Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Cucurbitacins constitute a group of cucumber-derived dietary lipids, highly oxidized tetracyclic triterpenoids, with potential medical uses. These compounds are known to interact with a variety of recognized cellular targets to impede the growth of cancer cells.
- cucurbitacins
- anti-proliferation
- apoptotic
1. Apoptotic and Cell-Cycle Arrest
Apoptotic cell death can be triggered in cancer through internal and extrinsic processes, which converge on the control of caspase-dependent proteolysis of cellular proteins and DNA fragmentation [30,31,32]. Similarly, all tumor types have abnormal cell-cycle progression activity, which acts as a catalyst for carcinogenesis [33]. Recent research has shown that a variety of biological processes are regulated by cell-cycle proteins [34,35,36]. Therefore, numerous chemo-preventive FDA-drugs have been shown to mediate antitumor effects either via activation of apoptotic or cell-cycle arrest (Figure 2) signaling pathways [37,38,39]. For instance, results from Li et al. (2018) revealed that cucurbitacin I caused lung cancer (A549) cells to undergo excessive ERS, CHOP-Bax and caspase-12-dependent ERS-associated apoptosis [40]. In colorectal cancer (SW480 and Caco-2) cells, treatment with cucurbitacin B resulted in cell-cycle arrest at the G1 phase as well as decreased Cyclin D1 and Cyclin E1 levels. Both CRC cell lines underwent in vitro cell death when exposed to CuB, which was accompanied by caspase-3 and cleaved PARP [41]. Using triple negative breast cancer (TNBC), cucurbitacin E strongly boosted JNK activation while considerably decreasing AKT and ERK activation in MDA-MB-468 cells. It also significantly decreased expression of Cyclin D1, Survivin, XIAP, Bcl2 and Mcl-1 [42]. In the pancreatic cancer cell line Capan-1, CuD induced cell-cycle arrest and death via the ROS/p38 pathway [43]. Cucurbitacin I-induced cell death in ovarian cancer (SKOV3) included apoptosis, as evidenced by upregulated caspase 3 and BAX and a decrease in Bcl2 [21]. Flow cytometric measurement of DNA content and RT-PCR analyses suggested that cucurbitacin B caused G2/M arrest in human breast cancer cell lines (MDA-MB-231 and MCF-7) through elevated p21 expression [44]. Huang et al. showed that in human bladder cancer (T24) cells, cucurbitacin E-induced G2/M arrest was accompanied by a significant rise in p53 and p21 levels and a fall in the levels of STAT3, cyclin-dependent kinase 1 (CDK1) and cyclin B [45]. In addition, cucurbitacin E-induced G2/M phase arrest and death of T24 cells also depended on Fas/CD95 and mitochondria-dependent apoptotic pathways. Similarly, using other cancerous cell lines, cucurbitacins target the cell-cycle actions that involves growth inhibition, cell-cycle arrest at G2/M phase and induction of apoptosis [46]. Cucurbitacin I has been observed to suppress phosphotyrosine STAT3 in human cancerous lung cells [47]. Recently, it was observed to promote gastric cancer cell apoptosis by inducing the production of cellular ROS, as well as the endoplasmic reticulum stress pathway [40,48]. While cucurbitacin B, E and I have been observed to inhibit both JAK2 and STAT3 activation, cucurbitacin A and I have been reported to inhibit JAK2 and STAT3, respectively [47]. Treating Hep-2 cells with different concentrations of cucurbitacin B for various time intervals showed reduction in cell proliferation, cell-cycle distribution, and increased cell apoptosis in cancerous cell lines [46]. This study also stated that cucurbitacin B exhibited significant efficacy in inhibiting cell growth, arresting cell cycle at the G2/M phase, and inducing apoptosis in a dose- and time-dependent manner [46]. Similarly, cucurbitacins B, D, E were observed to inhibit proteins such as JAK-STAT3. They also inhibited mitogen-activated protein kinases (MAPK)- signaling pathways and tumor angiogenesis [48]. A study conducted on human umbilical vascular endothelial cell lines revealed cucurbitacin to significantly inhibit the proliferation, migration, and angiogenesis. It also blocked essential proteins such as Jak2-signal transducer, vascular endothelial growth factor receptor (VEGFR) and STAT3 signaling pathways [49]. Such studies have highlighted that the main mechanism involved in imparting the anti-tumorigenic potentials of cucurbitacins involves inhibition of the JAK/STAT3 signaling pathway, which plays an essential role in activation, proliferation, and maintenance of cancerous cells [14]. Another recent study has shown that treatment with 8 µM cucurbitacin IIb for 24 h remarkably inhibited the proliferation of HeLa and A549 tumor cells, with IC50 values of 7.3 and 7.8 µM, respectively, while increasing total apoptosis by 56.9 and 52.3%, respectively [50]. Another pathway by which cucurbitacin IIb induces apoptosis and cell-cycle arrest is by the regulating EGFR/MAPK pathway [51]. Similarly, cucurbitacin D was observed to regulate the levels of oncogenic signaling cascades, JAK/STAT, Wnt/β-catenin and associated non-coding RNAs in many cancer cell lines [52]. Recent studies have shown that CuIIb and cucurbitacin B induced apoptosis in cervical cancer cell lines by Nrf2 inhibition, whereas in lung cancer cell lines cucurbitacin B was responsible for suppressing growth and inducing apoptotic death by impeding IL-6/STAT3 signaling [21,53].
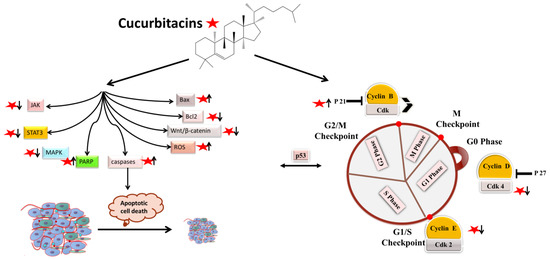
Figure 2. Molecular targets of cucurbitacins in modulating cell-cycle progression and inducing apoptotic cell death.
2. Antiangiogenic and Antimetastatic Mechanisms
The physiological process by which new blood vessels develop from pre-existing vessels is known as angiogenesis. Anti-angiogenesis causes suppression of tumor growth because of hunger and toxic waste buildup in its microenvironment [54,55]. The development and metastasis of the tumor have a major impact on the cancer vasculature (Figure 3). Vascular endothelial growth factors (VEGFs) are crucial protein regulators of angiogenesis and metastasis. Studies have shown that inhibiting the VEGFR2-mediated JAK/STAT3 pathway is considered as an effective approach to suppress angiogenesis [49]. Though many studies about the mechanism of cucurbitacins and angiogenesis are not well known, few studies have still shown that cucurbitacins such as cucurbitacin B, cucurbitacin D, cucurbitacin E and cucurbitacin I possess anti-angiogenesis properties [56,57]. CuB significantly inhibited angiogenesis, metastasis, and vascular development in dose-dependent manner in in vivo models and chick embryos [56]. CuE significantly inhibited human umbilical vascular endothelial cell (HUVEC) proliferation and angiogenesis by targeting the VEGFR2-mediated Jak2/STAT3 signaling pathway [49]. CuB has been observed to inhibit ERK1/2, prevent Raf-MEK-ERK from activating STAT3, which ultimately plays a key role in angiogenesis [58]. A similar effect of CuB was seen also in human breast cancer cell lines. It successfully inhibited angiogenesis by targeting the FAK/MMP-9 signaling axis [59]. CuB showed antimetastatic activity and targeted angiogenesis also in paclitaxel resistant A2780/Taxol ovarian cancer cells. It also suppressed angiogenesis by downregulating the expression of HIF-1 targets, VEGF, VEGFR2 phosphorylation and erythropoietin [56,60]. Another study revealed the effective use of CuE for anti-angiogenesis in Huh7 cells. It decreased the tube formation in HUVECs and was also responsible for inhibiting the process of neo-vascularization in CAM assays [61]. A recent study showed that CuE modulated the JAK/STAT3 pathways, which regulated the angiogenesis [62]. CuE has been also involved in inhibiting the KDR/VEGFR2-mediated pathway of angiogenesis [63]. Treating A549 cells with cucurbitacins for ~21 days showed positive results for inhibiting metastasis by regulating the levels of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D11 [64]. Similarly, other cucurbitacins were observed to inhibit angiogenesis in MDA-MB-231 and MCF-7 cancer cells by inhibiting the JAK/STAT pathways [65].
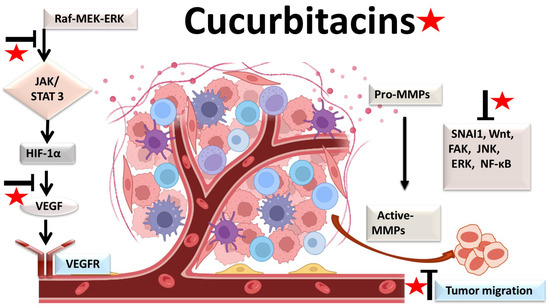
Figure 3. Molecular targets of cucurbitacins in suppressing angiogenesis and impeding cancer cell metastasis.
3. Anti-Inflammatory Mechanisms
Most malignancies’ growth and malignant progression are correlated with inflammation [66,67,68]. Both intrinsic and extrinsic inflammations have the potential to inhibit the immune system, which creates an ideal environment for the growth of tumors [69,70,71]. As a result, focusing on inflammation is a tempting strategy for both cancer therapy and cancer prevention [69,72]. Cucurbitacins have been observed to interact with proteins associated with inflammatory (Figure 4) pathways such as interleukins (IL)-6, IL-5, IL-1β, IL-12, IL-13 in a dose-dependent manner [73]. Dietary cucurbitacin E has been shown to reduce inflammation and immunosuppression by downregulating the NF-κB signaling pathway [74]. CuB has been studied to possess protective effects by reducing inflammatory responses on sepsis-induced acute lung injury in in vivo rat models. It significantly reduced the levels of TNF-α, IL-6, cytokine secretion and accumulation of inflammatory cells. It also regulated the levels of Ca2+, which play an essential role in inflammatory responses [75]. CuB inhibited inflammatory responses through targeting the SIRT1/IGFBPrP1/TGF β1 axis. It downregulated the expression levels of TGF β1, IGFBPrP1, and upregulated the expression of SIRT 1 [76]. Similarly, CuE decreased the levels of pro-inflammatory cytokines, such as IL-17 and IFN-γ, as well as the activities of the STAT3 and IL-17A-promoter in allo-reactive T cells [77]. CuE has been shown to inhibit skin inflammation and fibrosis by regulating the expression of α-Sma and Col-I in mice models [76]. Recently, it has also been demonstrated that CuE ameliorated lipopolysaccharide-evoked injuries and inflammation in bronchial epithelial cells by regulating the TLR4-NF-κB signaling. It was responsible for suppressing levels of inflammatory cytokine production, TNF-α, IL-6 and IL-8 [78]. Cucurbitacin B was observed to directly bind to toll-like receptor 4 (TLR4) and activate NLRP3 inflammasome, which further ultimately executed pyroptosis in A549 cells. CuB treatment has been observed to upregulate the protein expressions of IL-1β, GSDMD, HMGB1 and led to inhibition of generation of mitochondrial ROS and pyroptosis [79]. CuB was reported to sensitize CD133+ HepG2 cells in in vitro and in vivo models [80].
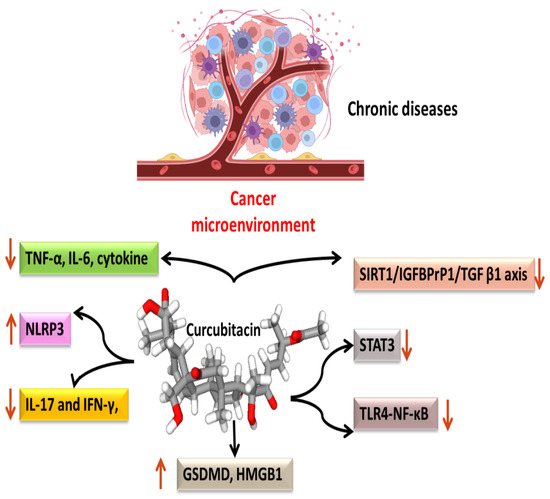
Figure 4. Anti-inflammatory targets of cucurbitacins in malignant cells.
This entry is adapted from the peer-reviewed paper 10.3390/biom13010057
This entry is offline, you can click here to edit this entry!
