Obesity is a complex multifactorial disease involving environmental factors, genetic predispositions, and human behaviors. Obesity has been commonly associated with muscle dysfunction and given the important metabolic roles and contribution to physical activity, maintaining muscle health is key to attenuation and prevention. A possible therapeutic strategy involves the implementation of a behavior intervention known as time-restricted feeding (TRF) which has garnered attention from the scientific community due to its associated benefits in metabolism and attenuation of obesity among others.
- obesity
- time-restricted feeding/feeding
- skeletal muscle disorders/aging
1. Current Knowledge Regarding the Benefits of Time-Restricted Feeding/Eating and Obesity
TRF (time-restricted feeding, a term used for animals) or TRE (time-restricted eating, a term used for humans) is an intervention that has gained attention in the scientific community due to its potential as a therapeutic alternative in attenuating obesity and metabolic disease [1]. The general principle of TRF/TRE involves a consolidation of daily caloric intake to the active hours or phases of the day with no alteration to total caloric consumption [2,3]. TRF’s known benefits include the attenuation of obesity-associated phenotypes [4] and improved metabolic function [5]. The beneficial effects have been attributed to a realignment of feeding time with the circadian clock and/or potentially through reduction of insulin resistance [2,3,6,7]. However, attention to the potential mechanisms behind TRF regarding skeletal muscle metabolism, structure, and function and its potential role in the management of obesity has just recently emerged.
Classically, obesity is defined as abnormal or excessive fat accumulation or an energy imbalance that impairs an individual’s health, usually accompanied by the deposition of lipids in non-adipose tissues, such as the liver, heart, and skeletal muscle [8,9]. As skeletal muscle (referred to as muscle) represents approximately 40% of total body mass in a healthy individual [10], it is quantitatively the most important site when considering the detrimental impacts of obesity on physical and metabolic activities. Known causes of obesity include aging, a lifestyle of chronic high-fat or calorie diets, genetic predisposition, and circadian disruption (Figure 1) [11,12,13,14]. The aged population continues to be a growing population with over two billion people in the world projected to be older than 60 by 2050 [15]. The prominence of the Western “American” diet, characterized by low consumption of fruits and vegetables and contrastingly high consumption of fat and sodium, are major contributors to obesity [16]. Crucially, genetic factors also play a role in determining people’s response to the obesogenic environment, as twin and family studies have estimated the BMI heritability to be >40% [17]. Circadian disruption has also emerged as a new concern due to an astounding number (16%) of American workers involved in non-daytime working hours [18] indicated by a recent meta-analysis to be positively associated with overweight risk [19,20,21,22,23].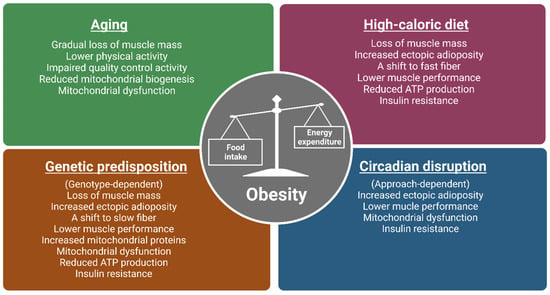
Figure 1. The impacts of various obesogenic challenges on skeletal muscle structure, function, and energy metabolism. Major causes of obesity include aging, a lifestyle of chronic high-calorie diets, genetic predisposition, and circadian disruption. Studies from human and animal models (mice and flies) show that the above-mentioned obesogenic challenges have common and distinct impacts on skeletal muscle structure, function, and metabolism. As muscle phenotypes are genotype-dependent in genetically obese models, only observations from ob/ob, M4RKO mutant mice, and Sk2 (obesogenic flies lacking sphingosine kinase 2; Sk2) mutant flies are included. Moreover, as circadian disruption can be induced by light, feeding patterns, and clock gene manipulation, muscle phenotypes can be approach dependent. Images created in Biorender.
Obesity is commonly associated with increased adiposity, leading to overall muscle-related dysfunction [24]. Further, obesity has been associated with detrimental impacts on the muscle’s metabolic role in maintaining insulin sensitivity, regulating ectopic lipid deposition, and regulating energy balance [9,24,25,26]. Interestingly, imposing TRE on obese or overweight adults with concurrent exercise has been found to help reduce fat mass and increase lean mass [27]. TRF also showed benefits in muscle performance, structure, and metabolism in Drosophila melanogaster under genetic and diet-induced obesity conditions [28]. Interestingly, a recent ongoing study in our lab using Drosophila supports the idea that various etiologies of obesity may lead to differences in mechanisms of TRF-induced benefits in muscle [29,30]. In light of this, this review aims to help distinguish between various causes of obesity and assess possible differences of mechanisms in TRF attenuation of muscle function to help address obesity effectively in a population inclusive of various metabolic backgrounds.
The development of obesity is complex and multiple different etiologies have been associated with obesity including poor diet quality, genetic predisposition, aging, and circadian disruption among other things [19,23]. Interestingly, differing mechanisms may underlie the progression of obesity unique to the metabolic challenge (e.g., diet, genetics, aging, or circadian rhythm disruption). For example, chronic lifestyles of high-fat diets can induce overconsumption due to differences in appetite suppression [22], while aging can lead to changes in hormonal production and favor sedentary lifestyles [21]. While differences between metabolic challenges are known at the whole body level, differences in mechanisms are now being explored in skeletal muscle function and metabolism. Another point of interest is whether a time-restricted intervention can lead to challenge-specific mechanisms in skeletal muscle modulation, which are protective against obesity.
Current obesity prevention and treatment strategies involve lifestyle changes including calorie restriction and prescription weight-loss medication [31]. These avenues of treatment, however, may come with limited long-term success or include the potential for harmful side effects [32] although some forms of caloric energy restriction have been met with modest success (5–10%) in short-term weight loss [33]. However, other individuals on caloric energy restriction regimens have shown levels of adherence to typically last 1–4 months [34] generally followed by significant weight gain within 1 year [35]. Additionally, anti-obesity drugs which suppress appetite or inhibit fat absorption come with potentially dangerous side effects such as increased cardiovascular and cancer risks among many others [36]. TRE may provide individuals with another viable form of therapy suitable for those unable to adhere to calorie restriction and concerned with the potential side effects of pharmacological therapy.
2. Aging Linkage to Obesity through Sarcopenia and Mitochondrial Dysfunction in Muscle
A key feature of aging is the gradual loss of total muscle mass and reduction in physical function, which are known characteristics of sarcopenia [37]. The underlying pathophysiology of sarcopenia has been described to be caused by age-related declines in anabolic hormone concentrations in serum such as testosterone, human growth hormone, and insulin-like growth factor-1 which contribute to muscle development, maintenance, and rejuvenation [38,39,40]. Sarcopenia can lead to lower levels of physical activity and impaired metabolism [41,42]. Further, sarcopenia-associated pathology can impact an individual ability to perform physical activities leading to less energy expenditure [43]. Reductions in energy expenditure lead to an energy imbalance and is a hallmark of obesity [44], suggesting that the pathophysiology of sarcopenia can have important implications related to the development of obesity. Also, with age, an accumulation of damaged cells and proteins can occur due to a loss of function in mechanisms involved in protein quality control including autophagy, proteasomal degradation, and chaperone-mediated folding [45]. This can lead to increased amounts of damaged mitochondria [46] and subsequently, impaired metabolism manifested by a decrease in resting metabolic rate and overall dysfunction in mitochondrial bioenergetics and lower ATP production [47]. In human studies, there is an observable loss of mitochondrial respiratory activity in aging human skeletal muscle in otherwise healthy men and women [48,49]. Protein levels of a mitochondrial biogenesis regulator, peroxisome proliferator-activated receptor gamma co-activator 1α (PGC-1α) were also found to be reduced which correlated with slower walking speed in healthy adults [50]. From our lab, a recent study measuring the effects of aging and other metabolic challenges observed that Drosophila muscle performance was drastically reduced supporting the idea that aging leads to a reduction of muscle physical ability [28]. Addressing the topics of proteostasis and mitochondrial dysfunction in skeletal muscle within the aging population may alleviate factors leading to sarcopenia and subsequent obesity.
3. Time-Restricted Dietary Regimens Attenuation of Aging through Regulation of Muscle Mass and Ectopic Lipid Deposition
TRE is a noteworthy therapeutic strategy in combatting age-related sarcopenia [51]. Protein intake is a primary anabolic stimulus [52] and anabolic response is relatively diminished in elderly individuals compared to young adults [52]. With the assumption that sufficient protein is included in meals, imposing TRE through consolidation of meals leading to larger protein boluses per meal could aid in meeting levels of optimal muscle stimulation for synthesis. While currently there is a lack of evidence in humans investigating the effects of TRE in muscle under aging, a study reported that TRE and concurrent exercise with a group of participants having a mean age of 44 found success in reducing fat mass and increasing lean mass in overweight and obese adults [27]. Contrastingly, a study measuring healthy younger adults found that pacing the intake of protein over a longer window throughout the day after exercise was observed to lead to greater protein synthesis rates [53]. Whether TRE conclusively increases muscle mass under aging remains to be explored however, it is to note that the latter study only measured outcomes in younger healthy adults. Another human study related to TRE in overweight sedentary adults over 65 years of age found a modest improvement in walking speed [54]. Also, a human study found that TRE also led to improvement in skeletal muscle insulin sensitivity and anabolic sensitivity in relatively young men [55]. Lastly, another study with overweight participants having a mean age of 38 demonstrated that TRE affected the periodicity of metabolites of amino acids [56]. With newly emerging studies, mechanisms related to time-restricted regimens and their effects on skeletal muscle and sarcopenic obesity is undeniably growing. Our lab has not evaluated changes in muscle mass under TRF in flies, however, in a recent study, our lab observed improved muscle performance in aged Drosophila under TRF [28]. Aged wild-type flies demonstrated a positive increase in muscle performance indicated by flight and climbing ability which mimicked the muscle performance of healthy controls [28]. Studies measuring the effects of TRF and TRE in aging are relatively new and further research is needed in ascertaining the effects of time-restricted regimens on muscle mass in elderly obese individuals [57].
As mentioned previously, mitochondrial dysfunction can occur due to a lack of removal of damaged mitochondria commonly observed in aging. This can lead to inefficient metabolism of lipids and glucose resulting in higher ectopic lipid deposition found in the muscle. From our study, we found that ectopic lipid deposition measured by lipid droplet area was observed to be significantly decreased under TRF in 3-week- aged flies in WT, HFD and a genetic obese mutant [28]. Overall reduction of ectopic lipid deposition can attenuate anabolic resistance [58] altering muscle synthesis and may suggest that time-restricted regimens can attenuate loss of muscle mass in aging [58].
4. Chronic High-Fat Diets and Obesity Are Linked through Metabolic Inflexibility and Insulin Resistance
Lifestyles consisting of chronic high-fat diets have been shown to lead to various negative effects on skeletal muscle mass and performance in addition to associated metabolic functions [12,59]. Under healthy conditions, mitochondria utilize glucose and lipids effectively to generate usable energy and maintain cellular homeostasis. However, under HFD conditions, metabolic inflexibility occurs as a result of nutrient overload leading to the inability to switch between sources of fuel effectively for energy production due to competing high levels of both carbohydrates and fatty acids [60,61,62]. Downstream consequences may lead to impaired energy production and accumulation of glucose and lipids [63]. To support this, a recent study found that HFD fed Drosophila after 4 days displayed a marked reduction in ATP levels and mitochondrial respiration [61]. This suggests that under HFD, inefficient energy production in mitochondria may stem from metabolic inflexibility. A study from our lab also corroborates this finding in which ATP levels were also found to be reduced in HFD-fed Drosophila [29,30]. Relating to mitochondrial impairment and ATP reduction, a study in humans under an HFD where biopsies of human muscle were collected showed the downregulation of genes involved in oxidative phosphorylation [64]. Oxidative phosphorylation is an essential pathway involving oxido–redox reactions that can lead to the production of ATP. As ATP is a currency needed by cells to undergo processes of metabolism and allows contraction of skeletal muscle in physical activity [65], management of energy metabolism may be essential in mediating HFD pathology in muscle and the development of obesity.
Chronic high-fat diets are also commonly associated with insulin resistance [66]. With increases in circulating fatty acids, higher amounts of diacylglycerol (DAG) can lead to the activation of protein kinase C (PKC) known to disrupt the insulin signaling pathway and result in insulin resistance [67]. Interestingly, our previous study demonstrated that an insulin resistance marker measured by gene expression of Neural Lazarillo (NLaz) [68] was increased under obesogenic conditions including HFD in Drosophila muscle [28]. As we have reported [28], studies have hypothesized that insulin resistance in humans is commonly linked with mitochondrial dysfunction implying the importance of maintaining mitochondria function [69,70]. It is to note, however, that other studies have shown contradictory results as to whether mitochondrial dysfunction is actually linked with insulin resistance and further study may be needed to establish this connection [71]. The increase in muscle fat content has been shown to correlate with loss in strength, mobility, metabolism and lead to insulin resistance [72,73]. A study conducted on mice also showed that mice exposed to long-term HFDs exhibited atrophy in different leg muscles of mice in conjunction with a fiber-type shift [12]. Interestingly, fiber type shifts are commonly observed in skeletal muscle in cases of obesity with the shift being towards fast fiber types; slow fiber types are inversely correlated with body fat levels due to differences in oxidative capacity [74]. Altogether, the mentioned studies indicate that within chronic HFD, there are multiple ways in which insulin sensitivity and metabolism are impaired in addition to muscle composition and mass. Interventions that address mitochondrial inflexibility and insulin resistance are promising for alleviating metabolic and muscle impairments found in HFD-induced obesity. Though a high-fat diet has been linked with metabolic inflexibility and insulin resistance, nutritional components and quality of diets such as excess carbohydrates and lack of protein may also lead to similar phenotypes observed in HFD.
5. Time-Restricted Dietary Regimens May Attenuate the Effects of Chronic High-Fat Diets through the Activation of Energy Metabolism, Decreased Triglyceride Synthesis, and Increased Protein Synthesis
TRF regimen studies in general regarding an HFD have shown positive benefits using animal models in metabolic function and muscle physiology [28,75,76,77,78] and also in humans [79]. For example, a study evaluating the effectiveness of TRF on various diet types including HFD using 12-week-old male wild-type mice demonstrated improvement in overall metabolic parameters including insulin sensitivity, body fat accumulation, inflammation, and weight gain [4].
Of note, a study in HFD mice demonstrated that induction of fasting cycles was effective in limiting mitochondrial damage induced by HFD [78]. In an ongoing study in our lab we found that in HFD under TRF in muscle, the purine cycle, a key energy metabolic pathway in muscle was upregulated, confirmed with metabolite analyses [29,30]. As the purine cycle/metabolism has been associated with maintaining ATP in muscle [80] activation of this pathway via TRF may provide a solution for meeting ATP requirements under metabolic inflexibility in HFD. The significance of increased ATP levels requires validation in future studies but suggests a potential mechanism in how TRF circumnavigates around metabolic inflexibility to bolster energy production. Additionally, our lab measured ATP levels which were reduced under HFD in ALF conditions and were increased under TRF [29,30]. Future studies are needed to validate the role of ATP in HFD improvement under time-restricted regimens and potentially incorporate multiple time points to assess if there are changes in the rhythmicity of available ATP. The involvement of the purine cycle/metabolism in HFD has also been observed by a previous finding where mice under HFD displayed dampened circadian rhythmicity mostly in purine catabolism [81]. This study was not muscle-specific but may suggest that purine cycles to an extent are interconnected with HFD and TRF intervention. Another study using mice (C57BL6/J) under a western diet found that TRF benefits included an extension of muscle performance, motor coordination, and glucose regulation [82]. Interestingly, this study also observed increased immune function (to sepsis) which could potentially corroborate the finding of increased activation of the purine cycle which plays a role in immune function.
In a mouse study, the rhythm of protein synthesis through mTORC1 and its associated amino acids was increased under TRF [4]. As HFD has been correlated with muscle atrophy, modulation of protein synthesis under TRF is noteworthy as a potential countermeasure to addressing HFD-related muscle loss [12]. Although direct measurements of muscle mass were not made, a study in our lab observed that TRF is beneficial to preserving muscle performance in HFD as seen in Drosophila [28]. HFD flies under ad libitum feeding had a significant reduction in muscle performance compared to WT flies while flies under TRF imposition displayed significantly improved muscle performance [28]. Further, TRF improved metabolic parameters including body weight, ectopic triglyceride levels, and insulin sensitivity, and prevented compromised integrity of muscle and mitochondria [28]. Further tests are needed to conclude whether improvement of muscle integrity and mitochondria leads to the improvement seen in metabolic parameters.
6. Thrifty Genes, a Potential Etiology of Genetic-Induced Obesity
In the past, humans and their predecessors had limited food security as food preservation techniques were not yet established. In order to survive under periods of food shortage, genetic adaptations protective against times of famine arose. Subsequently, this selection pressure may have led to the overrepresentation of genetic variants that promote rapid eating and excessive energy storage [84] coined as the thrifty gene hypothesis [85]. Thrust into the current era of abundant food availability, once historical evolutionary advantages, which allowed humans to thrive under limited food security, now led to a metabolic disadvantage in the modern scene.
Genetic factors play a crucial role in contributing to weight gain and obesity. A systematic review of BMI heritability from 140,525 twins and 42,968 family members estimated that genetic contributions to BMI were 0.47–0.90 from twin studies and 0.24–0.81 from family studies [17]. With the advent of candidate gene studies, genome-wide linkage studies (GWLS), and genome-wide association studies (GWAS), the discovery of candidate genes and loci associated with obesity risk and BMI has been significantly accelerated. To date, >250 loci in the human genome have been reported linked with BMI and obesity risk [86]. The most-studied gene mutations contributing to obesity are mainly located in the leptin/melanocortin pathway which controls appetite and metabolism and regulates energy balance and homeostasis. Within the leptin/melanocortin pathway, leptin is secreted by adipocytes, crosses the blood-brain barrier, and binds to leptin receptors in two subsets of neurons (NPY/AgRP and POMC/CART) in the hypothalamus. NPY/AgRP and POMC/CART control appetite and produce neuropeptides that activate the family of MCRs (MC1R to MC5R), which play crucial roles in energy balance and homeostasis. In addition, leptin can bind to its receptors on skeletal muscle and exert direct physiological effects on glucose and lipid metabolism [87].
7. Time-Restricted Dietary Regimen Benefits in A Genetic-Induced Obesity Model
TRF-mediated changes have been demonstrated in genetically obese Drosophila Sk2 mutant flies [28]. An intervention of 12 h active-phase TRF can improve muscle performance, suppress ectopic intramuscular fat deposits, attenuate mitochondrial aberrations, reduce markers of insulin resistance [28], and increase ATP levels in muscle [29,30]. Furthermore, time-series muscle transcriptome data have shown distinct upregulation of genes associated with AMPK-signaling pathways including glycolysis, glycogen metabolism, TCA, ETC in genetically obese Sk2 fly but not in the high-fat-diet-induced obese fly [29,30]. Rhythmic genes involved in glycolysis and glycogen metabolism have peaked expression levels at the end of the active phase [29,30]. Corroborating our observations, a study looking at MC4RKO obese mice also found significant improvement in substrate and energy metabolism [88] which may suggest modulations in mitochondrial function due to TRF. Previous studies suggest that TRF is beneficial in a limited amount of genetic obese models however, more studies are needed to evaluate TRF benefits in a broader range of genetic-induced obesity models.
8. Conclusion
This entry provides an overview of challenge specific benefits of TRF mainly in skeletal muscle function and metabolism, which play important roles in managing obesity. Studies have found that TRF under aging can lead to anabolic sensitivity, insulin sensitivity, and improved uptake of amino acids and that TRF can lead to larger protein intake due to consolidated feeding times allowing improved muscle protein synthesis. Additionally, from our own studies, we found that Drosophila displayed improved muscle performance and muscle integrity. TRF in HFD displayed improvements in muscle performance, insulin sensitivity, and reduced inflammation and our study found modulations in gene expression relating to the purine cycle, and levels of ATP in addition to improved muscle performance. Studies regarding genetic-induced obesity demonstrated possible mechanisms related to energy and lipid metabolism in addition to mitochondrial function and mitochondrial integrity. A recent study in our lab found that adenosine monophosphate kinase (AMPK) was upregulated in addition to downstream pathways such as TCA, glycogen metabolism, glycolysis, and ETC which was also supported by metabolite analyses [29,30]. As previously noted, obesity is a complex multifactorial disease that involves components related to environmental factors, genetic predispositions, and human behaviors. Obesity has been commonly associated with muscle dysfunction and given the important metabolic roles and contribution to physical activity, maintaining muscle health is key to attenuation and prevention. To fully address muscle dysfunction and exacerbation of obesity, common hallmarks within various metabolic challenges leading up to muscle dysfunction and obesity should be investigated to identify therapeutic targets for obesity caused by different challenges.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24010422
