Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Oaks (Quercus spp.) are a major component of subtropical and temperate forests in the Northern Hemisphere. There are approximately 464 species, and they are dominant tree species in ecosystems. Oaks have a long history of cultivation in Europe, North America, and other continents. They are also cultivated and distributed in most provinces of China.
- oak trees
- germplasm resources
- distribution in China
1. Germplasm Resources of Oaks
1.1. Indigenous Oak Species in China
There are 450–500 species of oaks (Quercus spp.) in the world. Due to their high adaptability to different climatic and soil conditions, oaks are widely distributed in Asia, Europe, North America, and Africa, in the northern hemisphere. Previous studies have reported 66 species of oak trees in China, including 51 species, 14 varieties, and one forma. A more detailed literature review confirmed that there are 67 lineages of Quercus in China, including 52 species, 14 varieties, and one forma [1]. After deleting the unaccepted species names or reduplicated synonyms, there are 60 lineages of Quercus in China, including 49 species, 7 varieties, and 4 subgenera. (Table 1). Although previous reports categorized Q. palustris and Q. robur as indigenous oak species in China, other studies have shown that these two species were introduced to China from North America in the early 20th century [2]. Until now, Q. palustris and Q. robur have been considered foreign-introduced species in most studies, while some reports believe they were transplanted from the Shandong Province and Northeast China. However, no wild trees have been reported in these areas. Recent studies have also classified Q. sichourensis and Q. edithiae as being in the Quercus genus [3]. In North and Northeast China, there are Q. wutaishanica, Q. mongolica, Q. dentata, Q. aliena var. acutiserrata, Q. aliena, and Q. variabilis, among other species. These species provide critical habitats for oak silkworms, and approximately 70,000 tons of tussah cocoons are produced each year. In the Henan and Shandong Province, Q. acutissima, Q. variabilis, Q. aliena var. acutiserrata, and Q. chenii are the main species of oak, with 50,000 tons of tussah cocoons produced each year. In the Southwest of China, including Guizhou, Sichuan, and Shanxi Province, Q. acutissima, Q. variabilis, Q. wutaishanica, and Q. fabri are distributed, and approximately 50,000 tons of tussah cocoons are produced annually [4][5].
1.2. Imported Oak Tree Species
Although oaks are abundant in China, as a major forest tree species and urban greening species, they have not been extensively studied. In China, the number of tree species available for urban landscaping is quite low. To expand oak tree populations and utilize their resources effectively, China introduced oak trees as greening tree species. The oak trees were imported from Europe and America in the mid-19th century, through foreign missionaries, businessmen, and students [6]. However, oak trees were not systematically introduced or studied in China until in the 1960s, when the Chinese Academy of Forestry introduced more than 10 species of Quercus from the United States [7]. This provided abundant materials for the germplasm breeding improvement of oaks in China. Some companies and scientific research institutions have also conducted studies on species introduction. To date, approximately 35 species of Quercus have been imported from Europe and America (Table 1). The successful species introduction, domestication, and biological and ecological evaluation of these foreign oaks have been studied [8]. Many of them have been successfully domesticated; for example, Q. texana shows good tolerance to waterlogging, and Q. virginiana shows high tolerance to drought, salinity, and heavy metal stress [9]. These species have great potential for applications in coastal protection and mining area greening. Meanwhile, several foreign-introduced oaks (e.g., Q. cinerea also named Q. incana, Q. cuneata, and Q. fraineitto) were not suitable for the local climate [10].
Table 1. Germplasm resources of oaks in China.
| No. | Species Name | No. | Species Name |
|---|---|---|---|
| 1 | Quercus acrodonta Seemen | 49 | Quercus yiwuensis Huang |
| 2 | Quercus acutissima Carruth. | 50 | Quercus yunnanensis Franch. (Formal name: Quercus dentata subsp. yunnanensis (Franch.) Menitsky) |
| 3 | Quercus aliena Blume | 51 | Quercus acutissima var. septentrionalis Liou (Formal name: Quercus acutissima subsp. acutissima) |
| 4 | Quercus aquifolioides Rehder. et E.H.Wilson. | 52 | Quercus acutissima var. depressinucata H.W.Jen et R.Q.Gao (Formal name: Quercus acutissima subsp. acutissima) |
| 5 | Quercus baronii Skan | 53 | Quercus aliena var. pekingensis Schottky |
| 6 | Quercus bawanglingensis Huang, Li et Xing | 54 | Quercus aliena var. pekingensis f. jeholensis (Liou et Li) H.Wei Jen et L.M.Wang (Formal name: Quercus aliena var. pekingensis Schottky) |
| 7 | Quercus chenii Nakai | 55 | Quercus aliena var. acutiserrata Maxim. |
| 8 | Quercus cocciferoides Hand.-Mazz. | 56 | Quercus baronii var. capillata (Kozlova) Liou |
| 9 | Quercus dentata Thunb. | 57 | Quercus cocciferoides var. taliensis (A.Camus) Y.C.Hsu et H.Wei Jen |
| 10 | Quercus dolicholepis A. Camus | 58 | Quercus mongolica var. crispula (Blume) H.Ohashi |
| Quercus dolicholepis var. elliptica Y. C. Hsu et H. W. Jen (Formal name: Quercus dolicholepis A. Camus) |
59 | Quercus mongolica var. mongolica | |
| 11 | Quercus edithiae Skan | 59 | Quercus serrata Murray |
| 12 | Quercus engleriana Seem. | Quercus glandulifera var. stellatopilosa W.H.Zhang (Formal name: Quercus serrata Murray) | |
| 13 | Quercus fabri Hance | Quercus serrata var. brevipetiolata (A.DC.) Nakai (Formal name: Quercus serrata Murray) | |
| 14 | Quercus × fangshanensis Liou | Quercus serrata var. tomentosa (B.C.Ding et T.B.Chao) Y.C.Hsu et H.Wei Jen (Formal name: Quercus serrata Murray) |
|
| 15 | Quercus × fenchengensis H. W. Jen et L. M. Wang | 60 | Quercus senescens var. muliensis (Hu) Y.C.Hsu et H.Wei Jen |
| 16 | Quercus franchetii Skan | 61 | Quercus palustris Münchh. [6] |
| 17 | Quercus fimbriata Y.C.Hsu et H.Wei Jen | 62 | Quercus robur L. [7] |
| 18 | Quercus gilliana Rehder. et E.H.Wilson. | 63 | Quercus suber L. [2] |
| 19 | Quercus griffithii Hook. f. et Thomson ex Miq. | 64 | Quercus texana Buckley [6] |
| 20 | Quercus guyavifolia H. Lév. | 65 | Quercus shumardii Buckley [10] |
| 21 | Quercus × hopeiensis Liou | 66 | Quercus nigra L. [9][11] |
| 22 | Quercus kingiana Craib | 67 | Quercus phellos L. [6][7] |
| 23 | Quercus kongshanensis Y.C.Hsu et H.W.Jen | 68 | Quercus virginiana Mill. [6][10] |
| 24 | Quercus lanceolata M.Martens et Galeotti ex A.DC. | 69 | Quercus coccinea Münchh. [7] |
| 25 | Quercus lodicosa O.E.Warb. et E.F.Warb. | 70 | Quercus rubra L. [7] |
| 26 | Quercus longispica (Hand.-Mazz.) A.Camus | 71 | Quercus falcata Michx. [6] |
| 27 | Quercus malacotricha A.Camus | 72 | Quercus petraea subsp. Petraea [6] |
| 28 | Quercus marlipoensis Hu et W.C.Cheng | 73 | Quercus velutina Lam. [7][11] |
| 29 | Quercus mongolica Fisch. ex Ledeb. | 74 | Quercus stellata Wangenh. [11] |
| 30 | Quercus × mongolicodentata Nakai | 75 | Quercus macrocarpa Michx. [11] |
| 31 | Quercus monimotricha Hand.-Mazz. | 76 | Quercus alba L. [11] |
| 32 | Quercus monnula Y.C.Hsu et H.Wei Jen | 77 | Quercus laurifolia Michx. [9] |
| 33 | Quercus oxyphylla (E.H.Wilson) Hand.-Mazz. | 78 | Quercus × schuettei Trel. [12] |
| 34 | Quercus pannosa Hand.-Mazz. | 79 | Quercus michauxii Nutt. [12] |
| 35 | Quercus phillyraeoides A. Gray | 80 | Quercus lyrata Walter [12] |
| 36 | Quercus pseudosemecarpifolia A. Camus | 81 | Quercus ithaburensis subsp. macrolepis (Kotschy) Hedge et Yalt. [13] |
| 37 | Quercus rehderiana Hand.-Mazz. | 82 | Quercus bicolor Willd. [14] |
| 38 | Quercus semecarpifolia Sm. | 83 | Quercus cerris L. [14] |
| 39 | Quercus senescens Hand.-Mazz. | 84 | Quercus ellipsoidalis E.J.Hill. [14] |
| 40 | Quercus setulosa Hickel et A.Camus | 85 | Quercus gambellii [14] |
| 41 | Quercus sichourensis (Y.C.Hsu) C.C.Huang et Y.T.Chang | 86 | Quercus glauca [14] |
| 42 | Quercus spinosa David | 87 | Quercus imbricaria [14] |
| 43 | Quercus tungmaiensis Y.T.Chang | 88 | Quercus libani [14] |
| 44 | Quercus dentata subsp. stewardii (Rehder) A.Camus | 89 | Quercus muehlenbergii [14] |
| 45 | Quercus tarokoensis Hayata | 90 | Quercus petaea [14] |
| 46 | Quercus utilis Hu et Cheng | 91 | Quercus prinus L. [14] |
| 47 | Quercus variabilis Blume | 92 | Quercus salicina Blume [14] |
| Quercus variabilis var. pyramidalis T.B.Chao, Z.I.Chang et W.C.Li (Formal name: Quercus variabilis Blume) |
93 | Quercus velutina [14] | |
| 48 | Quercus wutaishanica Mayr | 94 | Quercus stellata var. margaretta [14] |
Note: 1–60 represent the native oak tree species in China; 61–94 represent the oak tree species introduced from abroad. The species names in former studies are modified as accepted names.
2. Distribution of Oak Trees
2.1. Historical Distribution of Oaks in China
Oaks are widely distributed in Asia, Europe, North America, and Africa, and are particularly abundant in China, the United States, Russia, and India. Fossils of oaks are also widely distributed throughout geological history, and they are a dominant plant group in strata in the Northern Hemisphere from the Eocene epoch [15]. Archaeological data indicate that oaks were already widely distributed in China in ancient times. The Eocene-epoch Q. rhombifolia fossil found in Fushun, Liaoning Province, is the earliest fossil record of oak tree leaves in China. Quercus sect. Heterobalanus (Oerst.) species are montane sclerophyllous oaks in the Hengduan Mountains and have high tolerance to low temperatures, drought, soil impoverishment, and strong ultraviolet radiation. They are the dominant species, and their fossil record extends back to the Miocene. Early fossils of this sclerophyllous oak were found in Xigaze, the Tibet Autonomous Region (late Miocene), which was named Quercus tibetensis H. Xu, T. Su et Z.K. Zhou sp. nov. [16]. Other Sect. Brachylepides oak fossils were found in Xiaolongtan Basin, Yunnan province [17]. The earliest fossil of a deciduous oak was found in the Miocene epoch flora in Dunhua, Jilin province. This origin is later than that of the evergreen oaks. From archaeological research, the origin of Quercus may have been in the early stages of the Palaeocene, followed by accelerated species differentiation in the Eocene or Oligocene in East Asia, Europe, and North America. Quercus praedelavayi Xing Y.W. et Zhou Z.K. sp. nov. s from the upper Miocene was found in southwestern China [18]. Quercus heqingensis n. sp. from the late Pliocene was found in Heqing, Yunan province, China [19].
Deciduous broad-leaved tree species (especially Quercus) were dominant in vast regions of China. This was confirmed by archeological discoveries, including the 7000-year-old Xinle site in Shenyang, and the Chahai site in Fuxin County, Liaoning [20]. The analysis of charcoal fragments excavated from the Xiajiadian site (3500–4000 years ago) in Chifeng, Inner Mongolia, showed that the loess hills had a relatively warm and humid climate at the time, and that the zonal vegetation consisted of Q. mongolica and Pinus tabulaeformis forests [21]. Starch grains found on the surface of stone tools from the Shangzhai site (7000 years ago) in Pinggu, Beijing, show that the North China Plain was inhabited by deciduous broad-leaved zonal vegetation, consisting of oak species (such as Q. mongolica, Q. aliena, and Q. acutissima). The charcoal remains from the Beiqian site in Jimo, Shandong, showed that Quercus plants (especially Q. acutissima) have been the dominant species in the Jiaodong Peninsula since the Beixin cultural period (7000 years ago) [20].
2.2. Current Distribution of Oaks in China
Deciduous oaks are widely distributed in China, and form narrow belts in northern areas; in contrast, evergreen oaks are moderately distributed in southern China [22]. Deciduous oaks are dominant and constructive species (the main species in forest construction) in deciduous broad-leaved forests and mixed broadleaf-conifer forests in temperate zone and warm temperate regions, especially in North China. Their altitudinal distribution ranges from a few meters to 3500 m above sea level. China has three regions with concentrated distributions of oaks: (1) the Liaodong and Jiaodong peninsulas are hilly areas inhabited by deciduous oaks (mainly Q. mongolica, Q. wutaishanica, and Q. acutissima); (2) the Funiu and Dabie mountain areas show the highest diversity of Quercus species in China (18 species in total, including almost all species found in eastern, western, southern, and northern China); and (3) a wide range of mountainous areas in Sichuan, Yunnan, and Guizhou, which are inhabited by several unique species of oak trees (18 species known to date) [1] (Figure 1).
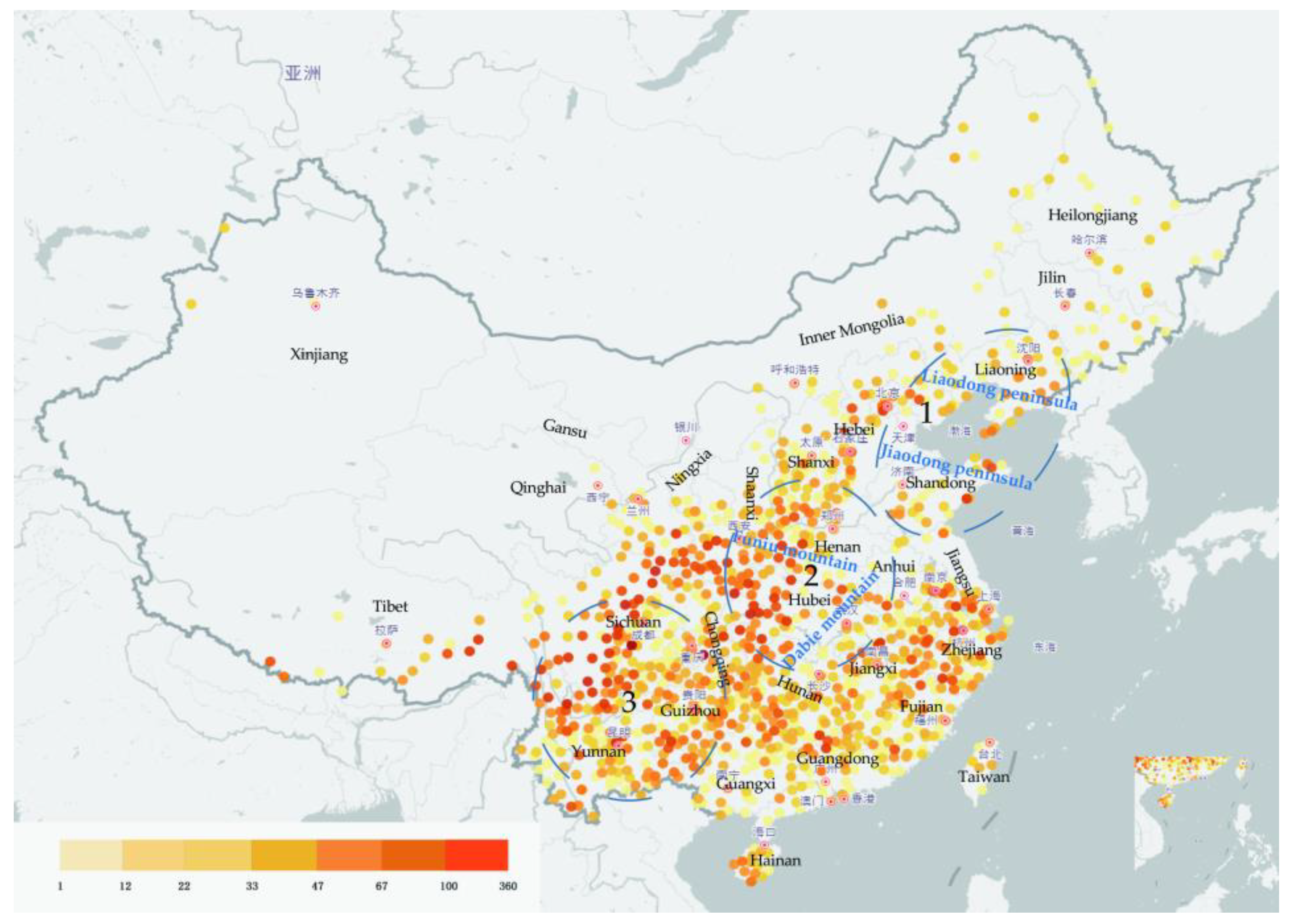
Figure 1. Oak distribution in China based on occurrence records obtained from the National Specimen Information Infrastructure database (www.nsii.org.cn, accessed on 23 December 2022). The different colors represent the collected plant specimen numbers (this reflects the abundance of Quercus samples in this area). Tree concentrated distributions in general areas are also shown in the picture.
The total oak forest area in China is 16.72 million hm2, based on the 8th National Forest Resources Survey. Most of these are natural forests, and the area of oak trees is 16.1 million hm2. The area of artificial oak forest is 0.61 million hm2. The land area occupied by oak tree forests exceeds 100,000 hm2 in 17 provincial regions, 500,000 hm2 in 10 regions, and 1000,000 hm2 in five regions (including Heilongjiang, Jilin, Liaoning, Hebei, and Inner Mongolia) [1]. Oak tree forests account for the highest proportion of dominant tree forests. The top ten tree species are oak, fir, larch, birch, poplar, masson pine, eucalyptus, spruce, Yunnan pine, and cypress, which account for 46.3% of the total forest area in China. There are about 20 species of deciduous oaks, being the main dominant species in temperate zones with broadleaved deciduous forest and mixed broadleaf-conifer forest. Q. acutissima, Q. variabilis, and Q. dentata are widely distributed in Northeast, Southeast, and Southern China. Q. wutaishanica and Q. mongolica, as representatives of deciduous oaks, are widely distributed in Northeast and North China and also located in Sichuan and Hubei provinces. In Yunnan Province, broadleaf oak timber reserves have reached 0.15 billion m3, which represents 43% of broadleaf tree timber reserves. Q. mongolica is mostly distributed in Northeast China and east Inner Mongolia. There are 411,000 hm2 oak forests in Jilin Province, which accounts for 7% of the local forest area. In Liaoning Province, oak forests account for 43.5% of the local forest area, which is 1.06 million hm2. In Hebei Province, oak forests total 0.9 million hm2 [23]. Based on the newest National Forest Resources Survey in 2019, Q. mongolica was the fifth most important tree (based on numbers), representing 8.294 billion trees and 0.583 billion m3 timber reserves. Q. wutaishanica, in the thirteenth most important position, has 2.647 billion trees and 0.183 billion m3 timber reserves.
3. Ecological Adaptability of Oak Trees
3.1. Morphological and Physiological Adaptability of Oaks
Oak trees vary in their leaf size and shape (Figure 2). This variation might reflect adaptations or plastic responses to different environments [24][25][26]. Leaf variation is influenced by genetic and environmental factors [27]. Oaks are deep-rooted plants. Their root systems are well-developed and deeply distributed, and the main roots of one-year-old trees can reach a soil depth of up to 100 cm [5]. In Q. variabilis, the main root length of young seedlings is 10 cm on the 58th day (no fibrous roots) and 50 cm on the 73rd day (with a large number of fibrous roots). The main roots of mature Q. variabilis can reach 6–7 m deep [1]. The well-developed root systems of oaks are important for soil and water conservation in mountainous areas. Roots also have varying degrees of plasticity, to adapt to environmental stress based on morphological or physiological plasticity and root chemical changes [28]. In addition, oak trees are obligate mycorrhizal symbionts. Their root hairs are 100–150 μm long and develop an ectomycorrhizal symbiosis with certain fungi. The mycelia of mycorrhizal fungi surround the root hair. They enter the cortex and invade the intercellular spaces. The mycelia extend outwards on the root surface in the form of villi and absorb water and nutrients from the soil, to maintain the growth of the oak [29]. Annual seedlings inoculated with mycorrhizal fungi can attain approximately double the biomass of control groups. For example, Q. wutaishanica inoculated with symbiotic fungi (such as Comphidius viscidus and Russula foeten) showed improved seedling growth, net photosynthetic rate, and total nitrogen and phosphorus contents [30]. Mycorrhizae affect the absorption of phosphorus fertilizers and have an antibiotic effect, thus protecting the roots of oaks from infestation by root rot fungi [1]. Mycelia can also secrete various types of extracellular enzymes that promote the decomposition of organic matter in the soil [31][32]. Based on these functions, mycorrhizal fungi can be used for the seedling culture and repopulation of oak trees.

Figure 2. Morphology and diversity of leaves and acorns among a few oak species in China.
Most oaks are heliophilous or neutral species that do not have strict soil condition requirements for growth. They grow rapidly in moist, fertile, and well-drained neutral or slightly acidic sandy loam soil (particularly in ravines and foothills). They can also tolerate stress and soil infertility, because of their deep-rootedness and mycorrhizal formations. In addition, acorns have a strong sprouting ability, while trees are not transplantation-resistant. Oaks are adaptable to a wide range of temperatures. Deciduous species of oak trees (such as Q. mongolica, Q. wutaishanica, and Q. dentata) are quite cold-resistant, whereas evergreen species are relatively demanding, requiring warmer temperature conditions [33]. Deciduous species are also relatively drought-resistant, whereas evergreen species are moisture-loving. The drought-tolerant species of oaks indigenous to China include Q. acutissima, Q. variabilis, Q. wutaishanica, and Q. mongolica. Moisture-loving species in southern China include Q. fabri, Q. serrata, and Q. chenii. Most foreign-introduced species of oaks are hygrocolous and highly moisture-resistant. In general, oaks exhibit strong resistance to wind, fire, pollution, and smoke. Species such as Q. variabilis, Q. acutissima, Q. wutaishanica, Q. suber, and Q. mongolica have a thick bark and exhibit fire resistance, which makes them ideal fire-resistant tree species [34]. Owing to its resistance to smoke and poisonous gas, Q. mongolica is a dominant tree species in the greening and isolation belts of industrial and mining areas [35]. Some species of oaks also exhibit saline-alkaline tolerance, such as Q. texana and Q. nigra [36]. In addition, Q. variabilis can absorb and accumulate heavy metals in suburban areas [37].
3.2. Climate Change Influences
Oak is one of the most diverse and ecologically important trees in the Northern hemisphere. They exhibit high tolerance to different environments and have proved useful in evolutionary mechanism research [38][39]. Climate change effects the oak pollen season, especially the start dates and season lengths [40]. In the Mediterranean basin, Q. pubescens, as an downy oak, is often used for anti-drought research in morphoanatomy, physiology, and genetic evolution. A significantly earlier senescence increased the sugar content in leaves, to maintain a higher photosynthetic potential [41]. Drought induced an increase in oxidative pressure from the transcript level [42]. Under a constant CO2 concentration, the net primary production was positively affected by longer vegetation periods and negatively by respiration costs in European oak trees [43]. In the Mediterranean region, climate change induces heat waves and droughts, disturbing forest species and affecting productivity. The cork oak (Q. suber) is resilient and cork growth rapidly recovers when droughts finish [44]. Under long-term environmental changes, trees mainly rely on phenotypic changes. In two oaks, Q. pyrenaica (more tolerant to drought) and Q. petraea (less tolerant to drought), Q. petraea displayed a greater response to moisture availability, by triggering a tighter stomatal control across genetic groups [45].
Oaks comprise 13% of the natural forest in China. Studies specifically addressing oaks adaptation to climate change in China are needed. Greenhouse gases will affect the species geographical distribution and change the richness distribution pattern. Based on 35 oak species and data of 19 bioclimatic variables in China, the Quercus distribution will migrate to high altitudes or high latitudes, from being primarily distributed in the area of southwestern China. A high percentage of species loss will happen in mountainous areas, while other regions will gain species due to a northward shift in the years 2050 and 2070 [46].
4. Threats to Oak Trees
There are several diseases that can influence oak trees, including leaf, trunk, root, and acorn diseases. Leaf diseases are the most common, and most pathogens are fungi. The infected leaves exhibit growth deficiencies and death. Powdery mildew [47], brown leaf spot, rust disease, and “frog eye” disease mainly affect healthy leaves [48] (Figure 3). White rot disease is found in the trunk and roots.

Figure 3. Partial leaf diseases and oak tree pests.
There were 624 kinds of pests reported in 2010 as damaging the leaves, trunks, and acorns in oak trees [49]. Regarding leaf pests, Malacosoma neustria, Camptoloma interiorata, Parasa consocia, Phalera assimilis, Phalerodonta albibasis, and Fentonia ocypete greatly impact leaf growth. In recent years, an emerging oak pest, Rhynchaenus maculosus, has caused spectacular damage in Jilin, Heilongjiang, and Liaoning Province. This pest had no reports till 2012 from when it was first recorded as a new species in China in 1987. It can induce leaf damage symptoms, including blister-like blotches on leaf margins. This pest is an univoltine insect and overwinters as an adult in the leaf litter. Both the larvae and adults can influence leaf growth. The leaf damage in Liaoning province increased from 6.9% in 2016 to 15.4% in 2018 [50][51]. Common trunk and acorn pests include Mallambyx raddei, Laspeyresia splendana, and Curculio arakawai (Figure 3).
Other than disease, the illegal timber trade and harvesting for charcoal also threaten oak resources. Deforestation for farming and increasing mountain fires influence oak growth. For example, Quercus variabilis bark is widely used for cork production. Based on the red list statistics, these are 32 critically endangered species and 57 endangered species of Quercus (https://www.iucnredlist.org/) (accessed on 27 November 2022). In China, there are 12 species of oak trees whose numbers are decreasing, including Q. chenii, Q. edithiae, Q. fimbriata, Q. kingiana, Q. lodicosa, Q. marlipoensis, Q. mongolica, Q. sichourensis, Q. utilis, Q. macrocarpa, Q. alba, and Q. libani. Two of them, Q. fimbriata and Q. marlipoensis, are listed as critically endangered species. Four of these, Q. edithiae, Q. kingiana, Q. lodicosa, and Q. utilis, are listed as endangered species (https://www.iucnredlist.org/) (accessed on 27 November 2022).
This entry is adapted from the peer-reviewed paper 10.3390/biology12010076
References
- Jiang, Y.R.; Liu, W.; Wang, G.B.; Zhou, X.J.; Qin, L. Research Advances in Germplasm Resource and Utilization of Quercus L. Sci. Seric. 2019, 45, 577–585.
- Teng, G.B.; Wang, Q.C.; Cao, Y.; Yang, H.; Feng, J. Research Progress of oak Introduction in China. Liaoning For. Sci. Technol. 2016, 5, 52–55.
- Su, H.L.; Yang, Y.C.; Ju, M.M.; Li, H.M.; Zhao, G.F. Characterization of the complete plastid genome of Quercus sichourensis. Conserv. Genet. Resour. 2019, 11, 129–131.
- State Forestry Administration of China. China Forest Resources Report; China Forestry Publishing House: Beijing, China, 2014.
- Li, Q.; Li, R.P.; Ambühl, D.; Liu, Y.Q.; Li, M.W.; Qin, L. Nutrient composition of chinese oak silkworm, Antheraea pernyi, a traditional edible insect in China: A review. J. Insects Food Feed 2020, 6, 355–369.
- Liu, C.L.; Cao, J.W.; Wu, Y.; Peng, C.H.; Li, B.H. Introduction and seedling raising experiment of several foreign Quercus species. For. Sci. Technol. Dev. 2008, 22, 78–80.
- Huang, L.B.; Li, X.C.; Zhu, X.C.; Yan, J.F. Studies on Introduction of North American Oaks. J. For. Eng. 2005, 19, 30–34.
- Chen, Y.T.; Chen, Y.C.; Huang, Y.Q.; Sun, H.J.; Chen, D.F. Preliminary study on Quercus virginiana introduction in eastern China. For. Res. 2007, 20, 542–546.
- Shuang, D.L.; Sun, L.S.; Kong, F.G.; He, Y.; Du, T.N.; An, L.H.; Wang, F. Analysis of Taizishan area waterlogging resistance introduced colired leaf oak. Hubei For. Sci. Technol. 2017, 46, 20–22.
- Chen, Y.T.; Sun, H.J.; Wang, S.F.; Shi, X. Growth performances of five north american oak species. For. Res. 2013, 26, 344–351.
- Huang, L.B.; Li, X.C.; Wang, Q.M. Preliminary report on seeding trials for introduction of seven exotic Quercus spp. J. Jiangsu For. Sci. Technol. 2003, 30, 1–4.
- Yang, Z.Y.; Li, T.; Zheng, X.; Zhao, Q.Z.; Qiu, Y.B. Study on the introduction adaptability of seven oak species. Shandong For. Sci. Technol. 2015, 3, 57–85.
- Yu, J.B. Development actualities of and strategies for firewood forests of Quercus acutissima in nanqiao district of Chuzhou city. Anhui For. Sci. Technol. 2016, 42, 109–110.
- Tang, Y.D.; Zheng, H.J. The studies on the introduction of the Quercus genus. Beijing Landsc. Archit. 2003, 19, 31–36.
- Yang, J.; Guo, Y.F.; Chen, X.D.; Zhang, X.; Ju, M.M.; Bai, G.Q.; Liu, Z.L.; Zhao, G.F. Framework phylogeny, evolution and complex diversification of Chinese oaks. Plants 2020, 9, 1024.
- Xu, H.; Su, T.; Zhang, S.T.; Deng, M.; Zhou, Z.K. The first fossil record of ring-cupped oak (Quercus L. Subgenus Cyclobalanopsis (Oersted) Schneider) in Tibet and its paleoenvironmental implications. Palaeogeogr. Palaeocl. 2016, 442, 61–71.
- Pu, C.X.; Zhou, Z.K.; Luo, Y. A cladistics analysis of Quercus (Fagaceae) in China based on leaf epidermis and architecture. Acta Bot. Yunnanica 2002, 24, 689–698.
- Xing, Y.W.; Hu, J.J.; Jacques, F.M.B.; Wang, L.; Su, T.; Huang, Y.J.; Liu, Y.S.; Zhou, Z.K. A new Quercus species from the upper miocene of southwestern China and its ecological significance. Rev. Palaeobot. Palynol. 2013, 193, 99–109.
- Huang, H.; Hu, J.J.; Su, T.; Zhou, Z.K. The occurrence of Quercus heqingensis n.Sp. and its application to palaeo-CO2 estimates. Chin. Sci. Bull. 2016, 61, 1354–1364.
- Wang, Y.Q.; Wu, W.W.; Xin, Y.; Jin, G.Y.; Wang, H.Y. Research on the carbonized plant in Chahai site, Fuxin, Liaoning. North. Cult. Relics 2012, 4, 13–18.
- Wang, S.Z.; Wang, Z.L.; Xu, H. The ecological and climatic environments of lower Xiajiadian culture reflected from the first locality of Dashanqian in Chifeng city, Inner Mongolia. Huaxia Archaeol. 2004, 3, 44–51.
- Liu, M.S.; Hong, B.G. The anlaysis of distribution pattern of Fagaceae in China. J. Nanjing For. Univ. 1999, 23, 18–22.
- Zhang, J.X.; Wang, H.X.; Yang, H.F. Utility value and resource cultivation of oak trees. J. Hebei For. Sci. Technol. 2014, 3, 76–77.
- Li, Y.J.; Zhang, Y.Y.; Liao, P.C.; Wang, T.R.; Wang, X.Y.; Ueno, S.; Du, F.K. Genetic, geographic, and climatic factors jointly shape leaf morphology of an alpine oak, Quercus aquifolioides Rehder & E.H. Wilson. Ann. Forest Sci. 2021, 78, 64.
- Liu, Y.; Li, Y.; Song, J.; Zhang, R.; Yan, Y.; Wang, Y.; Du, F.K. Geometric morphometric analyses of leaf shapes in two sympatric Chinese oaks: Quercus dentata Thunberg and Quercus aliena Blume (Fagaceae). Ann. Forest Sci. 2018, 75, 90.
- Maya-García, R.; Torres-Miranda, A.; Cuevas-Reyes, P.; Oyama, K. Morphological differentiation among populations of Quercus elliptica Neé (Fagaceae) along an environmental gradient in Mexico and Central America. Bot. Sci. 2020, 98, 50–65.
- Cavender-Bares, J. Diversification, adaptation, and community assembly of the American oaks (Quercus), a model clade for integrating ecology and evolution. New Phytol. 2018, 221, 669–692.
- Suseela, V.; Tharayil, N.; Orr, G.; Hu, D. Chemical plasticity in the fine root construct of Quercus spp. Varies with root order and drought. New Phytol. 2020, 228, 1835–1851.
- Yamamoto, S.; Sato, H.; Tanabe, A.S.; Hidaka, A.; Kadowaki, K.; Toju, H. Spatial segregation and aggregation of ectomycorrhizal and root-endophytic fungi in the seedlings of two Quercus species. PLoS ONE 2014, 9, e96363.
- Yan, X.F.; Wang, Q. Effects of co-inoculation with two ectomycorrhizal fungi on Quercus liaotungensis seedlings. Chin. J. Plan Ecol. 2004, 28, 17–23.
- Peter, R.B.; John, H.M.; Marla, S.M.; Edward, H. Effects of nitrogen fertilization on growth and ectomycorrhizal formation of Quercus alba, Q. rubra, Q. falcata, and Q. falcata var. pagodifolia. Can. J. Bot. 1983, 61, 2507–2514.
- Toju, H.; Yamamoto, S.; Sato, H.; Tanabe, A.S.; Gilbert, G.S.; Kadowaki, K. Community composition of root-associated fungi in a Quercus-dominated temperate forest: “Codominance” of mycorrhizal and root-endophytic fungi. Ecol. Evol. 2013, 3, 1281–1293.
- Xia, K.; Daws, M.I.; Peng, L.L. Climate drives patterns of seed traits in Quercus species across China. New Phytol. 2022, 234, 1628–1638.
- Catry, F.X.; Francisco, M.; Pausas, J.G.; Fernandes, P.M.; Francisco, R.; Enrique, C.; Thomas, C. Cork oak vulnerability to fire: The role of bark harvesting, tree characteristics and abiotic factors. PLoS ONE 2012, 7, e39810.
- Ma, Y.Z.; Yang, X.L.; Xu, Y.S.; Feng, Z.Z. Response of key parameters of leaf photosynthetic models to increased ozone concentration in four common trees. Chin. J. Plan Ecol. 2022, 46, 321–329.
- Mcleod, K.W.; Mccarron, J.K.; Conner, W.H. Photosynthesis and water relations of four oak species: Impact of flooding and salinity. Trees 1999, 13, 178–187.
- Zhao, X.; Zheng, L.; Xia, X.; Yin, W.; Lei, J.; Shi, S.; Shi, X.; Li, H.; Li, Q.; Wei, Y.; et al. Responses and acclimation of Chinese cork oak (Quercus variabilis Bl.) to metal stress: The inducible antimony tolerance in oak trees. Environ. Sci. Pollut. Res. Int. 2015, 22, 11456–11466.
- Du, F.K.; Wang, T.; Wang, Y.; Ueno, S.; de Lafontaine, G. Contrasted patterns of local adaptation to climate change across the range of an evergreen oak, Quercus aquifolioides. Evol. Appl. 2020, 13, 2377–2391.
- Kremer, A. Microevolution of European temperate oaks in response to environmental changes. CR Biol. 2016, 339, 263–267.
- Zhang, Y.; Bielory, L.; Georgopoulos, P.G. Climate change effect on Betula (birch) and Quercus (oak) pollen seasons in the United States. Int. J. Biometeorol. 2014, 58, 909–919.
- Holland, V.; Koller, S.; Lukas, S.; Brüggemann, W. Drought- and frost-induced accumulation of soluble carbohydrates during accelerated senescence in Quercus pubescens. Trees 2016, 30, 215–226.
- Mevy, J.P.; Loriod, B.; Liu, X.; Corre, E.; Torres, M.; Büttner, M.; Haguenauer, A.; Reiter, I.M.; Fernandez, C.; Gauquelin, T. Response of Downy Oak (Quercus pubescens Willd.) to Climate Change: Transcriptome assembly, differential gene analysis and targeted metabolomics. Plants 2020, 9, 1149.
- De Wergifosse, L.; André, F.; Goosse, H.; Boczon, A.; Cecchini, S.; Ciceu, A.; Collalti, A.; Cools, N.; D’Andrea, E.; De Vos, B.; et al. Simulating tree growth response to climate change in structurally diverse oak and beech forests. Sci. Total Environ. 2022, 806, 150422.
- Leite, C.; Oliveira, V.; Miranda, I.; Pereira, H. Cork oak and climate change: Disentangling drought effects on cork chemical composition. Sci. Rep. 2020, 10, 7800.
- Dorado-Liñán, I.; Valbuena-Carabaña, M.; Cañellas, I.; Gil, L.; Gea-Izquierdo, G. Climate change synchronizes growth and iWUE across species in a temperate-submediterranean mixed oak forest. Front. Plant Sci. 2020, 11, 706.
- Sun, S.; Zhang, Y.; Huang, D.; Wang, H.; Cao, Q.; Fan, P.; Yang, N.; Zheng, P.; Wang, R. The effect of climate change on the richness distribution pattern of oaks (Quercus L.) in China. Sci. Total Environ. 2020, 744, 140786.
- Kasprzyk, W.; Baranowska, M.; Korzeniewicz, R.; Behnke-Borowczyk, J.; Kowalkowski, W. Effect of irrigation dose on powdery mildew incidence and root biomass of sessile oaks (Quercus petraea (Matt.) Liebl.). Plants 2022, 11, 1248.
- Wang, G.B.; Liu, W.; Zhang, B.R.; Qin, L. Analysis of Flora structure and diversity of Quercus wutaishanica Leaves Infected with Powdery Mildew. Sci. Seric. 2021, 47, 310–315.
- Li, X.S.; Wu, Y.; Dong, X.G.; Zhao, S.W.; Mu, X.Q.; Cheng, Z.L.; Zhao, N.; Teng, X.Y.; Shi, S.P.; Yang, R.S.; et al. Checklist of major oak pests in China (Ⅰ). Sinece Seric. 2010, 36, 330–336.
- Yang, R.S.; Ni, M.Y.; Gu, Y.J.; Xu, S.J.; Jin, Y.; Zhang, J.H.; Wang, Y.; Qin, L. Newly Emerging Pest in China, Rhynchaenus maculosus (Coleoptera: Curculionidae): Morphology and molecular identification with DNA barcoding. Insects 2021, 12, 568.
- Yang, R.S.; Qiu, P.C.; Gu, Y.J.; Ni, M.Y.; Xue, Z.H.; Han, J.H.; Jiang, Y.R.; Jin, Y.; Wang, Y.; Zhou, X.F.; et al. Biology of Rhynchaenus maculosus provides insights and implications for integrated management of this emerging pest. Sci. Rep. 2022, 12, 14650.
This entry is offline, you can click here to edit this entry!
