Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Lysosomal degradation, the common destination of autophagy and endocytosis, is one of the most important elements of eukaryotic metabolism. The small GTPases Rab39A and B are potential new effectors of this pathway, as their malfunction is implicated in severe human diseases like cancer and neurodegeneration.
- Rab39
- autophagy
- endocytosis
1. Introduction
Lysosomal degradation pathways, autophagy, and endocytosis are essential for the proper homeostasis of all eukaryotic cells. Lysosomal malfunctions often lead to, or aggravate, severe human pathologies, such as Alzheimer’s disease or cancer [1]. Understanding the molecular mechanisms that govern lysosomal processes is of great clinical importance. Autophagic and endocytic degradation both depend on two key events: lysosomal fusion, and the subsequent clearance. Despite the numerous factors that are already identified, new, yet unknown proteins and mechanisms might emerge, providing new insights that can be utilized in tackling such diseases.
Ras-related in brain (Rab) proteins play a central role in vesicular transport and fusion events. Several Rab proteins are confirmed to regulate lysosomal pathways, e.g., Rab2 and Rab7 regulate lysosomal fusion and degradation, while Rab5 mediates early-to-late endosomal maturation [2][3][4][5]. Rab proteins convey effector responses in an active, GTP-bound state, thus, the factors regulating GTP- and GDP-binding, also have an excessive regulatory role [6]. Rab2 and Rab39 share a common ancestor and, therefore, are closely related, but in contrast to Rab2, the involvement of Rab39 in lysosome formation is poorly understood [7].
Rab39 was originally characterized as a trans-Golgi protein, where it regulates vesicle tethering, with possible pleiotropic effects in other vesicle trafficking pathways, such as endocytosis [8]. Mammalian organisms have two Rab39 paralogs, RAB39A and RAB39B, and the mutation of either leads to distinct pathologies. RAB39A depletion was shown to decrease the colonization rate of certain cancer stem cells, while augmenting lipopolysaccharide-induced autophagosome formation in macrophages [9][10][11][12]. RAB39A also regulates phagosome acidification and caspase-1 activity, and is also localized to lysosomal associated membrane protein 2 (LAMP2)-containing phagosomes [9][12][13]. RAB39B, on the other hand, is involved in the pathogenesis of an early-onset Parkinson-like disease, Waisman syndrome [14]. Both paralogs are implicated in proper neuritogenesis [15][16]. Interestingly, a GDP/GTP exchange factor (GEF) of RAB39B, C9ORF72 was found to regulate autophagy in neuronal cultures [17].
2. Rab39 Mutation Alters the Endolysosomal Distribution
Researchers examined the morphology of the lysosomal compartment using two organs of Drosophila melanogaster larvae. The research used a CRISPR/Cas9 generated rab39 mutant allele [18], in which the rab39 coding sequence was replaced with an eyeless promoter-driven red fluorescent protein (RFP) gene. Thus, this was named allele rab39CRISPR-RFP (rab39C-R). For the assessment of endolysosomes, researchers utilized the larval garland nephrocytes that continuously take up haemolymph via endocytosis [19]. First, researchers examined the acidic organelles using LysoTracker Red (LTR), as it detects acidic endolysosomes in nephrocytes. LTR-positive puncta were smaller in number as well as in size in Rab39 mutant nephrocytes compared to the controls (Figure 1A,B,E).
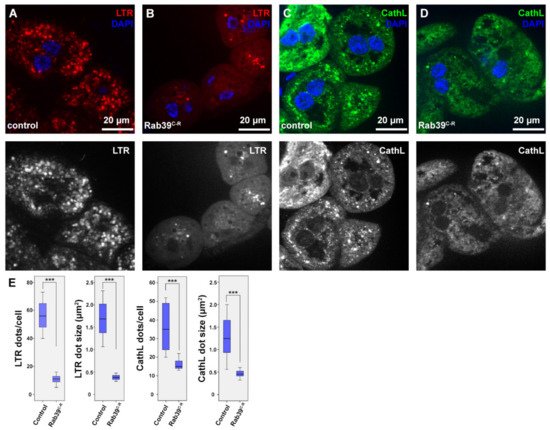
Figure 1. Endolysosomal distribution is altered in Rab39C-R garland nephrocytes of L3 stage wandering larvae. (A,B) Acidic organelles were marked using LTR to depict endolysosomes in control (A) and Rab39C-R (B) nephrocytes. Rab39 mutant nephrocytes contain significantly fewer and smaller LTR-positive vesicles. Please note that the smaller nuclei in B, when compared to A are only the result of natural deviation in nucleus size. (C,D) CathL was immunostained to show Golgi-derived vesicles, acidic and even non-acidic lysosomes in control (C) and Rab39 mutant (D) nephrocytes. CathL puncta were fewer and smaller in Rab39 mutant nephrocytes. (A–D) Respective red (A,B) and green (C–D) channels are shown in grayscale. (E) Quantifications of the LTR and CathL experiments, n = 10 cells. None of the samples showed Gaussian distribution. Medians are shown as horizontal black lines within the boxes. Bars show the upper and lower quartiles, and the whiskers plot the smallest and largest observations. *** p < 0.001. Heterozygous Rab39C-R animals in A,C,E were used as controls.
In order to see whether the scarcity of LTR-positive vesicles is a result of defective acidification, researchers performed an immunostaining against the lysosomal hydrolase Cathepsin-L (CathL) that detects both acidic and non-acidic lysosomes. The size and number of CathL-positive puncta were also smaller in Rab39C-R nephrocytes than in the control cells (Figure 1C–E). This suggests either an accelerated endolysosomal degradation or a reduced endocytic uptake or maturation, since lysosomal size increase is usually a sign of improper degradation [20][21].
3. Earlier Steps of Endocytosis Are Intact in Rab39 Mutant Nephrocytes
To assess what caused the observed lysosomal phenotype, it was examined that previous steps of endocytosis in Rab39 mutant cells. Early endosomes were detected via immunostaining against Rab5, and researchers saw no difference between mutant and control cells (Figure S1A,B). An immunostaining against late endosomal Rab7 was also performed, which again displayed an intact late endosomal compartment in Rab39C-R nephrocytes (Figure 2A,B,E). The study next measured endocytic function of nephrocytes. In this experiment, the larvae were grown on silver nitrate-containing food, as this heavy metal is taken up from the haemolymph by nephrocytes [22]. Since heavy metals cannot be degraded in lysosomes, nephrocytes sequester them into terminal or storage lysosomes, or residual bodies, as shown in Figure 2C in the case of control cells. In Rab39 deprived cells, however, silver distribution is altered (Figure 2D,E). The silver containing vesicles show a scattered distribution, while their size is also decreased. These data verify that Rab39 mutant cells can initiate endocytosis, and up to the stage of late endosomes there is no detectable malfunction in this vesicular transport route. According to the silver uptake experiment, the later stages of lysosomal maturation might be altered.
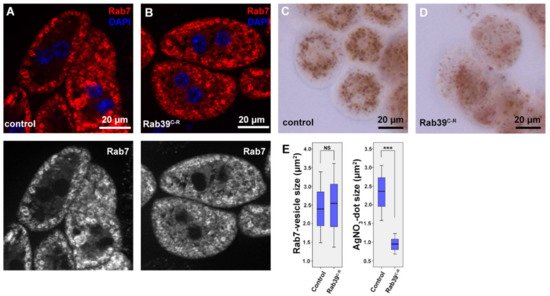
Figure 2. Endocytic uptake and maturation are unaffected in Rab39-depleted nephrocytes of L3 stage wandering larvae. (A,B) An immunostaining against late endosomal Rab7 was performed in control (A) and Rab39C-R (B) cells. No visible alteration was detectable upon Rab39 deficiency, suggesting that endosomal maturation undergoes properly. The respective red channels are shown in grayscale. (C,D) Silver nitrate was taken up by control (C) or mutant (D) garland nephrocytes, where silver was detected in the form of a brown precipitate. Rab39 mutant nephrocytes could also incorporate silver nitrate, but in smaller vesicles, meaning that endocytic uptake is unaltered. (E) Quantification of the experiments depicted in (A,D), n = 10 cells. None of the samples showed Gaussian distribution. Medians are shown as horizontal black lines within the boxes. Bars show the upper and lower quartiles, and the whiskers plot the smallest and largest observations. NS: non-significant (p ≥ 0.05), *** p < 0.001. Heterozygous Rab39C-R animals in A, C, E were used as control ones.
4. Rab39 Depletion Accelerates Endosomal Maturation
After having analyzed the nephrocyte morphology using fluorescent methods, their ultrastructure was also examined. A seemingly normal early and late endosomal compartment upon Rab39 depletion were found, as anticipated (Figure 3A,B). Nevertheless, the cytosol of the Rab39 mutant cells was abundant with small, electron-dense, lysosome-like vesicles (Figure 3B). These structures could be either Golgi-derived vesicles or some kind of lysosomes. Considering the small number of CathL-positive vesicles in mutant cells (Figure 1D), only a small portion of the observed vesicles could be Golgi-derived vesicles (also known as primary lysosomes), the majority are more likely to be endolysosomes that have undergone degradation and are depleted of CathL.
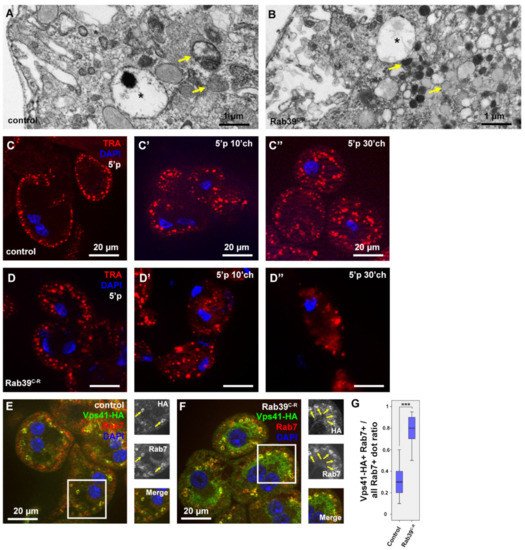
Figure 3. Endocytosis is accelerated in Rab39 depleted nephrocytes of L3 stage wandering larvae. (A,B) An ultrastructural analysis revealed normal late endosomal (marked with asterisks) size and distribution both in control (A) and Rab39 mutant (B) nephrocytes. In the mutant cells, however, only small, electron-dense lysosomes were present as opposed to control cells where lysosomes were larger. Two representative lysosomes are marked with arrows in both cases. (C,D) TRA was taken up by control (C) or Rab39 mutant (D) nephrocytes for 5 min, and it was chased 0 (C,D), 10 (C’,D’) or 30 min (C’’,D’’). In the mutant cells, TRA was internalized and degraded faster than in control cells. (E,F) The HA-tagged HOPS-subunit Vps41/Lt and late endosomal Rab7 were co-stained to detect their colocalization in control (E) and mutant cells (F). The colocalization increased upon Rab39 mutation. Boxed regions are enlarged in insets in (E,F) and colocalizing signals are marked with arrows. (G) Quantification of the experiments depicted in (E,F), n = 10 cells. None of the samples showed Gaussian distribution. Medians are shown as horizontal black lines within the boxes. Bars show the upper and lower quartiles, and the whiskers plot the smallest and largest observations. ***: p < 0.001. Heterozygous Rab39C-R animals with (E,G) or without (A,C) the indicated transgenes were used as controls.
To assess the degradation efficiency of Rab39 mutant cells, a fluorescent tracer, Texas Red-Avidin D (TRA), was added to the media in which the garland cells were incubated for 5 min (pulse). Afterward, the tracer was allowed to be internalized and degraded for an additional 0, 10, or 30 min (chase) by removing TRA from the media but keeping the cells within the media. In control cells after the 5 min pulse, TRA appeared in the outermost layer of the nephrocytes (Figure 3C). After the 10 min chase, TRA was observed closer to the nuclei and in larger vesicles that are presumably late endosomes and lysosomes (Figure 3C’) [4]. After 30 min of chase, TRA reached the perinuclear cytoplasm and most likely was sequestered by endolysosomes (Figure 3C’’) [23]. In Rab39 mutant cells, even after only the 5 min pulse, TRA was already present in larger vesicles that might be late endosomes (Figure 3D). It reached the perinuclear region after 10 min of chase and already started to be degraded, as the signal started to be vague and diffuse (3D’). After 30 min of chase, the TRA signal was considerably fainter in Rab39 mutant nephrocytes compared to the control cells, as most of the tracer was degraded and quenched by this time (3D’’). This suggests that TRA reaches lysosomes, and it is likely removed by accelerated lysosomal clearance in Rab39 mutants.
Increased lysosomal activity implies an elevated level of endosomal maturation, thus researchers assessed the colocalization between HOPS and late endosomal Rab7. In order to localize HOPS, a upstream activating sequence (UAS)-driven HA (hemagglutinin) epitope-tagged recombinant version of one of its subunits, Vps41/Light (Lt) was used [24]. Increased colocalization between Vps41-HA and Rab7 upon Rab39 depletion in garland nephrocytes (Figure 3E,G), suggesting an accelerated late endosomal maturation that probably conveys late endosomes towards lysosomal fusion at a higher rate.
This entry is adapted from the peer-reviewed paper 10.3390/ijms221910635
References
- Ballabio, A.; Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118.
- Hegedűs, K.; Takáts, S.; Boda, A.; Jipa, A.; Nagy, P.; Varga, K.; Kovács, A.L.; Juhász, G. The Ccz1-Mon1-Rab7 module and Rab5 control distinct steps of autophagy. Mol. Biol. Cell 2016, 27, 3132–3142.
- Fujita, N.; Huang, W.; Lin, T.-h.; Groulx, J.-F.; Jean, S.; Nguyen, J.; Kuchitsu, Y.; Koyama-Honda, I.; Mizushima, N.; Fukuda, M. Genetic screen in Drosophila muscle identifies autophagy-mediated T-tubule remodeling and a Rab2 role in autophagy. Elife 2017, 6, e23367.
- Lőrincz, P.; Tóth, S.; Benkő, P.; Lakatos, Z.; Boda, A.; Glatz, G.; Zobel, M.; Bisi, S.; Hegedűs, K.; Takáts, S. Rab2 promotes autophagic and endocytic lysosomal degradation. J. Cell Biol. 2017, 216, 1937–1947.
- Bucci, C.; Parton, R.G.; Mather, I.H.; Stunnenberg, H.; Simons, K.; Hoflack, B.; Zerial, M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 1992, 70, 715–728.
- Barr, F.; Lambright, D.G. Rab gefs and gaps. Curr. Opin. Cell Biol. 2010, 22, 461–470.
- Klöpper, T.H.; Kienle, N.; Fasshauer, D.; Munro, S. Untangling the evolution of Rab G proteins: Implications of a comprehensive genomic analysis. BMC Biol. 2012, 10, 71.
- Chen, T.; Han, Y.; Yang, M.; Zhang, W.; Li, N.; Wan, T.; Guo, J.; Cao, X. Rab39, a novel Golgi-associated Rab GTPase from human dendritic cells involved in cellular endocytosis. Biochem. Biophys. Res. Commun. 2003, 303, 1114–1120.
- Seto, S.; Sugaya, K.; Tsujimura, K.; Nagata, T.; Horii, T.; Koide, Y. Rab39a interacts with phosphatidylinositol 3-kinase and negatively regulates autophagy induced by lipopolysaccharide stimulation in macrophages. PLoS ONE 2013, 8, e83324.
- Chano, T.; Avnet, S. RAB39A: A Rab small GTPase with a prominent role in cancer stemness. J. Biochem. 2018, 164, 9–14.
- Chano, T.; Kita, H.; Avnet, S.; Lemma, S.; Baldini, N. Prominent role of RAB39A-RXRB axis in cancer development and stemness. Oncotarget 2018, 9, 9852.
- Seto, S.; Tsujimura, K.; Koide, Y. Rab GTPases regulating phagosome maturation are differentially recruited to mycobacterial phagosomes. Traffic 2011, 12, 407–420.
- Becker, C.E.; Creagh, E.M.; O’Neill, L.A. Rab39a binds caspase-1 and is required for caspase-1-dependent interleukin-1β secretion. J. Biol. Chem. 2009, 284, 34531–34537.
- Wilson, G.R.; Sim, J.C.; McLean, C.; Giannandrea, M.; Galea, C.A.; Riseley, J.R.; Stephenson, S.E.; Fitzpatrick, E.; Haas, S.A.; Pope, K. Mutations in RAB39B cause X-linked intellectual disability and early-onset Parkinson disease with α-synuclein pathology. Am. J. Hum. Genet. 2014, 95, 729–735.
- Giannandrea, M.; Bianchi, V.; Mignogna, M.L.; Sirri, A.; Carrabino, S.; D′Elia, E.; Vecellio, M.; Russo, S.; Cogliati, F.; Larizza, L. Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly. Am. J. Hum. Genet. 2010, 86, 185–195.
- Mori, Y.; Matsui, T.; Omote, D.; Fukuda, M. Small GTPase Rab39A interacts with UACA and regulates the retinoic acid-induced neurite morphology of Neuro2A cells. Biochem. Biophys. Res. Commun. 2013, 435, 113–119.
- Corbier, C.; Sellier, C. C9ORF72 is a GDP/GTP exchange factor for Rab8 and Rab39 and regulates autophagy. Small GTPases 2017, 8, 181–186.
- Kohrs, F.E.; Daumann, I.-M.; Pavlovic, B.; Jin, E.J.; Kiral, F.R.; Lin, S.-C.; Port, F.; Wolfenberg, H.; Mathejczyk, T.F.; Linneweber, G.A. Systematic functional analysis of rab GTPases reveals limits of neuronal robustness to environmental challenges in flies. Elife 2021, 10, e59594.
- Weavers, H.; Prieto-Sánchez, S.; Grawe, F.; Garcia-López, A.; Artero, R.; Wilsch-Braeuninger, M.; Ruiz-Gómez, M.; Skaer, H.; Denholm, B. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 2009, 457, 322–326.
- Mauvezin, C.; Nagy, P.; Juhász, G.; Neufeld, T.P. Autophagosome–lysosome fusion is independent of V-ATPase-mediated acidification. Nat. Commun. 2015, 6, 7007.
- Maruzs, T.; Lőrincz, P.; Szatmári, Z.; Széplaki, S.; Sándor, Z.; Lakatos, Z.; Puska, G.; Juhász, G.; Sass, M. Retromer ensures the degradation of autophagic cargo by maintaining lysosome function in Drosophila. Traffic 2015, 16, 1088–1107.
- Lőrincz, P.; Lakatos, Z.; Varga, Á.; Maruzs, T.; Simon-Vecsei, Z.; Darula, Z.; Benkő, P.; Csordás, G.; Lippai, M.; Andó, I. MiniCORVET is a Vps8-containing early endosomal tether in Drosophila. Elife 2016, 5, e14226.
- Kim, S.; Wairkar, Y.P.; Daniels, R.W.; DiAntonio, A. The novel endosomal membrane protein Ema interacts with the class C Vps–HOPS complex to promote endosomal maturation. J. Cell Biol. 2010, 188, 717–734.
- Lőrincz, P.; Kenéz, L.A.; Tóth, S.; Kiss, V.; Varga, Á.; Csizmadia, T.; Simon-Vecsei, Z.; Juhász, G. Vps8 overexpression inhibits HOPS-dependent trafficking routes by outcompeting Vps41/Lt. Elife 2019, 8, e45631.
This entry is offline, you can click here to edit this entry!
