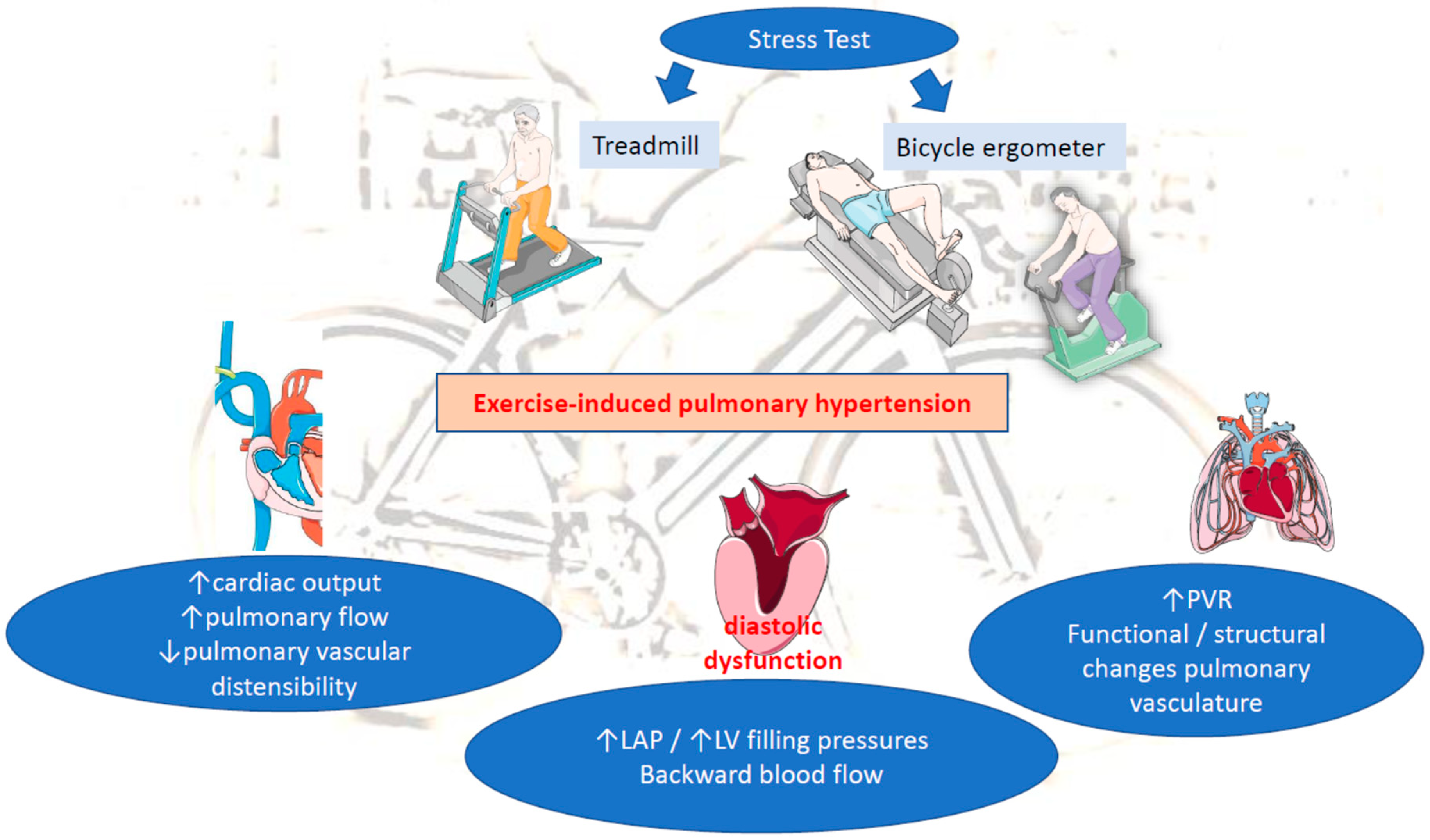
Exercise-induced pulmonary hypertension (EIPH) has been defined as an increase in mean pulmonary arterial pressure (mPAP) during exercise in otherwise normal values at rest. EIPH reflects heart and/or lung dysfunction and may precede the development of manifest pulmonary hypertension (PH) in a proportion of patients. It is also associated with decreased life expectancy in patients with heart failure with reduced ejection fraction (HFrEF) or left ventricle (LV) valvular diseases. Diastolic dysfunction exacerbated during exercise relates to increased LV filling pressure and left atrial pressure (LAP). In this context backward, transmitted pressure alone or accompanied with backward blood flow promotes EIPH.
- exercise-induced pulmonary hypertension (EIPH)
- pathophysiology
- right heart catheterization (RHC)
1. Introduction
2. Pathophysiology of EIPH
This entry is adapted from the peer-reviewed paper 10.3390/life13010128
References
- Kovacs, G.; Herve, P.; Barbera, J.A.; Chaouat, A.; Chemla, D.; Condliffe, R.; Garcia, G.; Grünig, E.; Howard, L.; Humbert, M.; et al. An official European Respiratory Society statement: Pulmonary haemodynamics during exercise. Eur. Respir. J. 2017, 50, 1700578.
- Kusunose, K.; Yamada, H.; Nishio, S.; Hirata, Y.; Saijo, Y.; Torii, Y.; Yamada, N.; Ise, T.; Yamaguchi, K.; Fukuda, D.; et al. Pulmonary Artery Hypertension-Specific Therapy Improves Exercise Tolerance and Outcomes in Exercise-Induced Pulmonary Hypertension. JACC Cardiovasc. Imaging 2019, 12, 2576–2579.
- Naeije, R.; Vanderpool, R.; Dhakal, B.P.; Saggar, R.; Saggar, R.; Vachiery, J.L.; Lewis, G.D. Exercise-induced pulmonary hypertension: Physiological basis and methodological concerns. Am. J. Respir. Crit. Care Med. 2013, 187, 576–583.
- Omote, K.; Sorimachi, H.; Obokata, M.; Reddy, Y.N.V.; Verbrugge, F.H.; Omar, M.; DuBrock, H.M.; Redfield, M.M.; A Borlaug, B. Pulmonary vascular disease in pulmonary hypertension due to left heart disease: Pathophysiologic implications. Eur. Heart J. 2022, 43, 3417–3431.
- Qutrio Baloch, Z.; Abbas, S.A.; Prasad, R.M.; Elamin, A.M.; Ali, A. Potential Role of Cardiopulmonary Exercise Testing as an Early Screening Tool for Patients with Suspected Pulmonary Hypertension Including Exercise-Induced Pulmonary Hypertension: Results from a Retrospective Analysis. Perm J. 2021, 25, 20.323.
- Wood, P. Pulmonary Hypertension with Special Reference to the Vasoconstrictive Factor. Heart 1958, 20, 557–570.
- Reeves, J.T.; Linehan, J.H.; Stenmark, K.R. Distensibility of the normal human lung circulation during exercise. Am. J. Physiol. Cell. Mol. Physiol. 2005, 288, L419–L425.
- Guseh, J.S. The Evolving Landscape of Exercise-Induced Pulmonary Hypertension. Curr. Treat. Options Cardiovasc. Med. 2016, 18, 41.
- Ha, J.-W.; Choi, D.; Park, S.; Shim, C.-Y.; Kim, J.-M.; Moon, S.-H.; Lee, H.-J.; Choi, E.-Y.; Chung, N. Determinants of exercise-induced pulmonary hypertension in patients with normal left ventricular ejection fraction. Heart 2008, 95, 490–494.
- Ojima, S.; Kubozono, T.; Saihara, K.; Miyauchi, T.; Kawasoe, S.; Kubota, K.; Shigemizu, S.; Ohtsubo, H.; Miyata, M.; Ohishi, M. Significant Clinical Indexes of Exercise-Induced Pulmonary Hypertension in Patients with Connective Tissue Disease. Circ. Rep. 2019, 1, 610–616.
- Voilliot, D.; Magne, J.; Dulgheru, R.; Kou, S.; Henri, C.; Laaraibi, S.; Sprynger, M.; Andre, B.; Pierard, L.A.; Lancellotti, P. Determinants of exercise-induced pulmonary arterial hypertension in systemic sclerosis. Int. J. Cardiol. 2014, 173, 373–379.
- Gargani, L.; Pignone, A.; Agoston, G.; Moreo, A.; Capati, E.; Badano, L.; Doveri, M.; Bazzichi, L.; Costantino, M.F.; Pavellini, A.; et al. Clinical and echocardiographic correlations of exercise-induced pulmonary hypertension in systemic sclerosis: A multicenter study. Am. Heart J. 2013, 165, 200–207.
- Suzuki, K.; Izumo, M.; Yoneyama, K.; Mizukoshi, K.; Kamijima, R.; Kou, S.; Takai, M.; Kida, K.; Watanabe, S.; Omiya, K.; et al. Influence of exercise-induced pulmonary hypertension on exercise capacity in asymptomatic degenerative mitral regurgitation. J. Cardiol. 2015, 66, 246–252.
- Schwaiblmair, M.; Faul, C.; von Scheidt, W.; Berghaus, T.M. Detection of Exercise-Induced Pulmonary Arterial Hypertension by Cardiopulmonary Exercise Testing. Clin. Cardiol. 2012, 35, 548–553.
- Miyanaga, S.; Kubota, K.; Iwatani, N.; Higo, K.; Miyata, M.; Horizoe, Y.; Ojima, S.; Kawasoe, S.; Kubozono, T.; Ohishi, M. Predictors of exercise-induced pulmonary hypertension in patients with connective tissue disease. Heart Vessel. 2019, 34, 1509–1518.
- Goda, A.; Takeuchi, K.; Kikuchi, H.; Finger, M.; Inami, T.; Sakata, K.; Soejima, K.; Satoh, T. Etiology of Exercise-Induced Pulmonary Hypertension Can Be Differentiated by Echocardiography—Insight from Patients with Chronic Pulmonary Thromboembolism With Normal Resting Hemodynamics by Balloon Pulmonary Angioplasty. Circ. J. 2019, 83, 2527–2536.
- Naeije, R.; Vonk Noordegraaf, A.; Kovacs, G. Exercise-induced pulmonary hypertension: At last! Eur. Respir. J. 2015, 46, 583–586.
- Fowler, R.M.; Maiorana, A.J.; Jenkins, S.C.; Gain, K.R.; O’Driscoll, G.; Gabbay, E. Implications of Exercise-Induced Pulmonary Arterial Hypertension. Med. Sci. Sports Exerc. 2011, 43, 983–989.
