Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Energy & Fuels
Hydrothermal carbonization converts high-moisture waste biomass into a valuable hydrochar unlocking multiple applications
- hydrothermal carbonization
- hydrochar
- biomass
- fuel
- Adsorbent
- Energy Storage
1. Solid Fuel and Combustion Properties of Hydrochar
Table 1 shows the fuel and combustion properties of select waste biomass-derived hydrochars at different HTC conditions. The HTC conditions are varied from 180–300 °C, 5 min–24 h, and 1:5–1:25 (solid: water w/w ratio) to synthesize energy-dense fuel [6,30,31]. The 0.5 h reaction time is reported as sufficient since the greatest extent of HTC reaction occurs within that specific period of time [32]. The elemental hydrogen-to-carbon ratio (H/C) and elemental oxygen-to-carbon ratio (O/C) of raw feedstocks vary between 0.12–2.11 and 0.73–0.88 for lignocellulose, and 0.12–2.10 and 0.38–0.90 for non-lignocellulose, respectively. It is observed that the H/C and O/C ratios for hydrochars vary between 0.80–1.58 and 0.19–0.75 for lignocellulose, 0.94–1.77 and 0.15–0.81 for non-lignocellulose, and 0.82–1.54 and 0.12–0.72 for combined lignocellulose:non-lignocellulose, respectively. The HTC decreases the H/C and O/C ratios for both lignocellulosic and non-lignocellulosic hydrochars; however, the reduction is more prominent for the combined lignocellulosic:non-lignocellulosic hydrochars. This is expected since HTC for a single feedstock creates an acidic environment through dehydration (formation of H2O) and decarboxylation reactions (formation of CO2 or carbonyl or carboxylic acids) [33,34,35,36] and thereby, the H/C and O/C ratios drop. However, in case of combined lignocellulose:non-lignocellulose HTC (also known as Co-HTC), one feedstock can act as a nucleation site for another, as found by Saba et al. [37] for coal–miscanthus blend HTC, which further augments condensation and polymerization of carbon-dense organics from process liquid to the hydrochar’s surface [36,38]. Xu et al. [39], on the other hand, showed that in the case of Co-HTC of polyvynyl chloride (PVC) and cotton textile waste, the surface –OH and C=O groups of hydrochar propagate C-Cl bonds and promote HTC through dehydration/aromatization reactions. In response, the solid hydrochar shows relatively higher carbon composition than hydrogen or oxygen. It was also observed that increasing HTC temperature increases the O/C and H/C ratios more, since the dehydration and decarboxylation reactions improve at a higher extent [6]. The lower H/C ratio also indicates higher aromatic compounds in hydrochar [40]. As suggested by the Van Krevelen diagram [41,42], the fuel quality of any solid is better if the fuel consists of lower H/C and O/C ratios. For example, walnut shell showed the lowest H/C ratio while tobacco stalk showed the lowest O/C ratio suggesting higher dehydration/demethylation reactions and decarboxylation/dehydration reactions, respectively [37]. In case of non-lignocellulose, the waste textile showed the lowest H/C while straw powder showed the lowest O/C. The HHVs reported in Table 1 also support the trend of O/C and H/C ratios for all types of feedstocks. For example, the HHV shows the lignocellulosic, non-lignocellulosic, and combined lignocellulosic:non-lignocellulosic hydrochars culminate to as high as 28.2, 27.0, and 27.6 MJ/kg, respectively, approaching the fuel quality of coal [43]. Orange peel shows the highest heating value, while sewage sludge shows the poorest heating value [44,45]. This is expected since sewage sludge experiences significant accumulation of ash at the expense of high volatiles after HTC, whereas orange peel experiences significant volatile loss which compliments to a significant increase in fixed carbon [44,45]. Since ash is an inorganic substance, it does not burn but, in fact, reduces the burning capacity, resulting in an overall drop in HHV [46].
The fuel ratio in Table 1 varied between 0.18–1.72 for lignocellulose, 0.09–1.03 for non-lignocellulose, and 0.22–1.22 for combined lignocellulose:non-lignocellulose. This gives an indication on the relative combustion behavior of the biomass. Table 1 shows that the ignition and burnout temperatures for lignocellulose reach as high as 371.3 °C and 750 °C, 388.8 and 832.9 °C for non-lignocellulose, and 354.9 °C and 585.3 °C for combined lignocellulose:non-lignocellulose, respectively. However, the raw feedstocks show 287.1 °C and 329.9 °C for lignocellulose and non-lignocellulose, respectively. In case of the combustibility index, the lignocellulose shows 12.3; however, the non-lignocellulose shows as high as 337. For Co-HTC, it shows 9.3 as the maximum combustibility index. It has been found that volatiles in the biomass first convert into pyrolysis gas as soon as those get exposed to the heat flux, which then react with surrounding oxygen and this phenomenon continues as the surface gets exposed more and more to the heat flux [47]. Liu et al. [48] also supported that lower volatiles in hydrochars make those more stable (supported by lower ignition and burnout temperature), and therefore, they show a less vigorous reaction (as the lower combustion index suggests in Table 1) than raw biomass. Lower volatiles also suggest less of a fire hazard risk than raw biomass [48,49]. In the case of Co-HTC, it is possible that both feedstocks show a synergistic effect on synthesizing stable fixed carbon that augments the thermal stability of the hydrochars. As an example (in Table 1), orange peel shows higher thermal stability than any other lignocellulose, whereas corn stover shows a longer burning time with a higher burnout temperature. Orange peel also shows >44% fixed carbon [45]; however, corn stover shows up to 32% only [50]. However, in the case of Co-HTC, Lin et al. [51] showed that increasing the composition of waste wood, waste paper, and waste food relative to waste textiles decreases the ignition temperature but increases the burnout temperature, resulting in an increased thermal stability and combustion, possibly due to increasing fuel ratio and ash composition. Overall, it has been found that the investigation of Co-HTC has increased by 3.6 times from 2017 to 2021 [29], which could be an emerging future study in upcoming years.
Table 1. Fuel and combustion properties of hydrochar.
| Feedstock | Reaction Temperature; Time; Solid:Water Ratio | H/C; O/C | Fuel Ratio | HHV (MJ/kg) | Ignition Temperature, Ti (°C) |
Burnout Temperature, Tb (°C) |
Combustibility Index, Si × 10−7 (%2/ °C3.min2) |
References |
|---|---|---|---|---|---|---|---|---|
| Walnut shell | 180–300 °C; 1–6 h; 1:6–1:5–1:10 | 0.12–1.51 *; 0.73–0.84 * 0.89–1.58; 0.26–0.75 |
0.33 * 1.58–1.72 |
12.7–18.9 * 12.7–28.0 |
[52,53] | |||
| Peanut shell | 220 °C; 12 h; 1:7.5 | 1.58 *; 0.75 * 0.99; 0.27 |
0.36 * 0.76 |
12.6 * 28.1 |
249.7 * 362.7 |
485.8 * 550.9 |
2.7 * 1.1 |
[45] |
| Orange peel | 220 °C; 12 h; 1:7.5 | 1.76 *; 0.86 * 1.02; 0.27 |
0.23 * 0.83 |
12.1 * 28.2 |
197.0 * 371.3 |
492.9 * 539.9 |
3.4 * 1.3 |
[45] |
| Rice straw | 220 °C; 12 h; 1:7.5 | 1.74 *; 0.86 * 1.11; 0.28 |
0.22 * 0.22 |
16.9 * 20.6 |
243.6 * 369.1 |
461.9 * 524.9 |
3.1 * 0.8 |
[45] |
| Corn stover | 200–260 °C;0.5–1 h; 1:7.5–1:10 | 1.40 *; 0.83 * 1.30–0.80; 0.68–0.35 |
0.22 * 0.18–0.67 |
16.8–22.4 * 19.2–22.8 |
198.8–275.0 * 228.9–300.0 |
492.7–535.0 * 603.2–750.0 |
6.90–12.3 * 1.20–6.6 |
[50,54] |
| Corn stalk | 190–240 °C; 0.5–10 h; 1:6–1:10 | 1.63–1.72 *; 0.75–0.80 * 1.10–1.38; 0.32–0.55 |
0.09 * 0.33 |
17.2–17.6 * 19.7–24.7 |
248 * 295–309 |
536 * 561–629 |
6.24 * 1.71–8.0 |
[55,56] |
| Grape marc | 180–260 °C; 0.5–8 h; 1:3–1:7.5 | - 1.36–1.50; 0.53–0.60 |
20.6–21.6 * 20.9–26.3 |
215.3 * 233.5 |
517.7 * 531.0 |
4.5 * 4.2 |
[54,57,58] | |
| Straw powder | 240 °C; 1.5 h; 1:25 | 1.51 *; 0.61 * 1.17; 0.36 |
17.5 * 22.2 |
[59] | ||||
| Wood | 180–260 °C; 0.5–1.5 h; 1:10–1:12 | 0.12–2.11 *; 0.73–0.88 * 0.95–1.35; 0.36–0.58 |
0.24–0.40 * 0.27–1.53 |
18.0–19.0 * 19.5–24.0 |
279.0 * 278.9 |
617.0 * 636.5 |
[9,51] | |
| Tobacco stalk | 180–260 °C; 2–12 h; 1:20 | 1.57 *; 0.70 * 1.51–0.97; 0.19–0.68 |
0.20 * 0.21–1.15 |
18.8 * 18.7–27.2 |
287.1 * 294.4–342.8 |
541.0 * 529.4–558.5 |
[60] | |
| Saw dust | 220 °C; 10 h; 1:6 | 1.67 *; 0.75 * 1.25; 0.42 |
0.08 * 0.33 |
18.7 * 24.4 |
[56] | |||
| Sewage sludge | 180–280 °C; 2–12 h; 1:7.5–1:9 | 2.07 *; 0.52 * 1.77; 0.36 |
0.12 * 0.22 |
9.3 * 6.0 |
212.1–228.3 * 193.6–243.5 |
499.5–839.1 * 408.2–832.9 |
0.4–337 * 0.1–131 |
[44,45] |
| Urban waste/yard waste | 160–260 °C; 2–24 h; 1:10 | 1.46 *; 0.84 * 1.02–1.37; 0.40–0.81 |
15.4 * 15.7–24.6 |
272.3 * 312.1–320.5 |
[61] | |||
| Polyvinyl chloride | 240 °C; 1.5 h; 1:25 | - 1.05; 0.15 |
22.5 * 25.9 |
[59] | ||||
| Cow manure | 180–260 °C; 0.083–1 h; 1:5 | 1.28 *; 0.58 * 1.10–1.22; 0.32–0.55 |
0.13 * 0.11–0.28 |
16.7–19.1 * 18.8–22.1 |
- 179.6 |
- 588.8 |
- 7.8 |
[54] |
| Sweet potato waste | 180–300 °C;0.5 h; 1:5 | 1.45 *; 0.53 * 0.94–1.35; 0.22–0.48 |
0.26 * 0.33–1.03 |
21.2 * 21.7–27.0 |
316.9 * 309.0–388.8 |
529.4 * 524.7–550.2 |
9.9 * 3.82–9.90 |
[62] |
| Paper sludge | 180–240 °C;0–2 h; 1:1 | 1.80 *; 0.68 * 1.60–1.40; 0.65–0.48 |
0.06 * 0.09–0.14 |
[63] | ||||
| Swine manure | 220 °C; 10 h; 1:6 | 1.85 *; 0.57 * 1.46; 0.26 |
0.01 * 0.15 |
18.1 * 23.1 |
[56] | |||
| Waste textile | 240 °C; 1.5 h; 1:12 | 0.12 *; 0.77 * - |
0.20 * 0.29 |
19.2 * - |
329.9 * 332.3 |
578.1 * 587.0 |
[51] | |
| Waste paper | 240 °C; 1.5 h; 1:12 | 2.10 *; 0.90 * - |
0.16 * 0.53 |
14.3 * - |
314.2 * 332.8 |
760.5 * 783.5 |
[51] | |
| Waste food | 240 °C; 1.5 h; 1:12 | 0.19–0.72 * - |
0.18 * 0.94 |
21.3 * - |
288.1 * 210.4 |
716.1 * 719.2 |
[51] | |
| Polyurethane | 200–260 °C; 0.5 h; 1:10 | 1.35 *; 0.38 * - |
0.03 * - |
34.1 * - |
315.0 - |
712.0 * - |
6.90 * - |
[50] |
| Straw powder and polyvinyl chloride | 240 °C; 0–2 h; 1:25 | - 1.01–1.27; 0.12–0.21 |
- 24.5–27.6 |
[59] | ||||
| Corn stover and cow manure (3:1–1:3) | 220 °C; 1 h; 1:5 | - 19.5–21.2 |
- 178.2–196.2 |
- 480.7–530.5 |
- 8.7–9.3 |
[54] | ||
| Grape marc and cow manure (3:1–1:3) | 220 °C; 1 h; 1:5 | - 21.1–23.1 |
- 200.9–354.9 |
- 492.7–585.3 |
- 4.7–7.6 |
[54] | ||
| Sewage sludge + Rice straw (3:1–1:3) | 220 °C; 12 h; 1:7.5 | - 1.17–1.49; 0.35–0.36 |
- 0.29–0.55 |
- 8.8–16.8 |
- 269.3–336.7 |
- 461.4–525.2 |
- 0.2–0.3 |
[45] |
| Sewage sludge + Orange peel (3:1–1:3) | 220 °C; 12 h; 1:7.5 | - 1.15–1.54; 0.21–0.29 |
- 0.34–0.63 |
- 10.1–21.0 |
- 266.2–338.8 |
- 479.2–508.3 |
- 0.2–0.6 |
[45] |
| Sewage sludge + Peanut shell (3:1–1:3) | 220 °C; 12 h; 1:7.5 | - 1.13–1.51; 0.28–0.34 |
- 0.39–0.79 |
- 10.4–21.7 |
- 276.2–349.2 |
- 467.5–525.2 |
- 0.3–0.6 |
[45] |
| Swine manure + sawdust (3:1–1:3) |
220 °C; 10 h; 1:6 | - 1.28–1.41; 0.31–0.37 |
- 0.24–0.36 |
- 23.2–24.1 |
[56] | |||
| Swine manure + corn stalk (3:1–1:3) |
220 °C; 10 h; 1:6 | - 1.30–1.45; 0.28–0.36 |
- 0.25–0.33 |
- 23.6–24.2 |
[56] | |||
| Waste textile + waste wood (3:1–1:3) | 240 °C; 1.5 h; 1:12 | - 0.90–1.20; 0.30–0.35 |
- 0.60–1.22 |
- 22.3–25.1 |
[51] | |||
| Waste textile + waste paper (3:1–1:3) | 240 °C; 1.5 h; 1:12 | - 0.95–1.20; 0.30–0.36 |
- 0.39–0.56 |
- 22.7–25.5 |
[51] | |||
| Waste textile + waste food (3:1–1:3) |
240 °C; 1.5 h; 1:12 | - 1.00–1.40; 0.20–0.40 |
- 0.54–0.85 |
- 22.0–26.9 |
[51] | |||
| Corn stover + polyurethane (5.7:1–19.0:1) | 200–260 °C; 0.5 h; 1:10 | - 0.82–1.38; 0.36–0.72 |
- 0.22–0.64 |
- 18.1–22.5 |
- 325.0 |
- 626.0–662.0 |
- 2.03–4.60 |
[50] |
* indicates values for raw.
2. Water Purification Using Hydrochar and Modified Hydrochar
Figure 2 shows an overview of hydrochar in the application of dye, heavy metal, and toxin removal from water. Therefore, a review of recent advances on these applications is warranted and further discussed below.
Figure 2. Contaminant removal from water by hydrochar.
3. Greenhouse Gas Adsorption by Activated Hydrochar
Figure 3 shows the application of hydrochar for greenhouse gas adsorption. Incessant anthropogenic emission of greenhouse gas (GHG), primarily constituted by carbon dioxide and methane, has resulted in global climate change with the grave consequence of threatening species extinction. This has motivated the scientific community to develop inexpensive adsorbents, to reduce the levels of CH4 and CO2 with capture technologies, based on sustainable biomass sources. Hence, in the process of developing functional adsorbents, hydrochars have successfully provided an avenue, as activation of such hydrochars have resulted in superior porosity and functionality, owing to the excellent capability of hydrochars to offer pore initiation while having a surface rich in surface functionalities. Pioneered by Fuertes and Sevilla [118], chemical activation of hydrochars resulted in a higher surface area than their conventionally activated counterparts, while hydrochars were demonstrated to be highly functional with a large amount of oxygen-containing groups [119]. Hence, the favorable characteristics of deriving porous activated hydrochars to be employed in GHG emission is extensively reviewed in the following subsections.
Figure 3. Greenhouse gas adsorption by porous hydrochar.
4. Hydrochar as Catalyst Support
Research has highlighted the potential use of a biomass-derived carbon-catalyst reaction where the carbon can be synthesized via HTC technique, resulting in hydrochar with active polar oxygenated functionalities [156]. Hydrochars are hence observed to be employed as a catalyst support in the heterogeneous catalysts. However, for its role as an acidic catalyst, the key step to improve the acidic sites on carbon is achieved when the carbon is treated with a chemical agent, such as H3PO4, H2SO4, HCl, or HClSO3 to improve its acidic sites [157,158]. Konwar et al. [159] shed light on the desired characteristics of a solid-acid catalyst which comprise high concentration of acidic sites, stability, and resistance to variations in pressures and temperatures. Hydrochar-derived carbon materials belong to a small group that have at least two of those traits, as is the case for sulfonic groups (–SO3H) functionalized hydrochar catalysts [160]. Sulfonated amorphous carbon, where the structure consists of -SO3H groups, presents much higher catalytic activity than other solid acid catalysts, providing feasibility in applications, such as biodiesel production [161]. Similarly, sulfonated catalysts derived via HTC have also been utilized for isomerization and hydrolysis.
In the case of sulfonated catalysts, surface porosity, including surface area, influences the catalytic activity as it indicates the number of active sites for an active group to anchor onto the carbon structure [162]. From the study [163], they found a large quantity of the SO3H group were able to be incorporated into the carbon lattice due to the meso- and macroporous structure of the catalyst. Although the sulfonation process decreases pore volume and pore size from 0.57 cm3/g to 0.51 cm3/g and 53–44 nm, respectively, higher than normal sulfonated glucose acid density can be obtained due to the bimodal porosity of the catalyst [164]. The porosity of the carbon material plays a significant role in anchoring the sulfonic group, thus affecting the catalytic activity of the sulfonated catalyst [165]. Therefore, in order to produce an excellent catalyst, the porosity of the carbon catalyst must not be taken for granted as it has a momentous impact on the catalytic performance. On the other hand, the stability of such catalysts can be controlled by tuning the carbon particle size of the catalyst. Decreasing the carbon particle size of the catalyst could increase the effectiveness of the sulfonation process and stability by improving contact between carbon particle surfaces with H2SO4 [165].
Apart from the sulfonated catalysts, carbonaceous catalysts consisting of transition metals (Cu, Co, Ni, Fe, etc.) and N coordination sites (N-doping) have also attracted attention as a substitute for Pt catalysts in the oxygen reduction reaction (ORR), oxidation, and desulfurization process. The utilization of Fe is advantageous, as standalone hydrochars show poor catalytic activity, whereas carbon-based materials prepared with FeCl3 show the highest catalytic activity [166]. Hydrochar’s applicability as a support for such heterogenous catalysts is also attributed to its enhanced metal dispersion ability, and the mutual reaction of metal with the carbon owing to its various surface oxygen-containing functionalities like hydroxyl, carboxyl, carbonyl, and lactone [167]. Such functionalities help enhance access of metal solutions into the hydrochar’s carbonaceous matrix as well as providing anchorage sites for the metal that can act as the active centers while functioning as catalysts [168]. In addition, hydrochar’s hydrophobicity provides excellent stability while confirming uniform metal dispersion, as observed by Ge at al. [169]. Chen et al. [170] derived thiourea-Fe(NO3)3-activated hydrochar and found that it offered a large surface area, pore volume, and other favorable surface functionalities (N and S functional groups) which improve complexation and electrostatic interaction for effective As(V) removal. Ma et al. [171] demonstrated that lignin-derived Fe-hydrochar could completely degrade phenol via its catalytic oxidation and exhibited stability upon three runs of regeneration. Moreover, as the hydrochar surface presents a higher degree of aromatization with plentiful oxygen-containing groups, it was conclusive to favor adsorption and degradation of organic pollutants [172]. For example, Dang et al. [173] correlated a lower HTC temperature to result in more abundant oxygen-containing functional groups. This increase of oxygen-containing functional groups generates more free radicals due to the presence of increased reactive–active moieties (RAMs) that could enhance DDT adsorption by providing more react sites for DDT adsorption. Another non-noble based catalyst is nickel-based, which is used for dry reforming of methane. Here, the hydrochar support is advantageous because of the core-shell structure that limits active metal sintering and hence, prevents carbon deposition. Han et al. [174] utilized sugarcane bagasse and revealed that nickel-based catalysts have excellent CH4 and CO2 conversions at 80.3% and 90.3%, respectively, at 850 °C. On the other hand, heteroatom doping, particularly by nitrogen, has the ability to create new defects, resulting in more active sites in the carbon matrix. This consequently enhance electrocatalytic activity during cases of ORR and oxygen evolution reaction (OER) [175,176]. Specifically, N-doping results in the transfer of a charge from carbon atoms to adjacent nitrogen atoms that can lead to redistribution of the electronic and spin culture of the sp2-hybridized carbon framework [177,178,179]. For example, Yu et al. [180] mediated nitrogen atoms in hydrochar that enhanced catalytic degradation of bisphenol A, bisphenol F, estrone, and 17β-estradiol where a higher surface area of hydrochar and graphitic-N species proved to be favorable. Similarly, the benefit of N-doping was demonstrated by Qiao et al. [181] where N-doping in the amorphous carbon structures could enhance ORR activity by creating nitrogenated active sites which promote electron transfer and also aid in protecting the Fe-derived active sites from catalyst poisoning by the byproducts of ORR reactions.
Another category of hydrochar-based catalyst being developed is composite photocatalysts where high charge transfer efficiency, high photocatalytic reaction, and excellent stability can be attained [182]. This is because the carbonaceous char can serve as an excellent electron bridge due to its large aromatic skeleton, abundant functional groups, good electronic conductivity, and environmental benignity [183]. The added bonus of hydrochar is the extra oxygen-containing functional groups, as mentioned before, which can act as an electron shuttle in photocatalysts that promote electron transfer while generating a greater number of oxygen reactive species by reacting with the hydration layer that promotes interfacial catalytic reaction. For example, Li et al. [182] concluded excellent photocatalytic activity in carbamazepine removal by microsphere-like hydrochar-based photocatalyst derived from glucose. Another type of photocatalyst is carbon dots (CD) produced via hydrothermal methods, which is beyond the scope of this manuscript.
In summary, as hydrochar catalyst support emerges and demonstrates versatile application in various fields, there is an opportunity of investigating tuning hydrochar’s particle size, optimizing transition metal/dopant concentration, and doping of other heteroatoms to assess the effects on surface porosity (SSA, total pore volume and pore size) and functionality, which have been successfully demonstrated to be vital in catalytic activity. It is also imperative to undermine the catalysis mechanism, to identify and reveal the role of active centers, for the applications observed in order to further improve the characteristics of the heterogenous catalyst based on hydrochars by modifying the key constituent (active center) and develop reaction models.
5. Electrochemical Applications of Hydrochar
Figure 4 shows an overview of hydrochar’s application as supercapacitor. As the dependency on energy surges, the importance to resort to renewable resources to meet these demands becomes absolute [201]. Fortunately, researchers have entertained studies demonstrating hydrochar as a high value material for electrochemical devices such as supercapacitors and batteries [202]. Both supercapacitors and batteries serve the purpose in providing energy with supercapacitors providing high power density and batteries supplying high energy density [201].
Figure 4. Hydrochar as supercapacitor.
Figure 5 shows a three-dimensional (3D) plot of BET-specific surface area and the pore size of hydrochar for supercapacitors versus the capacitance from recent articles. The 3D plot showed a positive influence of surface porosity of hydrochar as high surface area and micro-meso pore size (<2 nm, 2–50 nm) results in high electron storage. The driving force for excellent supercapacitor performance is owed to the presence of hierarchical porous carbon (micro/meso/macro) which support ionic mass transport, thus maximizing capacitance [203,204]. Fan et al. [205] investigated HTC and activation of alginic acid stable porous hollow carbon spheres (PHCSs). They observed well-balanced micro- and mesoporosity capable of maximum specific capacitance of 314 F/g and excellent electrochemical stability (5000 cycles) [205] (Figure 5). The study ascribed the superior performance to the high specific surface area (2421 m2/g) and large pore volume (1.61 cm3/g) [205]. Similarly, Li et al. [206] presented functional porous carbon derived from waste eucalyptus bark synthesized by hydrothermal treatment combined with KOH activation for toluene adsorption and aqueous symmetric supercapacitors. The findings showed that maximum specific capacitance of 263.2 F/g was attainable with excellent capacitance retention after 10,000 cycles along with high energy and power densities of 10.5 Wh kg−1 and 159.5 W kg−1, respectively [206] (Figure 5).
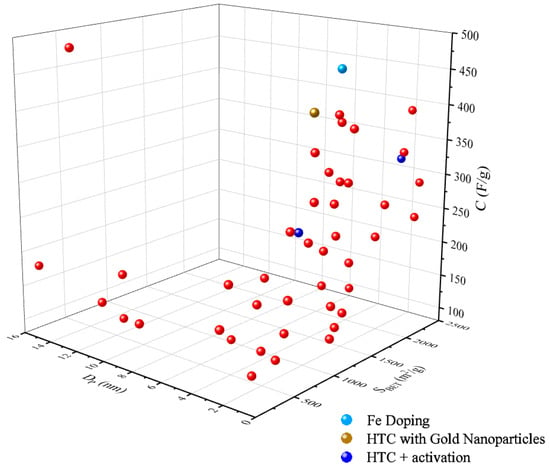
Figure 5. Distribution of the specific capacitance, C (F/g), of hydrochar for supercapacitor application with correlation to the pore size, Dp (nm), and BET-specific surface area, SBET (m2/g).
Recent studies have shown that improvement in the specific capacitance of hydrochar can be introduced by heteroatom doping the hydrochar to add pseudocapacitance [207,208]. Xu et al. [209] demonstrated the use of Fe-decorated porous carbon derived from bamboo synthesized via HTC with Fe-doping followed by mild chemical activation with KHCO3 for supercapacitor application. The study shows excellent electrochemical performance with maximum specific capacitance of 467 F/g with superb cyclic performance after 5000 cycles and maximum energy density of 20.31 W h kg−1 [209] (Figure 5). The performance was attributed to an extra pseudocapacitance supplied from the Fe oxides, sufficient specific surface area (1509.5 m2/g) with high electric conductivity and the carbon sphere architecture which facilitated better ion/electrolyte diffusion transportation [209]. Similarly, Esteban et al. [210] prepared tunable supercapacitor material via hydrothermal polymerization of glucose-stabilized gold nanoparticles displaying maximum specific capacitance of 436 F/g and energy and power densities of 7.2 Wh kg−1 and 24.9 W kg−1, respectively (Figure 5). The performance was due to the synergistic effect of the pore structure after the Au nanoparticles were melted onto the hydrochar along with the well dispersed presence of the Au within the material [210]. Moreover, hydrochar has proved promising as electrode material for electrochemical devices.
2.6. Role of Hydrochar as Soil Amendment and Carbon Sequestration
Figure 6 shows an overview of the application of hydrochar as soil amendment and carbon sequestration. Hydrochar can be considered as environment friendly soil amendment as it slowly releases the nutrient and sequester carbon. Hydrochar showed promising prospects of water and nutrient storage capacity, which enhances the microbial activity and hence, improves the soil fertility. As a result, the plant growth was positively affected due to applying the hydrochar in the soil.
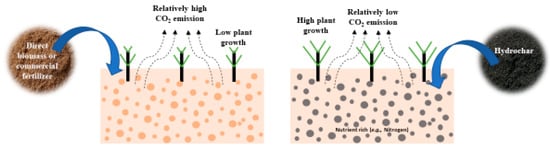
Figure 6. Hydrochar as soil amendment and carbon sequestration application.
Soil amendment on a global scale is becoming an important issue. Applying various ingredients, such as composts, mulches, manure, and organic fertilizer, could enhance soil fertility. However, only a small fraction of these organic compounds may be stabilized for the long term in soil depending on the climatic conditions [211]. On the other hand, hydrochar which contains more stable carbon can be used to promote nutrient acquisition and modify the chemical environment of soil [212,213]. It is also proven that the nitrogen present in hydrochar is not immobilized; rather, it is slowly released over time [212,214,215,216,217,218]. So, hydrochar can be used as soil amendment while slow-release nitrogen fertilizer is required. As the hydrochar is slow release and the organic compounds are fast release, the blends of hydrochar and organic compounds (e.g., animal manure, crop residues) may increase nutrients’ availability to the plant [219]. Hydrochar can be a potential source of not only carbon and nitrogen but also humic-like substances which are important for plant growth. For example, Bento et al. [220] found that applying bagasse-derived hydrochar significantly promotes maize seed germination due to the presence of amphiphilic moieties. Now, the question is: how does hydrochar improve the soil properties?
Due to having versatile physiochemical and morphological properties, hydrochar could be a promising soil amendment substance. For instance, adding hydrochar to the soil enhances the total porosity and water holding capacity [221,222]. Abel et al. [221] found that the water holding capacity increased due to the mixing of 2.5 wt% of corn silage-derived hydrochar with soil. The further addition of hydrochar does not improve the water holding capacity significantly because of the high organic content in hydrochar. A similar finding was observed by Kalderis et al. [223], where the authors reported that the water holding capacity increased until adding 5 wt% of orange peel-derived hydrochar; however, an insignificant change occurred after mixing additional hydrochar. Due to the hydrophobic nature of the hydrochar [220,224,225], the addition of excessive hydrochar in soil could increase the water repellency, resulting in an insignificant improvement of water holding capacity. Addition of hydrochar into the soil ultimately reduces the soil’s bulk density as the hydrochar is typically lighter than the soil [221]. In addition to the bulk density, the coarseness index, which describes the particle size distribution may be influenced by the soil–hydrochar mixer. For example, 30% (v/v) of dilution of hydrochar with soil showed the highest coarseness index with the lowest bulk density of the mixer.
Soil’s pH is one of the key factors to growing microbial community and plants, as it regulates the ion solubility in the soil [226]. In general, hydrochar is acidic and it becomes more acidic when it is produced at high temperatures [213,223,227,228]. Thus, applying hydrochar can make the soil alkalinity relatively low. Ren et al. [229] found a significant drop in soil pH after mixing the sewage sludge-derived hydrochar, although the hydrochar had lower pH compared to the soil’s initial pH. With the initial discrepancy in the pH between hydrochar and soil, more carboxylic functional groups formed over time due to the oxidation of the hydrochar surface resulting in a lower final pH.
Similar to the pH, electrical conductivity (which determines the level of soil salt content) is another key property of soil. When the salt content in the soil increases, it interrupts the water and nutrient balance which is detrimental to the plant. Belda et al. [230] reported the rising of electrical conductivity about 3-fold by applying 30% forest residue-derived hydrochar into the soil, while a 25% decrease was observed after using sewage sludge-derived hydrochar. The increase in electrical conductivity could be due to the higher organic matters in the forest residue-derived hydrochar compared to the soil, while the reduction could be due to the lower cation exchange capacity and oxygen-to-carbon ratio in the sewage sludge-derived hydrochar [231].
In addition to the soil’s physical and chemical properties, hydrochar has the potential to ameliorate the soil’s microorganisms as it could deliver essential nutrients and total organic carbons to the soil [213]. Although the potential impact of hydrochar on the microbial community is still in the initial stages, a few studies have found a positive impact of hydrochar on the growth of soil microbes [229,232,233]. For instance, Ren et al. [229] observed a substantial increase in the abundance of Archaea, Bacteria, and Bacillus in the soil after applying hydrochar. There could be several reasons for this increase in microbes. For example, hydrochar contains more organic matter and has a relatively more specific surface area compared to the soil which helps prevent the leaching of bacteria from soil. In contrast, Andert and Mumme [234] reported the adverse effect of hydrochar. For example, the application of hydrochar in soil reduced the Acidobacteria 5-to-6-fold compared to the control, whereas the abundance of Firmicutes was less than one-third. However, the excess of Bacteroidetes and Proteobacteria increased 2.4 and 1.6–1.7 times, respectively. The shift in this microbial community could be due to the easily degradable carbon and low pH of hydrochar. It also observed that the utilization of hydrochar derived from two different feedstocks had different effects on the soil microorganism community. For example, the abundance of ectomycorrhizal fungi was higher when paper mill biosludge-derived hydrochar was applied [235], whereas a negative effect was observed when spent brewer’s yeast-derived hydrochar was applied [236]. The probable cause of this different behavior could not only be due to the pH but also the physical and chemical properties, nutrients, and phytotoxicity of hydrochar.
The presence of high aliphatic and less aromatic carbon accelerated microbial degradation made the hydrochar less stable in soil compared to biochar [237,238]. It was also reported that the presence of high hydrophilic (e.g., hydroxyl, carbonyl, and carboxyl) functional groups, low C/N ratio, and low lignin content in the raw material enhance the degradability of the hydrochar [239]. However, a study by Schulze et al. [240] found that instead of lignin content, the reaction temperature and time are more important in determining the hydrochar stability. It has been noticed that higher reaction temperature led to improving the hydrochar stability by increasing the carbon content. Contrariwise, Malghani et al. [22] concluded that corn silage-based hydrochar protects the soil carbon from decomposition as the carbon presence in hydrochar gradually stabilizes after initial rapid decomposition [241,242]. It has been found that applying hydrochar along with fertilizer can generate noticeable greenhouse gas (GHG) emissions [230,234,243,244]. On the other hand, Yu et al. [245] found that hydrochar can reduce GHG emissions by avoiding the composting of fresh biowaste. Similarly, Adjuik et al. [246] concluded that the utilization of hydrochar as a soil amendment did not significantly improve the crop yield; instead, it reduced the soil GHG fluxes by about 34%.
Overall, hydrochar application in soil showed a positive effect on soil aggregation, as a result, it has a good potential for carbon sequestration [236,247,248]. In addition, this potential application could be further improved by removing (washing the hydrochar before using it) the superficially adsorbed labile components (responsible for biological decomposition) from the hydrochar [249].
7. Nutrient Recovery
Organic waste and biomass contain several nutrients which are essential for plants, apart from having a carbonaceous fraction. Among these nutrients, phosphorus, nitrogen, potassium, and calcium are predominantly found in waste biomass [250,251]. In some organic wastes, sodium is also found, which is considered a functional nutrient [252]. While nitrogen-, phosphorus-, and potassium-based fertilizer are manufactured through a chemical process, organic fertilizers are gaining traction for sustainable agricultural applications [250,253]. In the case of organic waste and biomass, thermochemical treatments are common methods for their scalability [4]. Among different nutrients, nitrogen is the most difficult one to recover considering its release into the atmosphere when high temperature treatment is applied [254]. On the other hand, phosphorus recovery is becoming significantly important due to the depletion of the worldwide reserve [255]. Hydrochar production from organic waste using HTC can provide a versatile solution to nutrient recovery from waste streams.
Nutrient recovery through HTC of organic waste can be achieved by two major pathways. The first pathway is to extract the minerals and nutrients from the parent feed which leaves the nutrients in the hydrochar and effluent liquid stream. Between the hydrochar and liquid effluent, hydrochar retains the major fraction of the plant nutrients [256,257]. Some of the nutrients are recovered from liquid streams (especially, most of the K and Na were found in the liquid phase) by chemical or biochemical processes [258,259,260,261,262]. Notably, the char which contains a major fraction of calcium (>50%), phosphorus (>91%), and nitrogen (>26%), can be utilized as soil replenishment, i.e., as biochar [256]. This is a direct method of nutrient recovery from the HTC process and hydrochar. As for the second pathway, it is achieved by using hydrochar as the mean for recovering nutrients from a waste stream (i.e., wastewater) [263]. In this way, hydrochar can be used for not only recovering nutrients but also purifying the effluent by reducing the undesired nutrient release to the atmosphere. Additional nutrient release to the atmosphere may cause eutrophication (i.e., algal bloom) [264]. The pathways for nutrient recovery from hydrochar are shown in Figure 7.
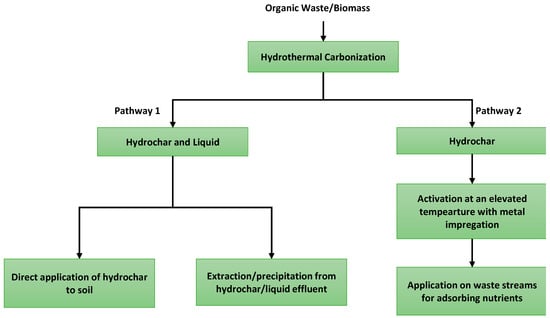
Figure 7. Pathways of nutrient recovery from hydrochar.
While both the methods are promising, the selection of a pathway is highly dependent on the feedstock and application criteria. The feedstock used in HTC may have a variable amount of nutrients and the speciation among different phases (i.e., solid, liquid, and gas) of HTC affects the amount of nutrients recovered [256,265,266]. The macro-nutrients such as nitrogen and phosphorus are usually targeted for recovery while potassium is also available in some feedstock (for pathway 1). For example, if the biochar is generated by pyrolysis (>650 °C) of organic feedstock, the recovery of nitrogen from biochar is not feasible due to its release in the gas phase [267]. As HTC uses low to moderate temperature for treatment, the resulting hydrochar can trap most of the nitrogen [254,256,267,268]. If the organic feedstock is thermochemically converted (i.e., combustion and gasification) at higher temperatures (>600 °C), most of the phosphorus ends up in ash as phosphate complex compounds [255,269]. It is to be noted that if the feedstock does not contain a significant amount of nutrient, pathway 1 would not be a feasible option.
As for pathway 2, recovering nutrients from a waste stream requires hydrochars with a large surface area, good wetting ability, and thermal stability for adsorbing nutrients [270]. In addition, the application of activated hydrochar is suitable if the phosphorus concentration is lower than 2000 mg P/g in the waste stream [271]. One method of increasing the surface is by activating the hydrochar which can increase the surface area by an order of magnitude (>2000 m2/g of hydrochar) [272,273]. It is to be noted that the pathway 2 is not limited by the feedstock nutrient content, however, but is dependent on the application media nutrient content and hydrochar properties. Impregnating magnesium and bismuth showed good results in adsorbing phosphorus from the wastewater with a phosphorus recovery varying from 40–125 mg/g of activated char [274,275]. While the advanced methods of hydrochar-based nutrient recovery are promising for recovering nutrients from waste streams, it is still early to determine their practical applicability in a commercial scale. Further studies are necessary for evaluating the techno-economic and environmental feasibility of these techniques.
To utilize the nutrients in the most efficient way, nutrients are to be recovered in an easily absorbable condition for plants. In the case of nitrogen, plants can uptake NO3− directly while some NH4+ ions can be slowly absorbed [276]. It is to be noted that a portion of the NH4+ ions can be hydrolyzed by microorganisms to NO3− while a part of it releases as NH3 to the atmosphere. On the other hand, phosphorus is preferred in phosphate compounds [277]. A typical method to recover phosphorus would be through a struvite precipitation process applicable to only liquid waste streams [278]. For direct applications of hydrochars, the available (i.e., free) phosphorous and nitrogen are critical. The retention of both phosphorus (as phosphate) and nitrogen (as nitrate, which is readily available for release) in the hydrochar at once may be difficult due to their opposing pH requirements for recovery. For example, low pH during HTC favors NH4+ ion and free ammonia formation while high pH facilitates the phosphate and gaseous ammonia formation [279]. The gaseous ammonia can be easily released to the atmosphere during the drying of hydrochar [280]. Apart from pH, another important factor for nutrient speciation is temperature during HTC. With the temperature increase, the pH of hydrochar increases by removing the carboxyl and hydroxyl groups [281,282], which indirectly affects the nutrient retention in the char. Moreover, a higher potassium (K) release was favored at a high temperature environment for hydrochar production [281]. As there are interacting parameters affecting the nutrient speciation in hydrochar, optimization is necessary to get to a point where nutrients can be recovered efficiently. Based on HTC experiments performed on anaerobic digestate, Stutzenstein et al. [280] found that the optimum temperature for hydrochar would be 230 °C, sacrificing the nitrogen recovery for the sake of phosphorus recovery (high pH). It is to be noted that the optimum operating parameters will vary with respect to the feedstock as well. As per the study by Dima et al. [256] on municipal solid waste, principal component analysis on several operating conditions yielded a negative impact of time and slightly positive impact of temperature on nutrient recovery in hydrochar. They also reported that potassium (K) and sodium (Na) ended up mostly in the liquid phase. The alkali metals are inorganically bound (as nitrates and chlorides) in the biomass, making them readily soluble in water [283,284].
It is to be remembered that there may be adverse effects in the case of direct application of hydrochar to soil if the free phosphorus is lower than the amount of phosphorus available in the soil. In such a case, it could adsorb phosphorus from the soil on its surface. According to a study by Fei et al. [285] on several types of chars (including hydrochars), up to 417 mg/kg of free phosphorus was available out of the total phosphorus content of 27,175 mg/kg of char. The free phosphorus was still higher than the soil phosphorus content, ensuring its suitability as slow-release fertilizer for the soil. Their study also revealed that P-laden (phosphorus adsorbed on the surface of hydrochar) chars were also suitable for application on soil as well.
Apart from direct application of hydrochar as a nutrient source, there are a few studies on acid leaching of nutrients from hydrochar for application in soil [286,287]. Ekanthalu et al. [287] showed that post-treatment of hydochars after HTC process could leach higher amounts of phosphorus in the liquid phase leading to an easier release to soil. Higher acid concentration provided a better leaching performance of the phosphorus (up to 100%), as expected [286].
Other than recovering nutrients in hydrochar, they could be also used as a means to recover nutrients from wastewater. The mechanism for this process is dependent on the physico-chemical properties of the hydrochar, which include calcium (Ca) and magnesium (Mg) content along with oxygen containing groups. The presence of Ca and Mg facilitates the capture of phosphorus while NH4-N is captured by the oxygen functional groups [288]. Although physisorption is not the dominant mechanism in this case, a high surface area facilitates the recovery process [289].
Both hydrochar and nutrient-adsorbed hydrochar are excellent candidates for soil remedying as slow-release fertilizers. However, due to several factors affecting the recovery and application of hydrochar to soil, additional systematic studies are needed for a better understanding of the underlying mechanisms.
This entry is adapted from the peer-reviewed paper 10.3390/en15249340
This entry is offline, you can click here to edit this entry!
