Polyphenols and their intermediate metabolites are natural compounds that are spread worldwide. Polyphenols are antioxidant agents beneficial for human health, but exposure to some of these compounds can be harmful to humans and the environment. A number of industries produce and discharge polyphenols in water effluents. These emissions pose serious environmental issues, causing the pollution of surface or groundwater (which are used to provide drinking water) or harming wildlife in the receiving ecosystems. The treatment of high-polyphenol-content waters is mandatory for many industries.
- polyphenols
- biological treatment
- bioreactors
- water
- aerobic granular sludge
1. Technologies to Remove Phenolic Compounds from Water Sources
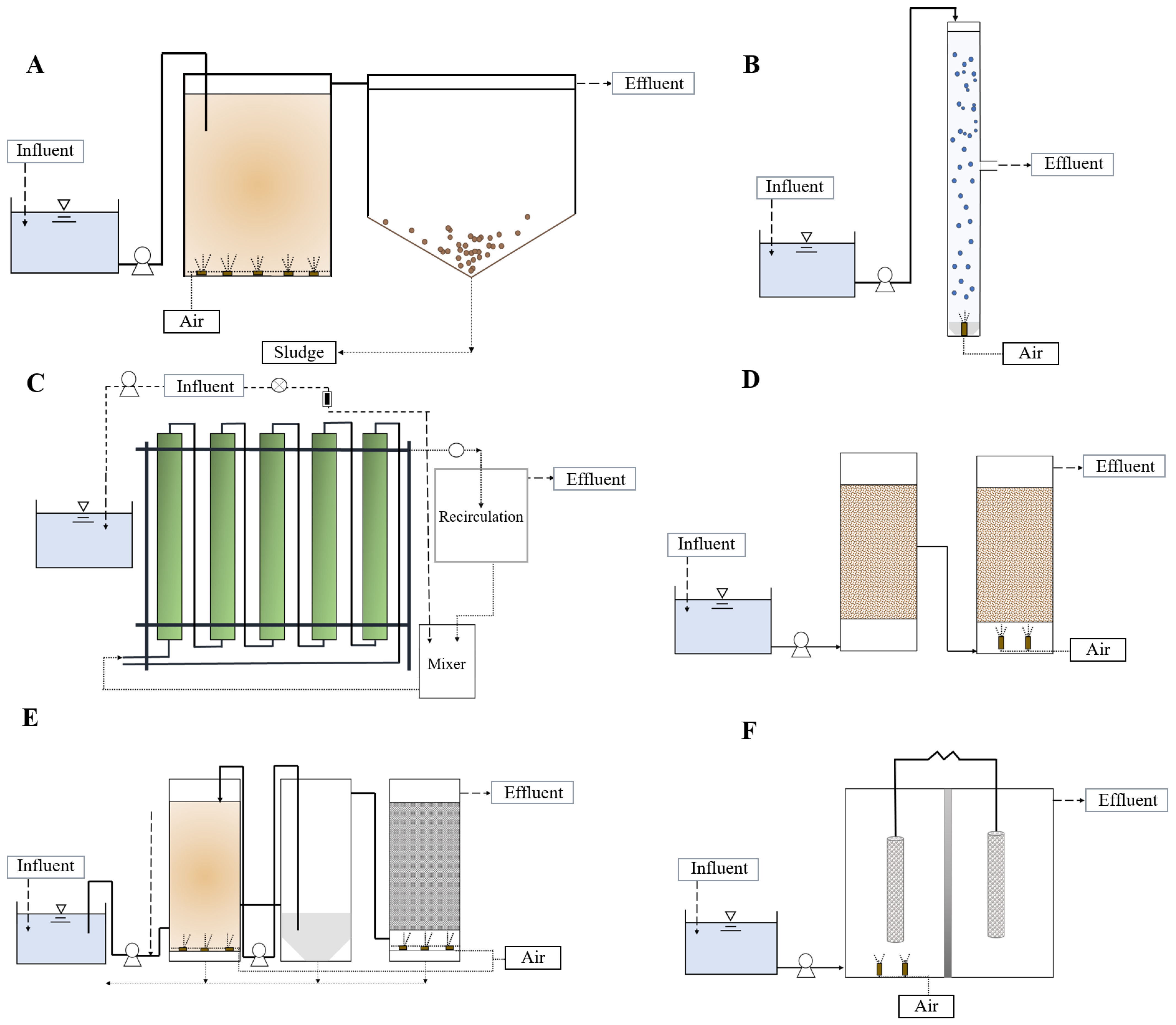
2. Feasible Biological Technologies for the Removal of Phenolic Compounds from Waters
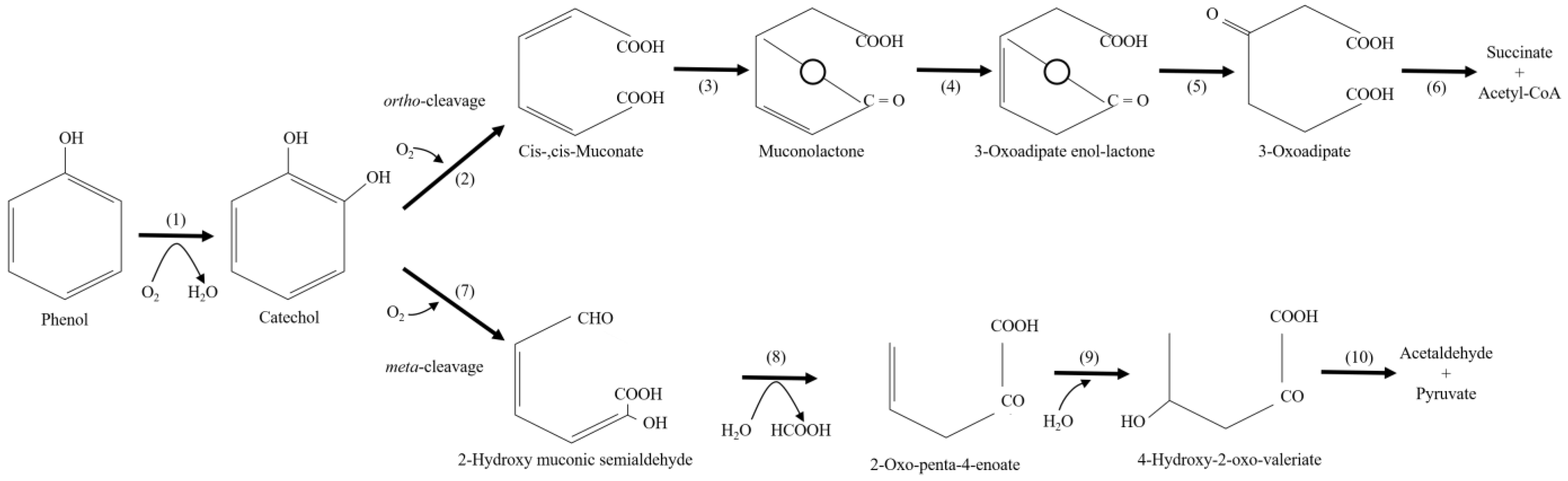
3. Microorganisms and Their Products Involved in Phenolic Biodegradation
This entry is adapted from the peer-reviewed paper 10.3390/molecules28010314
References
- Peeters, K.; Višnjevec, A.M.; Esakkimuthu, E.S.; Schwarzkopf, M.; Tavzes, Č. The Valorisation of Olive Mill Wastewater from Slovenian Istria by Fe3 O4 Particles to Recover Polyphenolic Compounds for the Chemical Specialties Sector. Molecules 2021, 26, 6946.
- Raza, W.; Lee, J.; Raza, N.; Luo, Y.; Kim, K.H.; Yang, J. Removal of Phenolic Compounds from Industrial Waste Water Based on Membrane-Based Technologies. J. Ind. Eng. Chem. 2019, 71, 1–18.
- Hasan, M.; Hakim, A.; Iqbal, A.; Bhuiyan, F.R.; Begum, M.K.; Sharmin, S.; Abir, R.A. Computational Study and Homology Modeling of Phenol Hydroxylase: Key Enzyme for Phenol Degradation. Int. J. Comput. Bioinform. Silico Model. 2015, 4, 691–698.
- Mohd, A. Presence of Phenol in Wastewater Effluent and Its Removal: An Overview. Int. J. Environ. Anal. Chem. 2022, 102, 1362–1384.
- Cladis, D.P.; Weaver, C.M.; Ferruzzi, M.G. (Poly)Phenol Toxicity in Vivo Following Oral Administration: A Targeted Narrative Review of (Poly)Phenols from Green Tea, Grape, and Anthocyanin-Rich Extracts. Phytother. Res. 2022, 36, 323–335.
- Xiao, J.; Xie, Y.; Han, Q.; Cao, H.; Wang, Y.; Nawaz, F.; Duan, F. Superoxide Radical-Mediated Photocatalytic Oxidation of Phenolic Compounds over Ag+/TiO2: Influence of Electron Donating and Withdrawing Substituents. J. Hazard. Mater. 2016, 304, 126–133.
- Mohammadi, S.; Kargari, A.; Sanaeepur, H.; Abbassian, K.; Najafi, A.; Mofarrah, E. Phenol Removal from Industrial Wastewaters: A Short Review. Desalination Water Treat 2015, 53, 2215–2234.
- Muñoz-Palazon, B.; Rodriguez-Sanchez, A.; Hurtado-Martinez, M.; de Castro, I.M.; Juarez-Jimenez, B.; Gonzalez-Martinez, A.; Gonzalez-Lopez, J. Performance and Microbial Community Structure of an Aerobic Granular Sludge System at Different Phenolic Acid Concentrations. J. Hazard. Mater. 2019, 376, 58–67.
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167.
- Vashi, H.; Iorhemen, O.T.; Tay, J.H. Aerobic Granulation: A Recent Development on the Biological Treatment of Pulp and Paper Wastewater. Environ. Technol. Innov. 2018, 9, 265–274.
- Jemaat, Z.; Suárez-Ojeda, M.E.; Pérez, J.; Carrera, J. Sequentially Alternating Pollutant Scenarios of Phenolic Compounds in a Continuous Aerobic Granular Sludge Reactor Performing Simultaneous Partial Nitritation and O-Cresol Biodegradation. Bioresour. Technol. 2014, 161, 354–361.
- Shi, X.; Leong, K.Y.; Ng, H.Y. Anaerobic Treatment of Pharmaceutical Wastewater: A Critical Review. Bioresour. Technol. 2017, 245, 1238–1244.
- Muñoz Sierra, J.D.; Oosterkamp, M.J.; Wang, W.; Spanjers, H.; van Lier, J.B. Comparative Performance of Upflow Anaerobic Sludge Blanket Reactor and Anaerobic Membrane Bioreactor Treating Phenolic Wastewater: Overcoming High Salinity. Chem. Eng. J. 2019, 366, 480–490.
- Muñoz Sierra, J.D.; Oosterkamp, M.J.; Wang, W.; Spanjers, H.; van Lier, J.B. Impact of Long-Term Salinity Exposure in Anaerobic Membrane Bioreactors Treating Phenolic Wastewater: Performance Robustness and Endured Microbial Community. Water Res. 2018, 141, 172–184.
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181.
- Huang, Y.; Hou, X.; Liu, S.; Ni, J. Correspondence Analysis of Bio-Refractory Compounds Degradation and Microbiological Community Distribution in Anaerobic Filter for Coking Wastewater Treatment. Chem. Eng. J. 2016, 304, 864–872.
- More, T.T.; Yadav, J.S.S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular Polymeric Substances of Bacteria and Their Potential Environmental Applications. J. Environ. Manag. 2014, 144, 1–25.
- Vashi, H.; Iorhemen, O.T.; Tay, J.H. Extensive Studies on the Treatment of Pulp Mill Wastewater Using Aerobic Granular Sludge (AGS) Technology. Chem. Eng. J. 2019, 359, 1175–1194.
- Gupta, S.; Nayak, A.; Roy, C.; Yadav, A.K. An Algal Assisted Constructed Wetland-Microbial Fuel Cell Integrated with Sand Filter for Efficient Wastewater Treatment and Electricity Production. Chemosphere 2021, 263, 128132.
- Rengaraj, S.; Moon, S.H.; Sivabalan, R.; Arabindoo, B.; Murugesan, V. Agricultural Solid Waste for the Removal of Organics: Adsorption of Phenol from Water and Wastewater by Palm Seed Coat Activated Carbon. Waste Manag. 2002, 22, 543–548.
- Juárez-Jiménez, B.; Manzanera, M.; Rodelas, B.; Martínez-Toledo, M.V.; Gonzalez-López, J.; Crognale, S.; Pesciaroli, C.; Fenice, M. Metabolic characterization of a strain (BM90) of Delftia tsuruhatensis showing highly diversified capacity to degrade low molecular weight phenols. Biodegradation 2010, 21, 475–489.
- Juarez Jimenez, B.; Reboleiro Rivas, P.; Gonzalez Lopez, J.; Pesciaroli, C.; Barghini, P.; Fenice, M. Immobilization of Delftia Tsuruhatensis in Macro-Porous Cellulose and Biodegradation of Phenolic Compounds in Repeated Batch Process. J. Biotechnol. 2012, 157, 148–153.
- Maza-Márquez, P.; González-Martínez, A.; Martínez-Toledo, M.V.; Fenice, M.; Lasserrot, A.; González-López, J. Biotreatment of Industrial Olive Washing Water by Synergetic Association of Microalgal-Bacterial Consortia in a Photobioreactor. Environ. Sci. Pollut. Res. 2017, 24, 527–538.
- Prasad, R.; Aranda, E. Approaches in Bioremediation: The New Era of Environmental Microbiology and Nanobiotechnology; Springer: Cham, Switzerland, 2018; ISBN 978-3-030-02368-3.
- Satish, K.; Neeraj; Viraj, K.M.; Santosh, K.K. Biodegradation of Phenol by Free and Immobilized Candida Tropicalis NPD1401. Afr. J. Biotechnol. 2018, 17, 57–64.
- Krastanov, A.; Alexieva, Z.; Yemendzhiev, H. Microbial Degradation of Phenol and Phenolic Derivatives. Eng. Life Sci. 2013, 13, 76–87.
- Arora, P.K.; Srivastava, A.; Singh, V.P. Bacterial Degradation of Nitrophenols and Their Derivatives. J. Hazard. Mater. 2014, 266, 42–59.
- Arora, P.K.; Srivastava, A.; Garg, S.K.; Singh, V.P. Recent Advances in Degradation of Chloronitrophenols. Bioresour. Technol. 2018, 250, 902–909.
- Arora, P.K.; Sasikala, C.; Ramana, C.V. Degradation of Chlorinated Nitroaromatic Compounds. Appl. Microbiol. Biotechnol. 2012, 93, 2265–2277.
- Singh, T.; Bhatiya, A.K.; Mishra, P.K.; Srivastava, N. An effective approach for the degradation of phenolic waste. In Abatement of Environmental Pollutants; Singh, P., Kumar, A., Borthaku, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–243.
- Arora, P.K.; Jain, R.K. Arthrobacter Nitrophenolicus Sp. Nov. a New 2-Chloro-4-Nitrophenol Degrading Bacterium Isolated from Contaminated Soil. 3 Biotech 2013, 3, 29–32.
- Arora, P.K.; Jain, R.K. Metabolism of 2-Chloro-4-Nitrophenol in a Gram Negative Bacterium, Burkholderia Sp. RKJ 800. PLoS ONE 2012, 7, e38676.
- Dulak, K.; Sordon, S.; Matera, A.; Kozak, B.; Huszcza, E.; Popłoński, J. Novel Flavonoid C-8 Hydroxylase from Rhodotorula Glutinis: Identification, Characterization and Substrate Scope. Microb. Cell Fact. 2022, 21, 175.
- Wilhelm, R.C.; Cyle, K.T.; Martinez, C.E.; Karasz, D.C.; Newman, J.D.; Buckley, D.H. Paraburkholderia Solitsugae Sp. Nov. and Paraburkholderia Elongata Sp. Nov., Phenolic Acid-Degrading Bacteria Isolated from Forest Soil and Emended Description of Paraburkholderia Madseniana. Int. J. Syst. Evol. Microbiol. 2020, 70, 5093–5105.
- Maza-Márquez, P.; Martínez-Toledo, M.V.; González-López, J.; Rodelas, B.; Juárez-Jiménez, B.; Fenice, M. Biodegradation of Olive Washing Wastewater Pollutants by Highly Efficient Phenol-Degrading Strains Selected from Adapted Bacterial Community. Int. Biodeterior. Biodegrad. 2013, 82, 192–198.
- Elmansour, T.E.; Mandi, L.; Ahmali, A.; Elghadraoui, A.; Aziz, F.; Hejjaj, A.; Del Bubba, M.; Ouazzani, N. Effect of Polyphenols on Activated Sludge Biomass during the Treatment of Highly Diluted Olive Mill Wastewaters: Biomass Dynamics and Purifying Performances. Water Sci. Technol. 2020, 82, 1416–1429.
- Martínková, L.; Kotik, M.; Marková, E.; Homolka, L. Biodegradation of Phenolic Compounds by Basidiomycota and Its Phenol Oxidases: A Review. Chemosphere 2016, 149, 373–382.
- Baldrian, P. Fungal Laccases-Occurrence and Properties. FEMS Microbiol. Rev. 2006, 30, 215–242.
- Ikehata, K.; Buchanan, I.D.; Smith, D.W. Recent Developments in the Production of Extracellular Fungal Peroxidases and Laccases for Waste Treatment. J. Environ. Eng. Sci. 2004, 3, 1–19.
- Shukla, A.C. Applied Mycology Entrepreneurship with Fungi; Springer: Cham, Switzerland, 2022; ISBN 9783030906481.
- Otto, B.; Schlosser, D. First Laccase in Green Algae: Purification and Characterization of an Extracellular Phenol Oxidase from Tetracystis Aeria. Planta 2014, 240, 1225–1236.
- Lindner, A.V.; Pleissner, D. Utilization of Phenolic Compounds by Microalgae. Algal. Res. 2019, 42, 101602.
- Wang, L.; Xue, C.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Strain Improvement of Chlorella Sp. for Phenol Biodegradation by Adaptive Laboratory Evolution. Bioresour. Technol. 2016, 205, 264–268.
- Di Caprio, F.; Scarponi, P.; Altimari, P.; Iaquaniello, G.; Pagnanelli, F. The Influence of Phenols Extracted from Olive Mill Wastewater on the Heterotrophic and Mixotrophic Growth of Scenedesmus sp. J. Chem. Technol. Biotechnol. 2018, 93, 3619–3626.
- Pinto, G.; Pollio, A.; Previtera, L.; Stanzione, M.; Temussi, F. Removal of Low Molecular Weight Phenols from Olive Oil Mill Wastewater Using Microalgae. Biotechnol. Lett. 2003, 25, 1657–1659.
