Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
MeHg is an environmental neurotoxin that can adversely affect the development of the nervous system. The molecular integrity of chromatin in the nucleus is an important target of MeHg. Low levels of MeHg trigger epigenetic mechanisms that may be involved in long-lasting and transgenerational neurotoxicity after exposure. Emerging evidence has shown that these mechanisms include histone modification, siRNA, and DNA methylation.
- methylmercury
- neurotoxicity
- transgenerational
1. Introduction
Methylmercury (MeHg) is an environmental neurotoxin that may cause long-lasting neurotoxicity in vulnerable populations, such as childbearing women and children [1]. MeHg is a persistent toxicant that is produced from microorganisms in the water system [2]. Environmental exposure to MeHg in the general population comes from eating fish that contain various levels of MeHg (0.02~0.55 ppm; US EPA guideline: 0.30 ppm) [3]. The developing nervous system is a preferential target of MeHg-induced toxicity [4]. The toxic mechanisms of MeHg involves the attachment of MeHg to thiol groups in biomolecules, forming various MeHg-SR complexes [2]. MeHg-SR complexes have lipophilic properties, which tend to partition into cellular lipid regions [5]. Indeed, the increase in partition coefficient after binding (even to cysteine) can explain the LAT1-independent entrance of MeHg in cells [5]. Exposure to this organic mercurial results in the distribution of the metal in the lysosome, mitochondria, and nucleus [6,7,8]. The mechanisms of MeHg-induced cytotoxicity are related to the disruption of homeostasis in a host of cellular physiological functions, including glutathione (GSH) depletion [9], calcium overload [10], loss of mitochondria membrane potential [11,12], and endoplasmic reticulum (ER) stress [13,14]. MeHg can readily cross the nuclear envelope to bind with chromatin components [15,16]. The process may not be involved in the acute toxicity of the metal; however, it could induce changes in the structure of chromatin, leading to long-lasting effects after exposure [17,18,19].
2. MeHg, DNA, and Chromatin
MeHg has a characteristic binding activity to the C- or N-containing moiety groups in the bases of DNA [20]. The physicochemical property of MeHg makes it very useful in the study of DNA structure. One of its compounds, methylmercury hydroxylate (MeHgOH), was used as a chemical probe to investigate DNA secondary structure and unpaired bases [21]. As it readily reacts with the purine and pyrimidine residues of nucleic acids, MeHgOH was also used as a reversible denaturing agent for DNA agarose gel electrophoresis [22]. The binding of MeHgOH to different bases in singular and duplex DNA was utilized for the detection and quantification of single-stranded DNA in duplex DNA samples [16]. These in vitro binding studies, which employed MeHgOH as the chemical species of MeHg, are in line with the genotoxic effects of MeHg [23,24,25,26,27]. The binding property to DNA can be changed with different ligands complexed to MeHg: for example, methylmercury chloride (MeHgCl), another widely used experimental chemical species. MeHgCl can interact with cysteine to form a major bioavailable form, MeHg-S-Cys [28]. The exchange of dissociable anions in MeHg complexes with thiol groups in other biomolecules dictates its molecular toxicity [2,29]. A cell culture study has shown that both MeHgOH and MeHgCl can cause cytotoxicity and genotoxicity, and MeHgCl is more toxic to SH-SY5Y cells than MeHgOH [30]. The differential toxic effects between MeHgOH and MeHgCl can be attributed to the stronger lipid partition coefficient of MeHgCl [5]; however, in a complex system in the cell, the genotoxic effects are probably mediated by their interaction with thiol-containing critical chromatin proteins (Figure 1) [2]. The integrity of DNA replication may be compromised after MeHg exposure, as corroborated in many studies on MeHg-induced genotoxicity [23,24,25,26,27]. These studies pave the way for the understanding of MeHg-induced genotoxic effects and its potential impact on the structure of nucleosomes and chromatin.
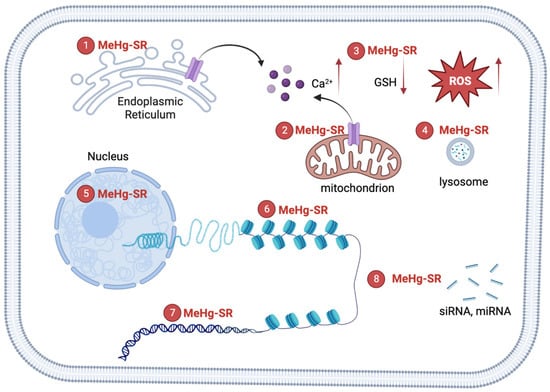
Figure 1. Mechanisms of MeHg-induced neurotoxicity. The formation of MeHg-SR complexes with endogenous thiol-containing biomolecules may increase its lipophilicity, resulting the distribution of the metal into hydrophobic compartments including mitochondria, lysosome, nucleus and other organelles. ① The complexation of MeHg with nascent proteins in the ER may cause ER stress. The black arrow (left): the flow of calcium ions from ER. ② MeHg-SR disrupts mitochondrial respiratory apparatus, leading to the elevation of reactive oxygen species (ROS). Multiples sources of Ca2+ contribute to MeHg-induced increase in intracellular Ca2+ [10]. The black arrow (right): the flow of calcium ions from mitochondrion. The red arrows (left): the increase of calcium ions; (middle): the decrease of GSH; (right): the increase of ROS. ③ The exchange of MeHg-SR with glutathione (GSH) results in reduction of GSH levels [9]. ④ The majority of MeHg-SR resides in lysosome, which corroborates the structural damage to biomolecules through attachment of the metal to thiol groups [8]. ⑤ MeHg-SR in the nucleus has the potential effects on the integrity of chromatin structures by complexing with histones or DNA [15,33,34]. ⑥ MeHg-RS changes histone post-translational modifications to affect the compactness of chromatin [15]. ⑦ A direct binging MeHg to bases of DNA constitutes the molecular basis for genotoxicity [20]. ⑧ MeHg-SR can interrupt biogenesis of siRNA and/or miRNA, leading to alterations in siRNA and/or miRNA-mediated gene regulations [40].
Although MeHg has a high affinity for sulfhydryl groups [31], its binding partners in the cellular system are also governed by a number of factors, such as exchange reactions and protein-specific structural and thermodynamical factors [2]. MeHg can bind with a variety of biomolecules in the nucleus, including DNA, histones, and non-histone protein components [15,32,33]. Likewise, many factors influence the propensity of the binding of MeHg to DNA bases, including the concentration of MeHg, temperature, base composition, ionic strength, and pH, to name a few [20]. A study in HeLa S3 suspension-culture cells has shown that the binding of MeHgCl to DNA and chromatin is a rapid process and could readily take place after the exposure [33]. A primary cell culture study in mouse fetal astrocyte has shown that MeHgCl exposure can competitively block the histone binding sites of the histone specific dye, N-(3-pyrene)maleimide, which specifically labels the cysteine groups in histone H3 of nucleosomes [15]. The blockage of the dye binding was gradually increased upon prolonged exposures in the in vitro model [15]. MeHg exposure also disrupts the complexing of histones with DNA. An in vitro study has shown that MeHgOH (1~10 μM) interferes with the binding of DNA by histones H3 and H4 [34]. A higher concentration of MeHgOH (10~32 μM) disrupted the complexing of DNA with the histones H2A and H2B [34]. The structure of chromatin plays an important role in gene regulation, which is predominantly regulated by histone proteins [35]. The post-translational modifications of histones, such as methylation and acetylation, regulates chromatin compactness, transcriptional activity, and genome functions [36]. Further, the regulation of gene expression at the transcriptional level involves epigenetic programs that are encoded by histone post-translational modifications, which determine the compactness of chromatin and transcriptional activity [37]. For example, increases in histone H3K4 methylation around the transcriptional start site (TSS) are associated with active transcription while high levels of H3K9 methylation at this region are associated with gene repression [38]. The regulatory mechanism during neuronal development and differentiation spatially and temporally invokes the modification of histones to fulfill gene regulation purposes [39]. Thus, the potential effects of MeHg on the post-translational modification of histones likely involves a direct binding of MeHg to the components of chromatin (particularly to thiol-containing proteins), leading to interference in chromatin compactness and structure. Though MeHg can interact with nucleophilic N-atoms found in nitrogenous bases forming the DNA nucleotides, the thiol-containing proteins found in the chromatin are possibly MeHg’s preferential targets. However, our knowledge on how MeHg disrupts the physiology and biochemistry of chromatin is still elusive. Recent experimental studies have shown that low levels of MeHg (nM) induce changes in histone post-translational modifications and DNA methylation, both of which may serve an important base for the long-lasting and transgenerational effects after the exposure [17,18].
This entry is adapted from the peer-reviewed paper 10.3390/toxics11010072
This entry is offline, you can click here to edit this entry!
