Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cucurbitacins constitute a group of cucumber-derived dietary lipids, highly oxidized tetracyclic triterpenoids, with potential medical uses. These compounds are known to interact with a variety of recognized cellular targets to impede the growth of cancer cells.
- cucurbitacins
- anti-proliferation
- apoptotic
1. Apoptotic and Cell-Cycle Arrest
Apoptotic cell death can be triggered in cancer through internal and extrinsic processes, which converge on the control of caspase-dependent proteolysis of cellular proteins and DNA fragmentation [1][2][3]. Similarly, all tumor types have abnormal cell-cycle progression activity, which acts as a catalyst for carcinogenesis [4]. Recent research has shown that a variety of biological processes are regulated by cell-cycle proteins [5][6][7]. Therefore, numerous chemo-preventive FDA-drugs have been shown to mediate antitumor effects either via activation of apoptotic or cell-cycle arrest (Figure 1) signaling pathways [8][9][10]. For instance, results from Li et al. (2018) revealed that cucurbitacin I caused lung cancer (A549) cells to undergo excessive ERS, CHOP-Bax and caspase-12-dependent ERS-associated apoptosis [11]. In colorectal cancer (SW480 and Caco-2) cells, treatment with cucurbitacin B resulted in cell-cycle arrest at the G1 phase as well as decreased Cyclin D1 and Cyclin E1 levels. Both CRC cell lines underwent in vitro cell death when exposed to cucurbitacin B (CuB), which was accompanied by caspase-3 and cleaved PARP [12]. Using triple negative breast cancer (TNBC), cucurbitacin E strongly boosted JNK activation while considerably decreasing AKT and ERK activation in MDA-MB-468 cells. It also significantly decreased expression of Cyclin D1, Survivin, XIAP, Bcl2 and Mcl-1 [13]. In the pancreatic cancer cell line Capan-1, CuD induced cell-cycle arrest and death via the ROS/p38 pathway [14]. Cucurbitacin I-induced cell death in ovarian cancer (SKOV3) included apoptosis, as evidenced by upregulated caspase 3 and BAX and a decrease in Bcl2 [15]. Flow cytometric measurement of DNA content and RT-PCR analyses suggested that cucurbitacin B caused G2/M arrest in human breast cancer cell lines (MDA-MB-231 and MCF-7) through elevated p21 expression [16]. Huang et al. showed that in human bladder cancer (T24) cells, cucurbitacin E-induced G2/M arrest was accompanied by a significant rise in p53 and p21 levels and a fall in the levels of STAT3, cyclin-dependent kinase 1 (CDK1) and cyclin B [17]. In addition, cucurbitacin E-induced G2/M phase arrest and death of T24 cells also depended on Fas/CD95 and mitochondria-dependent apoptotic pathways. Similarly, using other cancerous cell lines, cucurbitacins target the cell-cycle actions that involves growth inhibition, cell-cycle arrest at G2/M phase and induction of apoptosis [18]. Cucurbitacin I has been observed to suppress phosphotyrosine STAT3 in human cancerous lung cells [19]. Recently, it was observed to promote gastric cancer cell apoptosis by inducing the production of cellular ROS, as well as the endoplasmic reticulum stress pathway [11][20]. While cucurbitacin B, E and I have been observed to inhibit both JAK2 and STAT3 activation, cucurbitacin A and I have been reported to inhibit JAK2 and STAT3, respectively [19]. Treating Hep-2 cells with different concentrations of cucurbitacin B for various time intervals showed reduction in cell proliferation, cell-cycle distribution, and increased cell apoptosis in cancerous cell lines [18]. This study also stated that cucurbitacin B exhibited significant efficacy in inhibiting cell growth, arresting cell cycle at the G2/M phase, and inducing apoptosis in a dose- and time-dependent manner [18]. Similarly, cucurbitacins B, D, E were observed to inhibit proteins such as JAK-STAT3. They also inhibited mitogen-activated protein kinases (MAPK)- signaling pathways and tumor angiogenesis [20]. A study conducted on human umbilical vascular endothelial cell lines revealed cucurbitacin to significantly inhibit the proliferation, migration, and angiogenesis. It also blocked essential proteins such as Jak2-signal transducer, vascular endothelial growth factor receptor (VEGFR) and STAT3 signaling pathways [21]. Such studies have highlighted that the main mechanism involved in imparting the anti-tumorigenic potentials of cucurbitacins involves inhibition of the JAK/STAT3 signaling pathway, which plays an essential role in activation, proliferation, and maintenance of cancerous cells [22]. Another recent study has shown that treatment with 8 µM cucurbitacin IIb for 24 h remarkably inhibited the proliferation of HeLa and A549 tumor cells, with IC50 values of 7.3 and 7.8 µM, respectively, while increasing total apoptosis by 56.9 and 52.3%, respectively [23]. Another pathway by which cucurbitacin IIb induces apoptosis and cell-cycle arrest is by the regulating EGFR/MAPK pathway [24]. Similarly, cucurbitacin D was observed to regulate the levels of oncogenic signaling cascades, JAK/STAT, Wnt/β-catenin and associated non-coding RNAs in many cancer cell lines [25]. Recent studies have shown that CuIIb and cucurbitacin B induced apoptosis in cervical cancer cell lines by Nrf2 inhibition, whereas in lung cancer cell lines cucurbitacin B was responsible for suppressing growth and inducing apoptotic death by impeding IL-6/STAT3 signaling [15][26].
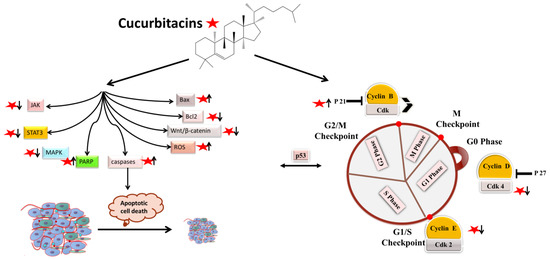
Figure 1. Molecular targets of cucurbitacins in modulating cell-cycle progression and inducing apoptotic cell death.
2. Antiangiogenic and Antimetastatic Mechanisms
The physiological process by which new blood vessels develop from pre-existing vessels is known as angiogenesis. Anti-angiogenesis causes suppression of tumor growth because of hunger and toxic waste buildup in its microenvironment [27][28]. The development and metastasis of the tumor have a major impact on the cancer vasculature (Figure 2). Vascular endothelial growth factors (VEGFs) are crucial protein regulators of angiogenesis and metastasis. Studies have shown that inhibiting the VEGFR2-mediated JAK/STAT3 pathway is considered as an effective approach to suppress angiogenesis [21]. Though many studies about the mechanism of cucurbitacins and angiogenesis are not well known, few studies have still shown that cucurbitacins such as cucurbitacin B, cucurbitacin D, cucurbitacin E and cucurbitacin I possess anti-angiogenesis properties [29][30]. CuB significantly inhibited angiogenesis, metastasis, and vascular development in dose-dependent manner in in vivo models and chick embryos [29]. CuE significantly inhibited human umbilical vascular endothelial cell (HUVEC) proliferation and angiogenesis by targeting the VEGFR2-mediated Jak2/STAT3 signaling pathway [21]. CuB has been observed to inhibit ERK1/2, prevent Raf-MEK-ERK from activating STAT3, which ultimately plays a key role in angiogenesis [31]. A similar effect of CuB was seen also in human breast cancer cell lines. It successfully inhibited angiogenesis by targeting the FAK/MMP-9 signaling axis [32]. CuB showed antimetastatic activity and targeted angiogenesis also in paclitaxel resistant A2780/Taxol ovarian cancer cells. It also suppressed angiogenesis by downregulating the expression of HIF-1 targets, VEGF, VEGFR2 phosphorylation and erythropoietin [29][33]. Another study revealed the effective use of CuE for anti-angiogenesis in Huh7 cells. It decreased the tube formation in HUVECs and was also responsible for inhibiting the process of neo-vascularization in CAM assays [34]. A recent study showed that CuE modulated the JAK/STAT3 pathways, which regulated the angiogenesis [35]. CuE has been also involved in inhibiting the KDR/VEGFR2-mediated pathway of angiogenesis [36]. Treating A549 cells with cucurbitacins for ~21 days showed positive results for inhibiting metastasis by regulating the levels of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D11 [37]. Similarly, other cucurbitacins were observed to inhibit angiogenesis in MDA-MB-231 and MCF-7 cancer cells by inhibiting the JAK/STAT pathways [38].
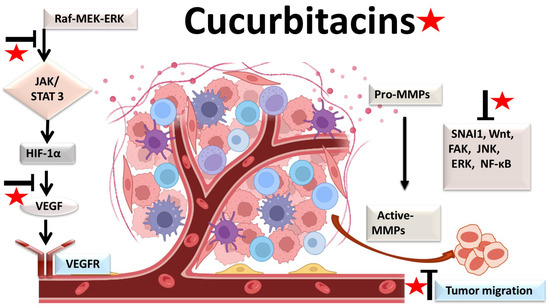
Figure 2. Molecular targets of cucurbitacins in suppressing angiogenesis and impeding cancer cell metastasis.
3. Anti-Inflammatory Mechanisms
Most malignancies’ growth and malignant progression are correlated with inflammation [39][40][41]. Both intrinsic and extrinsic inflammations have the potential to inhibit the immune system, which creates an ideal environment for the growth of tumors [42][43][44]. As a result, focusing on inflammation is a tempting strategy for both cancer therapy and cancer prevention [42][45]. Cucurbitacins have been observed to interact with proteins associated with inflammatory (Figure 3) pathways such as interleukins (IL)-6, IL-5, IL-1β, IL-12, IL-13 in a dose-dependent manner [46]. Dietary cucurbitacin E has been shown to reduce inflammation and immunosuppression by downregulating the NF-κB signaling pathway [47]. CuB has been studied to possess protective effects by reducing inflammatory responses on sepsis-induced acute lung injury in in vivo rat models. It significantly reduced the levels of TNF-α, IL-6, cytokine secretion and accumulation of inflammatory cells. It also regulated the levels of Ca2+, which play an essential role in inflammatory responses [48]. CuB inhibited inflammatory responses through targeting the SIRT1/IGFBPrP1/TGF β1 axis. It downregulated the expression levels of TGF β1, IGFBPrP1, and upregulated the expression of SIRT 1 [49]. Similarly, CuE decreased the levels of pro-inflammatory cytokines, such as IL-17 and IFN-γ, as well as the activities of the STAT3 and IL-17A-promoter in allo-reactive T cells [50]. CuE has been shown to inhibit skin inflammation and fibrosis by regulating the expression of α-Sma and Col-I in mice models [49]. Recently, it has also been demonstrated that CuE ameliorated lipopolysaccharide-evoked injuries and inflammation in bronchial epithelial cells by regulating the TLR4-NF-κB signaling. It was responsible for suppressing levels of inflammatory cytokine production, TNF-α, IL-6 and IL-8 [51]. Cucurbitacin B was observed to directly bind to toll-like receptor 4 (TLR4) and activate NLRP3 inflammasome, which further ultimately executed pyroptosis in A549 cells. CuB treatment has been observed to upregulate the protein expressions of IL-1β, GSDMD, HMGB1 and led to inhibition of generation of mitochondrial ROS and pyroptosis [52]. CuB was reported to sensitize CD133+ HepG2 cells in in vitro and in vivo models [53].
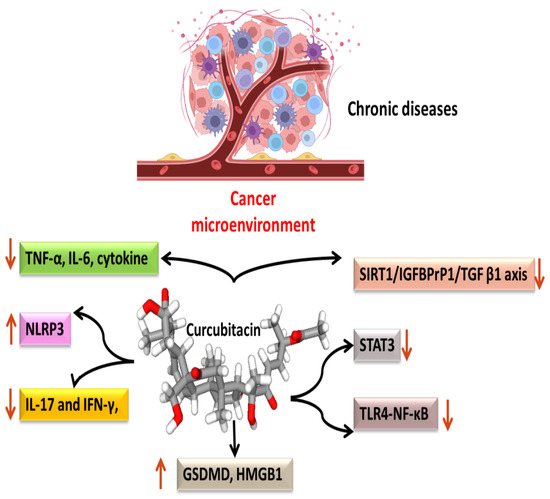
Figure 3. Anti-inflammatory targets of cucurbitacins in malignant cells.
This entry is adapted from the peer-reviewed paper 10.3390/biom13010057
References
- Manu, K.A.; Shanmugam, M.K.; Li, F.; Chen, L.; Siveen, K.S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2014, 92, 267–276.
- Sethi, G.; Kwang, S.A.; Sandur, S.K.; Lin, X.; Chaturvedi, M.M.; Aggarwal, B.B. Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-kappa B signaling pathway. J. Biol. Chem. 2006, 281, 23425–23435.
- Jung, Y.Y.; Um, J.Y.; Chinnathambi, A.; Govindasamy, C.; Sethi, G.; Ahn, K.S. Leelamine Modulates STAT5 Pathway Causing Both Autophagy and Apoptosis in Chronic Myelogenous Leukemia Cells. Biology 2022, 11, 366.
- Chopra, P.; Sethi, G.; Dastidar, S.G.; Ray, A. Polo-like kinase inhibitors: An emerging opportunity for cancer therapeutics. Expert Opin. Investig. Drugs 2010, 19, 27–43.
- Xiao, W.; Li, J.; Hu, J.; Wang, L.; Huang, J.R.; Sethi, G.; Ma, Z. Circular RNAs in cell cycle regulation: Mechanisms to clinical significance. Cell Prolif. 2021, 54, 13143.
- Raghunath, A.; Sundarraj, K.; Arfuso, F.; Sethi, G.; Perumal, E. Dysregulation of Nrf2 in Hepatocellular Carcinoma: Role in Cancer Progression and Chemoresistance. Cancers 2018, 10, 481.
- Ma, Z.; Xiang, X.; Li, S.; Xie, P.; Gong, Q.; Goh, B.C.; Wang, L. Targeting hypoxia-inducible factor-1, for cancer treatment: Recent advances in developing small-molecule inhibitors from natural compounds. Semin. Cancer Biol. 2022, 80, 379–390.
- Rajendran, P.; Ong, T.H.; Chen, L.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; et al. Suppression of signal transducer and activator of transcription 3 activation by butein inhibits growth of human hepatocellular carcinoma in vivo. Clin. Cancer Res. 2011, 17, 1425–1439.
- Ahn, K.S.; Sethi, G.; Aggarwal, B.B. Simvastatin potentiates TNF-alpha-induced apoptosis through the down-regulation of NF-kappaB-dependent antiapoptotic gene products: Role of IkappaBalpha kinase and TGF-beta-activated kinase-1. J. Immunol. 2007, 178, 2507–2516.
- Mohan, C.D.; Rangappa, S.; Nayak, S.C.; Jadimurthy, R.; Wang, L.; Sethi, G.; Garg, M.; Rangappa, K.S. Bacteria as a treasure house of secondary metabolites with anticancer potential. Semin. Cancer Biol. 2021, 86, 998–1013.
- Li, H.; Chen, H.; Li, R.; Xin, J.; Wu, S.; Lan, J.; Xue, K.; Li, X.; Zuo, C.; Jiang, W.; et al. Cucurbitacin I induces cancer cell death through the endoplasmic reticulum stress pathway. J. Cell. Biochem. 2018, 120, 2391–2403.
- Mao, D.; Liu, A.H.; Wang, Z.P.; Zhang, X.W.; Lu, H. Cucurbitacin B inhibits cell proliferation and induces cell apoptosis in colorectal cancer by modulating methylation status of BTG3. Neoplasma 2019, 66, 593–602.
- Kong, Y.; Chen, J.; Zhou, Z.; Xia, H.; Qiu, M.H.; Chen, C. Cucurbitacin E induces cell cycle G2/M phase arrest and apoptosis in triple negative breast cancer. PLoS ONE 2014, 9, e103760.
- Kim, M.S.; Lee, K.; Ku, J.M.; Choi, Y.J.; Mok, K.; Kim, D.; Cheon, C.; Ko, S.G. Cucurbitacin D Induces G2/M Phase Arrest and Apoptosis via the ROS/p38 Pathway in Capan-1 Pancreatic Cancer Cell Line. Evid. Based. Complement. Alternat. Med. 2020, 2020, 6571674.
- Vidal-Gutiérrez, M.; Torres-Moreno, H.; Arenas-Luna, V.; Loredo-Mendoza, M.L.; Tejeda-Dominguez, F.; Velazquez, C.; Vilegas, W.; Hernández-Gutiérrez, S.; Robles-Zepeda, R.E. Standardized phytopreparations and cucurbitacin IIb from Ibervillea sonorae (S. Watson) greene induce apoptosis in cervical cancer cells by Nrf2 inhibition. J. Ethnopharmacol. 2022, 298, 115606.
- Duangmano, S.; Sae-Lim, P.; Suksamrarn, A.; Patmasiriwat, P.; Domann, F.E. Cucurbitacin B Causes Increased Radiation Sensitivity of Human Breast Cancer Cells via G2/M Cell Cycle Arrest. J. Oncol. 2012, 2012, 601682.
- Huang, W.W.; Yang, J.S.; Lin, M.W.; Chen, P.Y.; Chiou, S.M.; Chueh, F.S.; Lan, Y.H.; Pai, S.J.; Tsuzuki, M.; Ho, W.J.; et al. Cucurbitacin E Induces G(2)/M Phase Arrest through STAT3/p53/p21 Signaling and Provokes Apoptosis via Fas/CD95 and Mitochondria-Dependent Pathways in Human Bladder Cancer T24 Cells. Evid. Based. Complement. Alternat. Med. 2012, 2012, 952762.
- Liu, T.; Zhang, M.; Zhang, H.; Sun, C.; Deng, Y. Inhibitory effects of cucurbitacin B on laryngeal squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2008, 265, 1225–1232.
- Blaskovich, M.A.; Sun, J.; Cantor, A.; Turkson, J.; Jove, R.; Sebti, S.M. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice-PubMed. Cancer Res. 2003, 63, 1270–1279.
- Liang, J.; Chen, D. Advances in research on the anticancer mechanism of the natural compound cucurbitacin from Cucurbitaceae plants: A review. Tradit. Chin. Med. 2019, 4, 68.
- Dong, Y.; Lu, B.; Zhang, X.; Zhang, J.; Lai, L.; Li, D.; Wu, Y.; Song, Y.; Luo, J.; Pang, X.; et al. Cucurbitacin E, a tetracyclic triterpenes compound from Chinese medicine, inhibits tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling pathway. Carcinogenesis 2010, 31, 2097–2104.
- Wu, D.; Wang, Z.; Lin, M.; Shang, Y.; Wang, F.; Zhou, J.Y.; Wang, F.; Zhang, X.; Luo, X.; Huang, W. In Vitro and In Vivo Antitumor Activity of Cucurbitacin C, a Novel Natural Product from Cucumber. Front. Pharmacol. 2019, 10, 1287.
- Torres-Moreno, H.; Marcotullio, M.C.; Velázquez, C.; Ianni, F.; Garibay-Escobar, A.; Robles-Zepeda, R.E. Cucurbitacin IIb, a steroidal triterpene from Ibervillea sonorae induces antiproliferative and apoptotic effects on cervical and lung cancer cells. Steroids 2020, 157, 108597.
- Liang, Y.; Zhang, T.; Ren, L.; Jing, S.; Li, Z.; Zuo, P.; Li, T.; Wang, Y.; Zhang, J.; Wei, Z. Cucurbitacin IIb induces apoptosis and cell cycle arrest through regulating EGFR/MAPK pathway. Environ. Toxicol. Pharmacol. 2021, 81, 103542.
- Lin, X.; Farooqi, A.A. Cucurbitacin mediated regulation of deregulated oncogenic signaling cascades and non-coding RNAs in different cancers: Spotlight on JAK/STAT, Wnt/β-catenin, mTOR, TRAIL-mediated pathways. Semin. Cancer Biol. 2021, 73, 302–309.
- Liu, J.H.; Li, C.; Cao, L.; Zhang, C.H.; Zhang, Z.H. Cucurbitacin B regulates lung cancer cell proliferation and apoptosis via inhibiting the IL-6/STAT3 pathway through the lncRNA XIST/miR-let-7c axis. Pharm. Biol. 2022, 60, 154–162.
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911.
- Wu, Q.; You, L.; Nepovimova, E.; Heger, Z.; Wu, W.; Kuca, K.; Adam, V. Hypoxia-inducible factors: Master regulators of hypoxic tumor immune escape. J. Hematol. Oncol. 2022, 15, 77.
- Piao, X.M.; Gao, F.; Zhu, J.X.; Wang, L.J.; Zhao, X.; Li, X.; Sheng, M.M.; Zhang, Y. Cucurbitacin B inhibits tumor angiogenesis by triggering the mitochondrial signaling pathway in endothelial cells. Int. J. Mol. Med. 2018, 42, 1018–1025.
- Sheikh, I.; Sharma, V.; Tuli, H.S.; Aggarwal, D.; Sankhyan, A.; Vyas, P.; Sharma, A.K.; Bishayee, A. Cancer Chemoprevention by Flavonoids, Dietary Polyphenols and Terpenoids. Biointerface Res. Appl. Chem. 2021, 11, 8502–8537.
- Yin, B.; Fang, D.M.; Zhou, X.L.; Gao, F. Natural products as important tyrosine kinase inhibitors. Eur. J. Med. Chem. 2019, 182, 111664.
- Sinha, S.; Khan, S.; Shukla, S.; Lakra, A.D.; Kumar, S.; Das, G.; Maurya, R.; Meeran, S.M. Cucurbitacin B inhibits breast cancer metastasis and angiogenesis through VEGF-mediated suppression of FAK/MMP-9 signaling axis. Int. J. Biochem. Cell Biol. 2016, 77, 41–56.
- Garg, S.; Kaul, S.C.; Wadhwa, R. Cucurbitacin B and cancer intervention: Chemistry, biology and mechanisms (review). Int. J. Oncol. 2018, 52, 19–37.
- Liu, Y.; Yang, H.; Guo, Q.; Liu, T.; Jiang, Y.; Zhao, M.; Zeng, K.; Tu, P. Cucurbitacin E Inhibits Huh7 Hepatoma Carcinoma Cell Proliferation and Metastasis via Suppressing MAPKs and JAK/STAT3 Pathways. Molecules 2020, 25, 560.
- Ramezani, M.; Hasani, M.; Ramezani, F.; Abdolmaleki, M.K. Cucurbitacins: A focus on Cucurbitacin E as a natural product and their biological activities. Pharm. Sci. 2020, 27, 1–13.
- Yun, W.; Dan, W.; Liu, J.; Guo, X.; Li, M.; He, Q. Investigation of the Mechanism of Traditional Chinese Medicines in Angiogenesis through Network Pharmacology and Data Mining. Evid.-Based Complement. Altern. Med. 2021, 2021, 5539970.
- Ren, Y.; Kinghorn, A.D. Natural Product Triterpenoids and Their Semi-Synthetic Derivatives with Potential Anticancer Activity. Planta Med. 2019, 85, 802–814.
- Sathya, T.N.; Mehta, V.A.; Senthil, D.; Navaneethakrishnan, K.; Murugan, S.; Kumaravel, T. Cytotoxicity evaluation of CELNORM, a nutritional health supplement, on MCF7 breast cancer cells. Indian J. Sci. Technol. 2020, 13, 3070–3075.
- Ong, P.S.; Wang, L.Z.; Dai, X.; Tseng, S.H.; Loo, S.J.; Sethi, G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharmacol. 2016, 7, 395.
- Ma, Z.; Wang, Y.Y.; Xin, H.W.; Wang, L.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Wang, H.; Tang, F.R.; Warrier, S.; et al. The expanding roles of long non-coding RNAs in the regulation of cancer stem cells. Int. J. Biochem. Cell Biol. 2019, 108, 17–20.
- Ashrafizadeh, M.; Zarrabi, A.; Mostafavi, E.; Aref, A.R.; Sethi, G.; Wang, L.; Tergaonkar, V. Non-coding RNA-based regulation of inflammation. Semin. Immunol. 2022, 59, 101606.
- Morgan, D.; Garg, M.; Tergaonkar, V.; Tan, S.Y.; Sethi, G. Pharmacological significance of the non-canonical NF-κB pathway in tumorigenesis. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188449.
- Cheng, J.T.; Wang, L.; Wang, H.; Tang, F.R.; Cai, W.Q.; Sethi, G.; Xin, H.W.; Ma, Z. Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells 2019, 8, 1178.
- Cai, W.; Chen, Z.X.; Rane, G.; Singh, S.S.; Choo, Z.; Wang, C.; Yuan, Y.; Tan, T.Z.; Arfuso, F.; Yap, C.T.; et al. Wanted DEAD/H or Alive: Helicases Winding Up in Cancers. J. Natl. Cancer Inst. 2017, 109, djw278.
- Ong, S.K.L.; Shanmugam, M.K.; Fan, L.; Fraser, S.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Bishayee, A. Focus on Formononetin: Anticancer Potential and Molecular Targets. Cancers 2019, 11, 611.
- Kapoor, N.; Ghorai, S.M.; Kushwaha, P.K.; Shukla, R.; Aggarwal, C.; Bandichhor, R. Plausible mechanisms explaining the role of cucurbitacins as potential therapeutic drugs against coronavirus 2019. Inform. Med. Unlocked 2020, 21, 100484.
- Xie, H.; Tuo, X.; Zhang, F.; Bowen, L.; Zhao, W.; Xu, Y. Dietary cucurbitacin E reduces high-strength altitude training induced oxidative stress, inflammation and immunosuppression. An. Acad. Bras. Cienc. 2020, 92, 1–14.
- Hua, S.; Liu, X.; Lv, S.; Wang, Z. Protective Effects of Cucurbitacin B on Acute Lung Injury Induced by Sepsis in Rats. Med. Sci. Monit. 2017, 23, 1355.
- Yang, L.; Ao, Q.; Zhong, Q.; Li, W.; Li, W. SIRT1/IGFBPrP1/TGF β1 axis involved in cucurbitacin B ameliorating concanavalin A-induced mice liver fibrosis. Basic Clin. Pharmacol. Toxicol. 2020, 127, 371–379.
- Kim, S.Y.; Park, M.J.; Kwon, J.E.; Jung, K.A.; Jhun, J.Y.; Lee, S.Y.; Seo, H.B.; Ryu, J.Y.; Beak, J.A.; Choi, J.Y.; et al. Cucurbitacin E ameliorates acute graft-versus-host disease by modulating Th17 cell subsets and inhibiting STAT3 activation. Immunol. Lett. 2018, 203, 62–69.
- Shang, J.; Liu, W.; Yin, C.; Chu, H.; Zhang, M. Cucurbitacin E ameliorates lipopolysaccharide-evoked injury, inflammation and MUC5AC expression in bronchial epithelial cells by restraining the HMGB1-TLR4-NF-κB signaling. Mol. Immunol. 2019, 114, 571–577.
- Yuan, R.; Zhao, W.; Wang, Q.Q.; He, J.; Han, S.; Gao, H.; Feng, Y.; Yang, S. Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol. Res. 2021, 170, 105748.
- Wang, X.; Bai, Y.; Yan, X.; Li, J.; Lin, B.; Dai, L.; Xu, C.; Li, H.; Li, D.; Yang, T.; et al. Cucurbitacin B exhibits antitumor effects on CD133+ HepG2 liver cancer stem cells by inhibiting JAK2/STAT3 signaling pathway. Anticancer. Drugs 2021, 32, 548–557.
This entry is offline, you can click here to edit this entry!
