Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Betula spp., Quercus spp., and Populus spp. are the most promising deciduous woody plants in forestry. These species were found to be sensitive to climate change that can badly affect their plantations. Thus, a deep understanding of genetic mechanisms of adaptation to adverse environmental conditions plays an important role in preventing the reduction of deciduous forest area. The stress responses of Betula spp., Quercus spp., and Populus spp. are described to drought and salt stresses
- oak
- salt stress
- drought
- birch
- poplar
- transcriptome
- differential expression analysis
1. Introduction
Deciduous woody plants are economically valuable tree species with a high potential for plantation forestry, covering large areas in the Eurasian continent. However, due to global climate change, including an increase in average annual temperature [1] and sea level rise [2], as well as low rates of reforestation after active felling, the areas under deciduous forests are declining every year. With respect to forest tree species such as birches, oaks, and poplars, the resistance to adverse environmental conditions is of particular importance.
Responses of deciduous woody plants to abiotic stress may depend both on the intensity of stress factors, such as drought [3][4][5], soil salinity [6][7], and tree species composition [8][9]. Abiotic stresses induce rapid tissue release of various reactive oxygen species (ROS), such as hydrogen peroxide (H2O2) and superoxide anion (O2−) [10], which negatively affect the structural integrity of the cell wall, carbohydrate metabolism, biosynthesis and folding of proteins, etc. In addition, there are changes in formation of roots [11][12], leaves [13], and wood [14], as well as in the susceptibility to pathogens and insects [15][16]. Thus, plants have developed a wide range of molecular mechanisms to support their growth and development, thereby reducing the cost of adaption to stress conditions, in particular, stomatal movement control to avoid water and electrolyte leakage and penetration of pathogens [17][18], accumulation of osmoprotectants [19][20], biosynthesis of cell wall compounds [21][22][23] and antioxidants [24][25], specific DNA loci associated with phenotypic traits important for drought tolerance [26], as well as stress memory systems based on epigenetic regulation [27].
A deeper understanding of these mechanisms has been made possible by advances in differential gene expression analysis using next generation sequencing that has greatly enhanced our current knowledge of the stress response of deciduous woody plants. Several recent reviews described physiological and molecular responses of woody plants to abiotic stress, but generally, without focusing on particular important species (e.g., [28][29][30][31]).
Birch (Betula spp.), oak (Quercus spp.), and poplar (Populus spp.) are among the most promising species for plantation forestry. Birches are most numerous in the boreal zone of Northern Europe [32]. Due to the increased cold tolerance and the ability to grow on poor soil, the birch habitat extends up to Central Siberia and has a higher altitude limit. In turn, oaks are widespread throughout most of Europe, stretching from the northern regions of Scotland to southern Turkey, as well as continental Russia as far as the Urals [33]. Oak’s taproots penetrate deep into the soil, giving them structural wind resistance and tolerance to moderate drought. Poplar is cosmopolitan and grows in Europe, Asia, North America, and East Africa [34]. Obviously, these species use different ecological strategies. Therefore, it can be assumed that they also have different traits of adaptation to stress.
2. Main Aspects of Adaptation to Drought and Salt Stress in Betula spp., Quercus spp., and Populus spp.
The response of deciduous woody plants to various abiotic stresses includes both regular activities and specific responses. The identified genes can be promising candidates for gene editing and targeted selection [35]. The main responses to drought and salt stresses of birches, oaks, and poplars were identified (Figure 1).
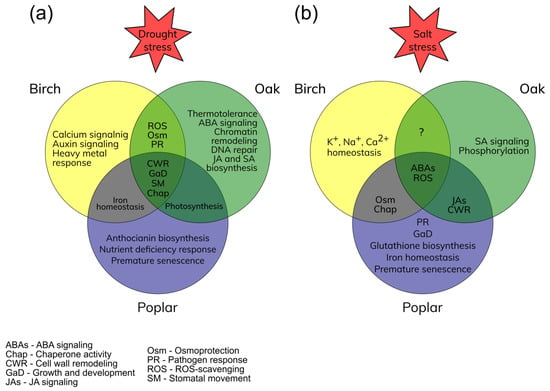
Figure 1. Main responses of birches, oaks, and poplars to drought (a) and salt (b) stress conditions. Overlapping circle parts represent the common stress responses.
During the early response of Betula spp. to drought, the transcription factors (TFs) of the ERF, NAC, WRKY, and AGL families associated with the prevention of water leakage were significantly up-regulated. Drought protection included mainly the control of stomatal movement and the osmotic stress response (Figure 1a). The biosynthesis and metabolism of jasmonic acid (JA), as well as signaling pathways with its participation, were also activated, which indicates the control of plant growth and development. It should be noted that stomatal movement mediated by abscisic acid (ABA) may be suppressed here in favor of an alternative pathway. Further exposure to drought, accompanied by ROS accumulation and impaired protein folding, promotes the induction of genes associated with chaperone activity (LEAs and HSP) and ROS scavenging through up-regulation of TFs of MYB and ERF families and the transcriptional activators of the PTI family. Long-term drought mediates the accumulation of proline and lignin in birch cells through the up-regulation of the TFs of the HOX and ERF families (Table S1).
Compared to birch, oak’s early reaction to drought is more pronounced, which is expressed in an increase in thermal tolerance and activation of JA and salicylic acid (SA) signaling pathways. (Figure 1a). Intensive cell wall remodeling, represented by monosaccharide polymerization and cellulose and lignin biosynthesis, was observed during long-term drought stress. During this drought period, ROS scavenging systems and antioxidant biosynthesis became significantly more active. Up-regulation of the genes associated with chromatin remodeling indicates formation of stress memory based on epigenetic regulation. It should be noted that DNA repair activity was also detected, which indicates that the long-term drought stress may affect the gene integrity in oak’s cells.
Poplar’s response to drought includes antioxidant activity, represented by anthocyanin biosynthesis, and a response to nutrient deficiency. It should be noted that the photosynthetic system is improved due to iron homeostasis, which makes poplars related to birches and oaks (Figure 1a). The ABA-mediated stomatal movement here can be suppressed by protein phosphatase activity (Table S1). In addition, TFs of the MYB, WRKY, and ZFP families were involved in root growth and tissue development to prevent water leakage (Table S1). It should be noted that drought-mediated premature senescence in poplar cells (Figure 1a) was manifested through the activation of DEGs associated with tissue senescence and accumulation of hydrogen peroxide (Table S1).
Under short-term salt stress in birch cells, with the leakage of water and electrolytes, the systems for maintaining ion homeostasis and the biosynthesis of osmoprotectants (proline and polyols) are activated (Figure 1b). The response to oxidative stress is represented by ROS scavenging (peroxidase, ascorbate oxidase, and flavonoid 3′,5′-hydroxylase). Stomatal movement here can be mediated through the ABA signaling pathway. The complex response to abiotic stress was regulated by the activation of TFs of the WRKY, ERF, ZIP, and AHL families. At the same time, the processes of development, reproduction, and growth are suppressed.
As with birch, oak’s response to salt stress also includes ROS scavenging and ABA signaling (Figure 1b). However, in this case, phytohormone signaling pathways (JA and SA) and TFs (MYB, NAC, WRKY, ABI, and ERF families) were significantly more activated (Figure 1b). It should be noted that common processes of the response to salt stress only between birch and oak have not been identified (Figure 1b). However, this does not indicate their absence. Research in this area should be continued.
In turn, the reaction of poplar to salt stress includes many common processes with birch and oak, such as osmoprotection, chaperone activity, and cell wall remodeling (Figure 1b). In addition, the early salt stress response also includes control of root growth and development, glutathione biosynthesis, and activation of genes associated with the response to pathogens. At the same time, up-regulation of some TFs of the MYB family associated with tolerance to iron deficiency may indicate adaptation of the photosynthetic system to salt stress (Figure 1b, Table S1).
It should also be noted several TFs, the action mechanism of which is of interest. In particular, the responses of Betula platyphylla to drought and PEG-mediated osmotic stress are very similar, when ERF2 plays a crucial role [36][37], involving in the chaperone activity, cell wall remodeling, and ROS scavenging. In both cases, ABA-mediated stomatal movement is suppressed by either negative regulation [37] or a decrease in ABA biosynthesis [36]. On the other hand, according to Yao et al. [7][38], ERF76 is also involved in primary salt stress response in poplar hybrid Populus simonii × Populus nigra, directly regulating activity of LEA, HSP, SOD, and POD and stomatal aperture. Given these results, it would be promising to investigate functions of these TFs and the mechanisms of their actions in tandem with other deciduous woody plants.
The same research can be carried out for WRKY6 and WRKY29. According to Jia et al. [37], WRKY6 performs the negative regulation of ABA-mediated stomatal movement in B. platyphylla under 20% PEG6000 treatment during 9 h. In turn, WRKY29 was also up-regulated in B. platyphylla under milder osmotic stress conditions (9% PEG6000), over 25 days [39]. Therefore, the mechanisms of separate and cooperative actions of WRKY6 and WRKY29 should be investigated in detail.
The bZIP4-PP2C-system mediating suppression of ABA signaling [40] was identified in Populus ussuriensis under PEG-mediated osmotic stress [41] and in B. platyphylla under salt stress [42]. Silencing this pathway can lead to increased sensitivity to ABA and, consequently, increased stress tolerance.
Finally, interestingly, NAC72 and HB7, which are involved in a drought response in Quercus robur [5] and B. platyphylla [36], stomatal movement in Arabidopsis thaliana [43], and osmotic stress in Populus spp. [44], were also up-regulated in P. simonii × P. nigra under salt stress [7]. This feature makes these genes a promising object of research in the field of response to abiotic stresses in deciduous woody plants.
Thus, according to the above, it can be assumed that the species Betula spp., Quercus spp., and Populus spp., although phylogenetically relatively distant from each other, may demonstrate similar molecular traits for adaptation to drought and salt stress. This feature can be explained both by sympatry [32][33] and by the overlap of their habitats [32][33][34].
This entry is adapted from the peer-reviewed paper 10.3390/f14010007
References
- Lindsey, R.; Dahlman, L. Climate Change: Global Temperature|NOAA Climate.gov. Available online: http://www.climate.gov/news-features/understanding-climate/climate-change-global-temperature (accessed on 27 September 2022).
- Lindsey, R. Climate Change: Global Sea Level|NOAA Climate.gov. Available online: http://www.climate.gov/news-features/understanding-climate/climate-change-global-sea-level (accessed on 27 September 2022).
- Guerrero-Sánchez, V.M.; Castillejo, M.Á.; López-Hidalgo, C.; Alconada, A.M.M.; Jorrín-Novo, J.V.; Rey, M.-D. Changes in the transcript and protein profiles of Quercus ilex seedlings in response to drought stress. J. Proteomics 2021, 243, 104263.
- Mevy, J.-P.; Loriod, B.; Liu, X.; Corre, E.; Torres, M.; Büttner, M.; Haguenauer, A.; Reiter, I.M.; Fernandez, C.; Gauquelin, T. Response of downy oak (Quercus pubescens Willd.) to climate change: Transcriptome assembly, differential gene analysis and targeted metabolomics. Plants 2020, 9, 1149.
- Madritsch, S.; Wischnitzki, E.; Kotrade, P.; Ashoub, A.; Burg, A.; Fluch, S.; Brüggemann, W.; Sehr, E.M. Elucidating drought stress tolerance in european oaks through cross-species transcriptomics. Genes Genomes Genet. 2019, 9, 3181–3199.
- Lei, X.; Liu, Z.; Xie, Q.; Fang, J.; Wang, C.; Li, J.; Wang, C.; Gao, C. Construction of two regulatory networks related to salt stress and lignocellulosic synthesis under salt stress based on a Populus davidiana × P. bolleana transcriptome analysis. Plant Mol. Biol. 2022, 109, 689–702.
- Yao, W.; Li, C.; Lin, S.; Wang, J.; Zhou, B.; Jiang, T. Transcriptome analysis of salt-responsive and wood-associated NACs in Populus simonii × Populus nigra. BMC Plant Biol. 2020, 20, 317.
- Kuchma, O.; Janz, D.; Leinemann, L.; Polle, A.; Krutovsky, K.V.; Gailing, O. Hybrid and environmental effects on gene expression in poplar clones in pure and mixed with black locust stands. Forests 2020, 11, 1075.
- Kuchma, O.; Rebola-Lichtenberg, J.; Janz, D.; Krutovsky, K.V.; Ammer, C.; Polle, A.; Gailing, O. Response of poplar leaf transcriptome to changed management and environmental conditions in pure and mixed with black locust stands. Forests 2022, 13, 147.
- Singh, P.; Arif, Y.; Miszczuk, E.; Bajguz, A.; Hayat, S. Specific roles of lipoxygenases in development and responses to stress in plants. Plants 2022, 11, 979.
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought? Front. Plant Sci. 2015, 6, 547.
- Kulczyk-Skrzeszewska, M.; Kieliszewska-Rokicka, B. Influence of drought and salt stress on the growth of young Populus nigra ‘Italica’ Plants and Associated Mycorrhizal Fungi and Non-Mycorrhizal Fungal Endophytes. New Forests 2022, 53, 679–694.
- Čehulić, I.; Sever, K.; Katičić Bogdan, I.; Jazbec, A.; Škvorc, Ž.; Bogdan, S. Drought impact on leaf phenology and spring frost susceptibility in a Quercus robur L. provenance trial. Forests 2019, 10, 50.
- Larysch, E.; Stangler, D.F.; Puhlmann, H.; Rathgeber, C.B.K.; Seifert, T.; Kahle, H.-P. The 2018 hot drought pushed conifer wood formation to the limit of its plasticity: Consequences for woody biomass production and tree ring structure. Plant Biol. (Stuttg) 2022, 24, 1171–1185.
- Heimonen, K.; Valtonen, A.; Kontunen-Soppela, S.; Keski-Saari, S.; Rousi, M.; Oksanen, E.; Roininen, H. Susceptibility of silver birch (Betula pendula) to herbivorous insects is associated with the size and phenology of birch–Implications for Climate Warming. Scand. J. For. Res. 2017, 32, 95–104.
- Hossain, M.; Veneklaas, E.J.; Hardy, G.E.S.J.; Poot, P. Tree host–pathogen interactions as influenced by drought timing: Linking physiological performance, biochemical defence and disease severity. Tree Physiol. 2019, 39, 6–18.
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694.
- Wang, Z.; Gou, X. The first line of defense: Receptor-like protein kinase-mediated stomatal immunity. Int. J. Mol. Sci. 2021, 23, 343.
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 1913306.
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 5186.
- Perrot, T.; Pauly, M.; Ramírez, V. Emerging roles of β-glucanases in plant development and adaptative responses. Plants 2022, 11, 1119.
- Serra, O.; Geldner, N. The Making of suberin. New Phytol. 2022, 235, 848–866.
- Wang, Y.; Gui, C.; Wu, J.; Gao, X.; Huang, T.; Cui, F.; Liu, H.; Sethupathy, S. Spatio-temporal modification of lignin biosynthesis in plants: A promising strategy for lignocellulose improvement and lignin valorization. Front. Bioeng. Biotechnol. 2022, 10, 917459.
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione metabolism in plants under stress: Beyond reactive oxygen species detoxification. Metabolites 2021, 11, 641.
- Raza, A.; Charagh, S.; García-Caparrós, P.; Rahman, M.A.; Ogwugwa, V.H.; Saeed, F.; Jin, W. Melatonin-mediated temperature stress tolerance in plants. GM Crops Food 2022, 13, 196–217.
- Noelle, N.M.; Weru, W.P.; Rodrigue, S.J.; Karlin, G. The effects of drought on rice cultivation in sub-saharan africa and its mitigation: A review. Afr. J. Agric. Res. 2018, 13, 1257–1271.
- Sadhukhan, A.; Prasad, S.S.; Mitra, J.; Siddiqui, N.; Sahoo, L.; Kobayashi, Y.; Koyama, H. How do plants remember drought? Planta 2022, 256, 7.
- Lobo, A.K.M.; Catarino, I.C.A.; Silva, E.A.; Centeno, D.C.; Domingues, D.S. Physiological and molecular responses of woody plants exposed to future atmospheric CO2 levels under abiotic stresses. Plants 2022, 11, 1880.
- Estravis-Barcala, M.; Mattera, M.G.; Soliani, C.; Bellora, N.; Opgenoorth, L.; Heer, K.; Arana, M.V. Molecular bases of responses to abiotic stress in trees. J. Exp. Bot. 2020, 71, 3765–3779.
- Polle, A.; Chen, S.L.; Eckert, C.; Harfouche, A. Engineering drought resistance in forest trees. Front. Plant Sci. 2019, 9, 1875.
- Yao, T.; Zhang, J.; Xie, M.; Yuan, G.; Tschaplinski, T.J.; Muchero, W.; Chen, J.-G. Transcriptional regulation of drought response in Arabidopsis and woody plants. Front. Plant Sci. 2021, 11, 572137.
- Beck, P.; Caudullo, G.; de Rigo, D.; Tinner, W. Betula pendula, Betula pubescens and Other Birches in Europe: Distribution, Habitat, Usage And Threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 70–73. ISBN 978-92-79-36740-3.
- Eaton, E.; Caudullo, G.; Oliveira, S.; de Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, Habitat, Usage And Threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 160–163. ISBN 978-92-79-36740-3.
- Stobrawa, K. Poplars (Populus Spp.): Ecological role, applications and scientific perspectives in the 21st century (review paper). Baltic Forestry 2014, 20, 204–213.
- Cao, H.X.; Vu, G.T.H.; Gailing, O. From genome sequencing to CRISPR-based genome editing for climate-resilient forest trees. Int. J. Mol. Sci. 2022, 23, 966.
- Wen, X.; Wang, J.; Zhang, D.; Wang, Y. A gene regulatory network controlled by BpERF2 and BpMYB102 in birch under drought conditions. Int. J. Mol. Sci. 2019, 20, 3071.
- Jia, Y.; Niu, Y.; Zhao, H.; Wang, Z.; Gao, C.; Wang, C.; Chen, S.; Wang, Y. Hierarchical transcription factor and regulatory network for drought response in Betula platyphylla. Hortic. Res. 2022, 9, uhac040.
- Yao, W.; Wang, L.; Zhou, B.; Wang, S.; Li, R.; Jiang, T. Over-expression of poplar transcription factor ERF76 gene confers salt tolerance in transgenic tobacco. J. Plant Physiol. 2016, 198, 23–31.
- Tan, Z.; Wen, X.; Wang, Y. Betula platyphylla BpHOX2 transcription factor binds to different cis-acting elements and confers osmotic tolerance. J. Integr. Plant Biol. 2020, 62, 1762–1779.
- Noh, M.; Huque, A.K.M.M.; Jung, K.W.; Kim, Y.Y.; Shin, J.S. A stress-responsive cam-binding transcription factor, BZIP4, confers abiotic stress resistance in Arabidopsis. J. Plant Biol. 2021, 64, 359–370.
- Li, W.; Liu, Z.; Feng, H.; Yang, J.; Li, C. Characterization of the gene expression profile response to drought stress in Populus ussuriensis using PacBio SMRT and Illumina Sequencing. Int. J. Mol. Sci. 2022, 23, 3840.
- Xing, B.; Gu, C.; Zhang, T.; Zhang, Q.; Yu, Q.; Jiang, J.; Liu, G. Functional study of BpPP2C1 revealed its role in salt stress in Betula platyphylla. Front. Plant Sci. 2021, 11, 617635.
- Ré, D.A.; Capella, M.; Bonaventure, G.; Chan, R.L. Arabidopsis AtHB7 and AtHB12 evolved divergently to fine tune processes associated with growth and responses to water stress. BMC Plant Biol. 2014, 14, 150.
- Zhu, Y.; Song, D.; Sun, J.; Wang, X.; Li, L. PtrHB7, a class III HD-Zip gene, plays a critical role in regulation of vascular cambium differentiation in Populus. Mol. Plant 2013, 6, 1331–1343.
This entry is offline, you can click here to edit this entry!
