through Sustainable Agricultural Practices and Its Evaluation by -Omics Approaches: A Perspective for the Environment, Food Quality and Human Safety. Microorganisms 2021, 9, 1400.
- Kakeh, J.; Gorji, M.; Mohammadi, M.H.; Asadi, H.; Khormali, F.; Sohrabi, M.; Cerdà, A. Biological Soil Crusts Determine Soil Properties and Salt Dynamics under Arid Climatic Condition in Qara Qir, Iran. Sci. Total Environ. 2020, 732, 139168.
- Kirsch, F.; Klähn, S.; Hagemann, M. Salt-Regulated Accumulation of the Compatible Solutes Sucrose and Glucosylglycerol in Cyanobacteria and Its Biotechnological Potential. Front. Microbiol. 2019, 10, 2139.
- Page, K.L.; Dang, Y.P.; Dalal, R.C.; Kopittke, P.M.; Menzies, N.W. The Impact, Identification and Management of Dispersive Soils in Rainfed Cropping Systems. Eur. J. Soil Sci. 2021, 72, 1655–1674.
- Yunusa, I.A.M.; Newton, P.J. Plants for Amelioration of Subsoil Constraints and Hydrological Control: The Primer-Plant Concept. Plant Soil 2003, 257, 261–281.
- Srivastava, P.K.; Gupta, M.; Shikha; Singh, N.; Tewari, S.K. Amelioration of Sodic Soil for Wheat Cultivation Using Bioaugmented Organic Soil Amendment: Novel Organic Soil Amendment for Sodic Soil. Land Degrad. Dev. 2016, 27, 1245–1254.
- Copeman, R.H.; Martin, C.A.; Stutz, J.C. Tomato Growth in Response to Salinity and Mycorrhizal Fungi from Saline or Nonsaline Soils. HortScience 1996, 31, 341–344.
- Singh, Y.P.; Nayak, A.K.; Sharma, D.K.; Singh, G.; Mishra, V.K.; Singh, D. Evaluation of Jatropha Curcas Genotypes for Rehabilitation of Degraded Sodic Lands: Rehabilitation of degraded sodic lands through Jatropha curcas. Land Degrad. Dev. 2015, 26, 510–520.
- Iannetta, M.; Colonna, N. Salinisation in the Mediterranean Context, Booklet in the Framework of the VI Framework Programma Priorità 1. In The Framework of the Vi Framework Programma Priorità, 1.1.6.3 Global Change and Ecosystems; EU SSA Lucinda Project, Ed.; European Commission Directorate-General for Research: Brussels, Belgium, 2009; p. 20.
- Ali, H. Practices of Irrigation & On-Farm Water Management: Volume 2; Springer: New York, NY, USA, 2014; ISBN 9781489981653.
- Qadir, M.; Ghafoor, A.; Murtaza, G. Amelioration Strategies for Saline Soils: A Review. Land Degrad. Dev. 2000, 11, 501–521.
- Rawat, L.; Singh, Y.; Shukla, N.; Kumar, J. Alleviation of the Adverse Effects of Salinity Stress in Wheat (Triticum aestivum L.) by Seed Biopriming with Salinity Tolerant Isolates of Trichoderma Harzianum. Plant Soil 2011, 347, 387–400.
- Mao, W.; Kang, S.; Wan, Y.; Sun, Y.; Li, X.; Wang, Y. Yellow River Sediment as a Soil Amendment for Amelioration of Saline Land in the Yellow River Delta: Yellow River Sediment as a Soil Amendment. Land Degrad. Dev. 2016, 27, 1595–1602.
- Flores, P.; Botella, M.Á.; Cerdá, A.; Martínez, V. Influence of Nitrate Level on Nitrate Assimilation in Tomato (Lycopersicon esculentum) Plants under Saline Stress. Can. J. Bot. 2004, 82, 207–213.
- Chatzigiannakis, E.; Ilias, A.; Panoras, A. Guide to Address the Loss of Organic Matter, Salinisation, Acidification and the Erosion of Agricultural Soils. In The Scope of Project LIFE So. S; Thessaloniki, Greece, 2012.
- Jiao, F.; Shi, X.R.; Han, F.P.; Yuan, Z.Y. Increasing Aridity, Temperature and Soil pH Induce Soil C-N-P Imbalance in Grasslands. Sci. Rep. 2016, 6, 19601.
- Reynolds, J.F.; Smith, D.M.S.; Lambin, E.F.; Turner, B.L., 2nd; Mortimore, M.; Batterbury, S.P.J.; Downing, T.E.; Dowlatabadi, H.; Fernández, R.J.; Herrick, J.E.; et al. Global Desertification: Building a Science for Dryland Development. Science 2007, 316, 847–851.
- Field, C.B.; Mooney, H.A. The Photosynthesis-Nitrogen Relationship in Wild Plants. In On the Economy of Plant Form and Function; Givnish, T.J., Ed.; Cambridge University Press: Cambridge, UK, 1986; pp. 25–55.
- Fageria, N.K.; Gheyi, H.R.; Moreira, A. Nutrient Bioavailability in Salt Affected Soils. J. Plant Nutr. 2011, 34, 945–962.
- Feng, K.; Lu, H.M.; Sheng, H.J.; Wang, X.L.; Mao, J. Effect of Organic Ligands on Biological Availability of Inorganic Phosphorus in Soils. Pedosphere 2004, 14, 85–92.
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971.
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and Characterization of a Phosphate-Solubilizing Halophilic Bacterium Kushneria Sp. YCWA18 from Daqiao Saltern on the Coast of Yellow Sea of China. Evid. Based. Complement. Alternat. Med. 2011, 2011, 615032.
- Moreno-Jiménez, E.; Plaza, C.; Saiz, H.; Manzano, R.; Flagmeier, M.; Maestre, F.T. Aridity and Reduced Soil Micronutrient Availability in Global Drylands. Nat. Sustain. 2019, 2, 371–377.
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral–Organic Associations: Formation, Properties, and Relevance in Soil Environments. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–140. ISBN 9780128021378.
- Kämpf, N.; Schwertmann, U. Goethite and Hematite in a Climosequence in Southern Brazil and Their Application in Classification of Kaolinitic Soils. Geoderma 1983, 29, 27–39.
- Voegelin, A.; Pfister, S.; Scheinost, A.C.; Marcus, M.A.; Kretzschmar, R. Changes in Zinc Speciation in Field Soil after Contamination with Zinc Oxide. Environ. Sci. Technol. 2005, 39, 6616–6623.
- Hassink, J. The Capacity of Soils to Preserve Organic C and N by Their Association with Clay and Silt Particles. Plant Soil 1997, 191, 77–87.
- Churchman, G.J. Game Changer in Soil Science. Functional Role of Clay Minerals in Soil. J. Plant Nutr. Soil Sci. 2018, 181, 99–103.
- Gabarrón-Galeote, M.A.; Trigalet, S.; van Wesemael, B. Effect of Land Abandonment on Soil Organic Carbon Fractions along a Mediterranean Precipitation Gradient. Geoderma 2015, 249–250, 69–78.
- Tiessen, H.; Stewart, J.W.B. Particle-Size Fractions and Their Use in Studies of Soil Organic Matter: II. Cultivation Effects on Organic Matter Composition in Size Fractions. Soil Sci. Soc. Am. J. 1983, 47, 509–514.
- Yost, J.L.; Hartemink, A.E. Effects of Carbon on Moisture Storage in Soils of the Wisconsin Central Sands, USA. Eur. J. Soil Sci. 2019, 70, 565–577.
- van Wambeke, A. Soils of the Tropics: Properties and Appraisals; McGraw-Hill Professional: New York, NY, USA, 1991; ISBN 9780070679467.
- Osman, K.T. Sandy Soils. In Sandy Soils; Osman, K.T., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 37–65.
- Jonczak, J. Vertical Distribution of Cu, Ni and Zn in Brunic Arenosols and Gleyic Podzols of the Supra-Flood Terrace of the Słupia River as Affected by Litho-Pedogenic Factors. For. Res. Pap. 2015, 75, 333–341.
- Kraft, G.J.; Clancy, K.; Mechenich, D.J.; Haucke, J. Irrigation Effects in the Northern Lake States: Wisconsin Central Sands Revisited. Ground Water 2012, 50, 308–318.
- Scott, D.F. Soil Wettability in Forested Catchments in South Africa; as Measured by Different Methods and as Affected by Vegetation Cover and Soil Characteristics. J. Hydrol. (Amst.) 2000, 231–232, 87–104.
- Fan, J.; Ding, W.; Xiang, J.; Qin, S.; Zhang, J.; Ziadi, N. Carbon Sequestration in an Intensively Cultivated Sandy Loam Soil in the North China Plain as Affected by Compost and Inorganic Fertilizer Application. Geoderma 2014, 230–231, 22–28.
- Kundu, S.; Bhattacharyya, R.; Prakash, V.; Ghosh, B.; Gupta, H. Carbon Sequestration and Relationship between Carbon Addition and Storage under Rainfed Soybean–Wheat Rotation in a Sandy Loam Soil of the Indian Himalayas. Soil Tillage Res. 2007, 92, 87–95.
- Mugnai, G.; Rossi, F.; Chamizo, S.; Adessi, A.; De Philippis, R. The Role of Grain Size and Inoculum Amount on Biocrust Formation by Leptolyngbya Ohadii. Catena 2020, 184, 104248.
- Román, J.R.; Chamizo, S.; Roncero-Ramos, B.; Adessi, A.; De Philippis, R.; Cantón, Y. Overcoming Field Barriers to Restore Dryland Soils by Cyanobacteria Inoculation. Soil Tillage Res. 2021, 207, 104799.
- Bu, C.; Wu, S.; Yang, Y.; Zheng, M. Identification of Factors Influencing the Restoration of Cyanobacteria-Dominated Biological Soil Crusts. PLoS ONE 2014, 9, e90049.
- Mugnai, G.; Rossi, F.; Martin Noah Linus Felde, V.J.; Colesie, C.; Büdel, B.; Peth, S.; Kaplan, A.; De Philippis, R. The Potential of the Cyanobacterium Leptolyngbya Ohadii as Inoculum for Stabilizing Bare Sandy Substrates. Soil Biol. Biochem. 2018, 127, 318–328.
- Peng, X.; Bruns, M.A. Development of a Nitrogen-Fixing Cyanobacterial Consortium for Surface Stabilization of Agricultural Soils. J. Appl. Phycol. 2019, 31, 1047–1056.
- Hall, A.J.; Richards, R.A. Prognosis for Genetic Improvement of Yield Potential and Water-Limited Yield of Major Grain Crops. Field Crops Res. 2013, 143, 18–33.
- Miner, G.L.; Hansen, N.C.; Inman, D.; Sherrod, L.A.; Peterson, G.A. Constraints of No-till Dryland Agroecosystems as Bioenergy Production Systems. Agron. J. 2013, 105, 364–376.
- Chi, Y.; Li, Z.; Zhang, G.; Zhao, L.; Gao, Y.; Wang, D.; Liu, L.; Cai, D.; Wu, Z. Inhibiting Desertification Using Aquatic Cyanobacteria Assisted by a Nanocomposite. ACS Sustain. Chem. Eng. 2020, 8, 3477–3486.
- Zhao, Y.; Wang, J. Mechanical Sand Fixing Is More Beneficial than Chemical Sand Fixing for Artificial Cyanobacteria Crust Colonization and Development in a Sand Desert. Appl. Soil Ecol. 2019, 140, 115–120.
- Kheirfam, H.; Asadzadeh, F. Stabilizing Sand from Dried-up Lakebeds against Wind Erosion by Accelerating Biological Soil Crust Development. Eur. J. Soil Biol. 2020, 98, 103189.
- Delgado, J.A.; Nearing, M.A.; Rice, C.W. Conservation Practices for Climate Change Adaptation. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2013; pp. 47–115. ISBN 9780124076853.
- Wang, X.B.; Cai, D.X.; Hoogmoed, W.B.; Oenema, O.; Perdok, U.D. Developments in Conservation Tillage in Rainfed Regions of North China. Soil Tillage Res. 2007, 93, 239–250.
- Spokas, L.; Steponavičius, D. Fuel Consumption during Cereal and Rape Harvesting and Methods of Its Reduction. J. Food Agric. Environ. 2011, 9, 257–263.
- Plaza-Bonilla, D.; Arrúe, J.L.; Cantero-Martínez, C.; Fanlo, R.; Iglesias, A.; Álvaro-Fuentes, J. Carbon Management in Dryland Agricultural Systems. A Review. Agron. Sustain. Dev. 2015, 35, 1319–1334.
- Lahmar, R. Adoption of Conservation Agriculture in Europe. Land Use Policy 2010, 27, 4–10.
- Sharma, A.K. Arid Zone Agroforestry: Dimensions and Directions for Sustainable Livelihoods. Available online: http://www.IUFRO-archive.boku.ac.at/iufro/taskforce/tfscipol/chennai-papers/faksharma.pdf (accessed on 12 December 2022).
- Sepehr, A.; Hassanzadeh, M.; Rodriguez-Caballero, E. The Protective Role of Cyanobacteria on Soil Stability in Two Aridisols in Northeastern Iran. Geoderma Reg. 2019, 16, e00201.
- Fischer, T.; Yair, A.; Veste, M.; Geppert, H. Hydraulic Properties of Biological Soil Crusts on Sand Dunes Studied by 13C-CP/MAS-NMR: A Comparison between an Arid and a Temperate Site. Catena 2013, 110, 155–160.
- Drahorad, S.L.; Jehn, F.U.; Ellerbrock, R.H.; Siemens, J.; Felix-Henningsen, P. Soil Organic Matter Content and Its Aliphatic Character Define the Hydrophobicity of Biocrusts in Different Successional Stages. Ecohydrology 2020, 13, e2232.
- Lázaro, R.; Mora, J.L. Sediment Content and Chemical Properties of Water Runoff on Biocrusts in Drylands. Biologia 2014, 69, 1539–1554.
- Mullins, C.E. Hardsetting. In Methods for Assesment of Soil Degradation. Advances in Soil Science, 19; Lal, R., Blum, W.H., Valentine, C., Stewart, B.A., Eds.; CRC Press: New York, NY, USA, 1997; pp. 109–128.
- Lima, H.V.; Silva, A.P.; Santos, M.C.; Cooper, M.; Romero, R.E. Micromorphology and Image Analysis of a Hardsetting Ultisol (Argissolo) in the State of Ceará (Brazil). Geoderma 2006, 132, 416–426.
- Hamza, M.A.; Anderson, W.K. Soil Compaction in Cropping Systems. Soil Tillage Res. 2005, 82, 121–145.
- Raper, R.L.; Schwab, E.B.; Balkcom, K.S.; Burmester, C.H.; Reeves, D.W. Effect of Annual, Biennial, and Triennial in-Row Subsoiling on Soil Compaction and Cotton Yield in Southeastern u.S. Silt Loam Soils. Appl. Eng. Agric. 2005, 21, 337–343.
- Carter, M.R. Soil Quality for Sustainable Land Management: Organic Matter and Aggregation Interactions That Maintain Soil Functions. Agron. J. 2002, 94, 38–47.
- Soane, B.D. The Role of Organic Matter in Soil Compactibility: A Review of Some Practical Aspects. Soil Tillage Res. 1990, 16, 179–201.
- Williams, S.M.; Weil, R.R. Crop Cover Root Channels May Alleviate Soil Compaction Effects on Soybean Crop. Soil Sci. Soc. Am. J. 2010, 68, 1403.
- Casby-Horton, S.; Herrero, J.; Rolong, N.A. Gypsum Soils—Their Morphology, Classification, Function, and Landscapes. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2015; pp. 231–290. ISBN 9780128021378.
- Eswaran, H.; Zi-Tong, G. Properties, Genesis, Classification, and Distribution of Soils with Gypsum. In SSSA Special Publications; Soil Science Society of America: Madison, WI, USA, 2015; pp. 89–119. ISBN 9780891189213.
- Herrero, J.; Porta, J.; Fédoroff, N. Hypergypsic Soil Micromorphology and Landscape Relationships in Northeastern Spain. Soil Sci. Soc. Am. J. 1992, 56, 1188–1194.
- Boixadera, J.; Poch, R.M.; Herrero, C. Soilscapes of Catalonia and Aragon (NE Spain): Tour Guide of the Annual Excursion of the Belgian Soil Science Society 1999; DARP-UdL-DGA: Lleida, Spain, 2000.
- Castañeda, C.; Moret-Fernández, D. Superficial Color Patches as a Visual Diagnostic Criterion for Agricultural Management. Pedosphere 2013, 23, 740–751.
- Van Alphen, J.G.; de Los Rios Romero, F. Gypsiferous Soils—Notes on Their Characteristics and Management; International Institute for Land Reclamation and Improvement: Wageningen, The Netherlands, 1971.
- Laya, D.; Van Ranst, E.; Herrero, J. A Modified Parametric Index to Estimate Yield Potentials for Irrigated Alfalfa on Soils with Gypsum in Quinto (Aragón, Spain). Geoderma 1998, 87, 111–122.
- Food and Agriculture Organization of the United Nations. Management of Gypsiferous Soils; Food & Agriculture Organization of the United Nations (FAO): Rome, Italy, 1990; ISBN 9789251029480.
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Pearson: Upper Saddle River, NJ, USA, 2007; ISBN 9780132279383.
- Monier, M.W.; Fawkia, L.; Alaa, Z. Management of Calcareous Soils in Arid Region. Int. J. Environ. Pollut. Env. Model. 2019, 2, 248–258.
- Pearce, R.C.; Li, Y.; Bush, L.P. Calcium and Bicarbonate Effects on the Growth and Nutrient Uptake of Burley Tobacco Seedlings: Float System1. J. Plant Nutr. 1999, 22, 1079–1090.
- Ramadan, H.; Koreish, E.; Gaber, H.; El–Fayoumy, M. Assessment and Comparison of Bio and Mineral Fertilization on Farm Profitability in Different Newly Reclaimed Soils. Alex. J. Agric. Res. 2002, 47, 133–146.
- He, Z.L.; Calvert, D.V.; Alva, A.K.; Li, Y.C.; Banks, D.J. Clinoptilolite Zeolite and Cellulose Amendments to Reduce Ammonia Volatilization in a Calcareous Sandy Soil. Plant Soil 2002, 247, 253–260.
- Badran, M. Effect of Nitrogenous and Phosphatic Fertilization on Some Economical Characters of Soybean Crawford Cultivar under Calcareous Soil Conditions. Egypt. J. Agric. Res. 2003, 81, 433–440.
- Attia, K. Effect of Farmyard Manure and Phosphorus Fertilization on Growth, Yield and N, P and Ca Content of Sesame Grown on a Sandy Calcareous Soil. Assiut. J. Agric. Sci 2001, 32, 141–151.
- El–Dsouky, M.; Attia, K. Effect of Inoculation with Phosphate Solubilizing Bacteria, Organic Manure and Phosphate Fertilization on Peanuts Grown on Sandy Calcareous Soil. Assiut. J. Agric Sci. 1999, 30, 177–188.
- Vásquez-Dean, J.; Maza, F.; Morel, I.; Pulgar, R.; González, M. Microbial Communities from Arid Environments on a Global Scale. A Systematic Review. Biol. Res. 2020, 53, 29.
- Ren, C.; Chen, J.; Lu, X.; Doughty, R.; Zhao, F.; Zhong, Z.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Responses of Soil Total Microbial Biomass and Community Compositions to Rainfall Reductions. Soil Biol. Biochem. 2018, 116, 4–10.
- Beier, C.; Beierkuhnlein, C.; Wohlgemuth, T.; Penuelas, J.; Emmett, B.; Körner, C.; de Boeck, H.; Christensen, J.H.; Leuzinger, S.; Janssens, I.A.; et al. Precipitation Manipulation Experiments--Challenges and Recommendations for the Future. Ecol. Lett. 2012, 15, 899–911.
- Manzoni, S.; Schaeffer, S.M.; Katul, G.; Porporato, A.; Schimel, J.P. A Theoretical Analysis of Microbial Eco-Physiological and Diffusion Limitations to Carbon Cycling in Drying Soils. Soil Biol. Biochem. 2014, 73, 69–83.
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial Stress-Response Physiology and Its Implications for Ecosystem Function. Ecology 2007, 88, 1386–1394.
- Rodriguez-Caballero, E.; Belnap, J.; Büdel, B.; Crutzen, P.J.; Andreae, M.O.; Pöschl, U.; Weber, B. Dryland Photoautotrophic Soil Surface Communities Endangered by Global Change. Nat. Geosci. 2018, 11, 185–189.
- Bowker, M.A.; Belnap, J.; Davidson, D.W.; Goldstein, H. Correlates of Biological Soil Crust Abundance across a Continuum of Spatial Scales: Support for a Hierarchical Conceptual Model: Scale-Dependent Soil Crust Distribution. J. Appl. Ecol. 2006, 43, 152–163.
- Mueller, R.C.; Belnap, J.; Kuske, C.R. Soil Bacterial and Fungal Community Responses to Nitrogen Addition across Soil Depth and Microhabitat in an Arid Shrubland. Front. Microbiol. 2015, 6, 891.
- Weijers, D.; Nemhauser, J.; Yang, Z. Auxin: Small Molecule, Big Impact. J. Exp. Bot. 2018, 69, 133–136.
- Gamalero, E.; Bona, E.; Todeschini, V.; Lingua, G. Saline and Arid Soils: Impact on Bacteria, Plants, and Their Interaction. Biology 2020, 9, 116.
- Dragoš, A.; Kovács, Á.T. The Peculiar Functions of the Bacterial Extracellular Matrix. Trends Microbiol. 2017, 25, 257–266.
- Roberson, E.B.; Firestone, M.K. Relationship between Desiccation and Exopolysaccharide Production in a Soil Pseudomonas Sp. Appl. Environ. Microbiol. 1992, 58, 1284–1291.
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides Producing Rhizobacteria and Their Role in Plant Growth and Drought Tolerance. J. Basic Microbiol. 2018, 58, 1009–1022.
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636.
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant Growth-Promoting Bacteria Confer Resistance in Tomato Plants to Salt Stress. Plant Physiol. Biochem. 2004, 42, 565–572.
- Upadhyay, S.K.; Singh, D.P. Effect of Salt-Tolerant Plant Growth-Promoting Rhizobacteria on Wheat Plants and Soil Health in a Saline Environment. Plant Biol. (Stuttg.) 2015, 17, 288–293.
- Ruben, J.A.; Bennett, A.F. Antiquity of the Vertebrate Pattern of Activity Metabolism and Its Possible Relation to Vertebrate Origins. Nature 1980, 286, 886–888.
This entry is offline, you can click here to edit this entry!
