Upper gastrointestinal (GI) malignancy is a leading cause of cancer-related morbidity and mortality. Upper endoscopy has an established role in diagnosing and staging upper GI cancers, screening for pre-malignant lesions, and providing palliation in cases of advanced malignancy. New advances in endoscopic techniques and technology have improved diagnostic accuracy and increased the therapeutic potential of upper endoscopy.
- upper gastrointestinal cancer
- endoscopy
- cancer
1. Introduction
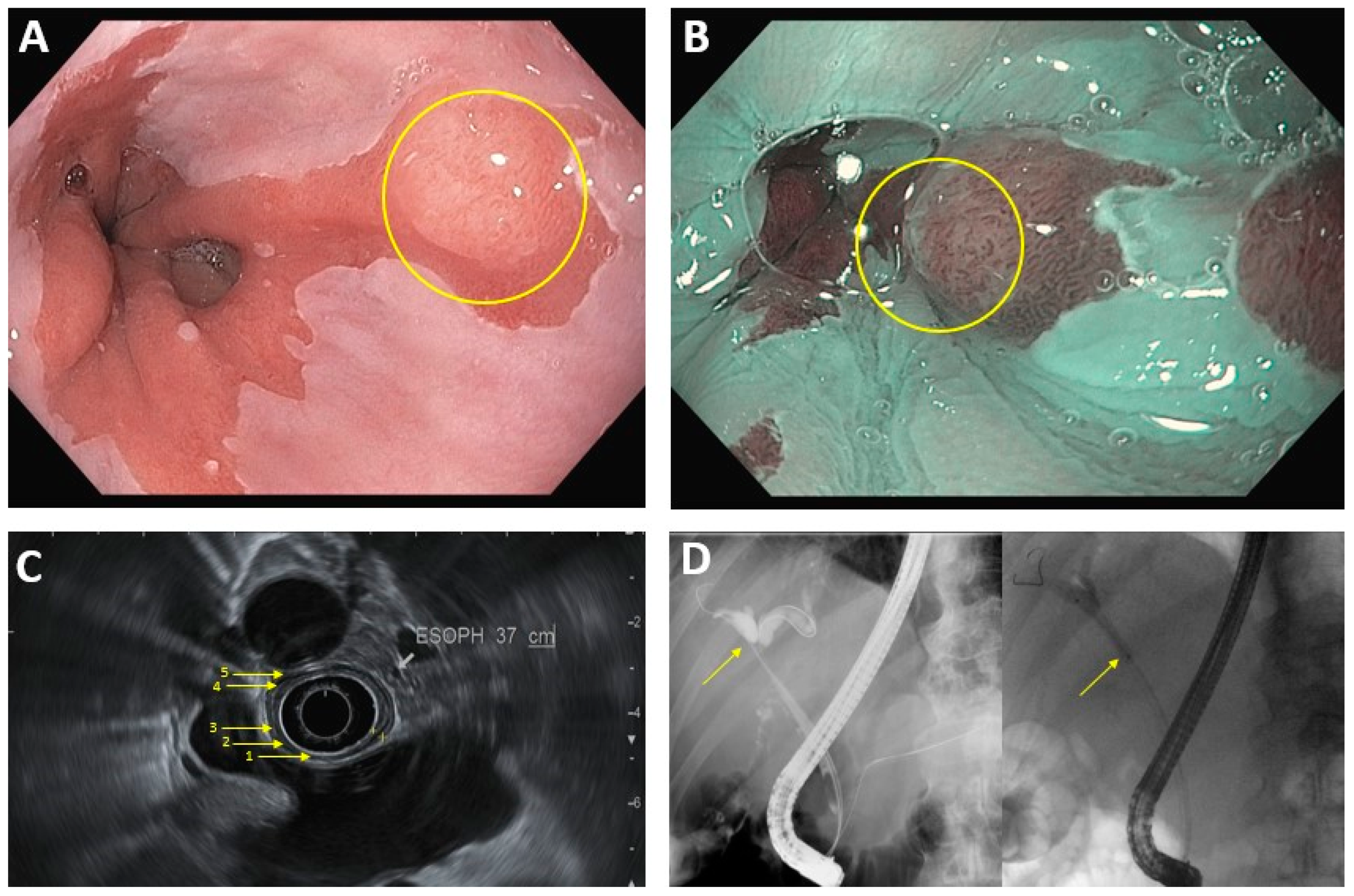
2. Diagnosis and Staging
2.1. Luminal Upper Gastrointestinal Cancer
| Staging Modalities | Endoscopic Treatment Options | |
|---|---|---|
| Luminal Upper GI cancer a | EUS: -First line for T-staging (sensitivity is 81% for stage T1/T2, >90% for T3/T4) and N-staging -EUS-FNA can help for N-staging via lymph node biopsy, although results can be technique-limited CT: -Used for M-staging -Lower sensitivity and specificity for N-staging, when compared to EUS |
Endoscopic techniques (EMR, ESD) generally feasible for T1a tumors ≤2 cm Surgical resection vs systemic therapy for larger and more advanced tumors |
| Extraluminal upper GI cancer b | EUS: -Sensitivity and specificity for T and N staging highest in pancreatic cancer (compared to gallbladder cancer or cholangiocarcinoma) Laparoscopy: -Most accurate diagnostic modality for gallbladder cancer and cholangiocarcinoma Cross-sectional imaging: -Modality of choice for diagnosing intrahepatic cholangiocarcinoma and evaluating resectability in pancreatic cancer -Used for M-staging |
Generally, endoscopy has only a palliative role (RFA, stenting) |
2.2. Pancreaticobiliary Cancer
3. Treatment of Cancer
3.1. Esophageal Squamous Cell Carcinoma/Adenocarcinoma
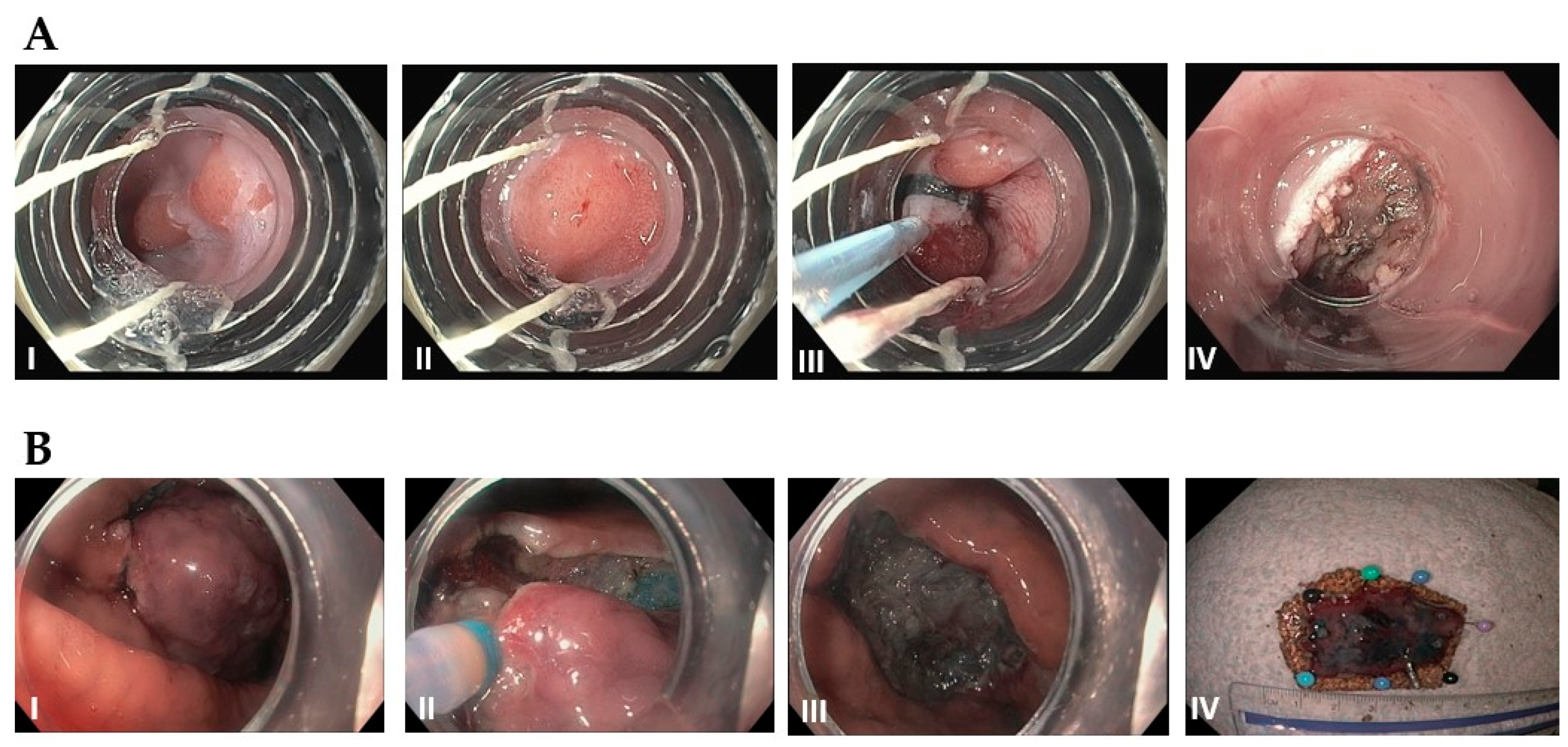
3.2. Gastric Cancer
3.3. Gastrointestinal Stromal Tumor
3.4. Pancreatic and Ampullary Cancer
3.5. Extrahepatic Cholangiocarcinoma
4. Conclusion
Recent advancements have made endoscopic treatments the preferred technique for resection of small focal tumors. Additionally, early studies in the areas of endoscopic intra-tumoral injections and EUS-guided brachytherapy seed placement have shown promising results. While further studies are needed to determine the clinical benefit of these procedures, these developments demonstrate the potential to supplement existing treatments and expand the ever increasing role for the endoscopic oncologist.
This entry is adapted from the peer-reviewed paper 10.3390/diseases11010003
References
- Axon, A.T.R. Fifty years of digestive endoscopy: Successes, setbacks, solutions and the future. Dig. Endosc. 2019, 32, 290–297.
- Teh, J.-L.; Shabbir, A.; Yuen, S.; So, J.B.-Y. Recent advances in diagnostic upper endoscopy. World J. Gastroenterol. 2020, 26, 433–447.
- Subramanian, V.; Ragunath, K. Advanced Endoscopic Imaging: A Review of Commercially Available Technologies. Clin. Gastroenterol. Hepatol. 2014, 12, 368–376.e1.
- Akarsu, M. Evaluation of New Technologies in Gastrointestinal Endoscopy. JSLS J. Soc. Laparoendosc. Surg. 2018, 22, e2017.00053.
- Shin, D.; Protano, M.-A.; Polydorides, A.D.; Dawsey, S.M.; Pierce, M.C.; Kim, M.K.; Schwarz, R.A.; Quang, T.; Parikh, N.; Bhutani, M.S.; et al. Quantitative Analysis of High-Resolution Microendoscopic Images for Diagnosis of Esophageal Squamous Cell Carcinoma. Clin. Gastroenterol. Hepatol. 2014, 13, 272–279.e2.
- Song, L.-M.W.K.; Banerjee, S.; Desilets, D.; Diehl, D.L.; Farraye, F.A.; Kaul, V.; Kethu, S.R.; Kwon, R.S.; Mamula, P.; Pedrosa, M.C.; et al. Autofluorescence imaging. Gastrointest. Endosc. 2011, 73, 647–650.
- Jang, J.-Y. The Past, Present, and Future of Image-Enhanced Endoscopy. Clin. Endosc. 2015, 48, 466–475.
- Shukla, R.; Abidi, W.M.; Richards-Kortum, R.; Anandasabapathy, S. Endoscopic imaging: How far are we from real-time histology? World J. Gastrointest. Endosc. 2011, 3, 183–194.
- Bhutani, M.S.; Cazacu, I.M.; Chavez, A.A.L.; Saftoiu, A.; Vilmann, P. A quarter century of EUS-FNA: Progress, milestones, and future directions. Endosc. Ultrasound 2018, 7, 141–160.
- Bang, J.Y.; Navaneethan, U.; Hasan, M.; Hawes, R.; Varadarajulu, S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: A randomized trial (with videos). Gastrointest. Endosc. 2018, 88, 9–17.
- Yousaf, M.N.; Ehsan, H.; Wahab, A.; Muneeb, A.; Chaudhary, F.S.; Williams, R.; Haas, C.J. Endoscopic retrograde cholangiopancreatography guided interventions in the management of pancreatic cancer. World J. Gastrointest. Endosc. 2020, 12, 323–340.
- Hanada, K.; Minami, T.; Shimizu, A.; Fukuhara, M.; Yano, S.; Sasaki, K.; Koda, M.; Sugiyama, K.; Yonehara, S.; Yanagisawa, A. Roles of ERCP in the Early Diagnosis of Pancreatic Cancer. Diagnostics 2019, 9, 30.
- Friedberg, S.R.; Lachter, J. Endoscopic ultrasound: Current roles and future directions. World J. Gastrointest. Endosc. 2017, 9, 499–505.
- Yeo, S.T.; Bray, N.; Haboubi, H.; Hoare, Z.; Edwards, R.T. Endoscopic ultrasound staging in patients with gastro-oesophageal cancers: A systematic review of economic evidence. BMC Cancer 2019, 19, 900.
- Bhutani, M.; Cazacu, I.; Singh, B.; Saftoiu, A. Recent developments in hepatopancreatobiliary EUS. Endosc. Ultrasound 2019, 8, 146–150.
- Early, D.S.; Ben-Menachem, T.; Decker, G.A.; Evans, J.A.; Fanelli, R.D.; Fisher, D.A.; Fukami, N.; Hwang, J.H.; Jain, R.; Jue, T.L.; et al. Appropriate use of GI endoscopy. Gastrointest. Endosc. 2012, 75, 1127–1131.
- Polkowski, M. Endosonographic staging of upper intestinal malignancy. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 649–661.
- Boniface, M.; Wani, S.; Schefter, T.; Koo, P.; Meguid, C.; Leong, S.; Kaplan, J.; Wingrove, L.; McCarter, M. Multidisciplinary management for esophageal and gastric cancer. Cancer Manag. Res. 2016, 8, 39–44.
- Pilonis, N.D.; Januszewicz, W.; di Pietro, M. Confocal laser endomicroscopy in gastro-intestinal endoscopy: Technical aspects and clinical applications. Transl. Gastroenterol. Hepatol. 2022, 7, 7.
- Gaddam, S.; Mathur, S.C.; Singh, M.; Arora, J.; Wani, S.B.; Gupta, N.; Overhiser, A.; Rastogi, A.; Singh, V.; Desai, N.; et al. Novel Probe-Based Confocal Laser Endomicroscopy Criteria and Interobserver Agreement for the Detection of Dysplasia in Barrett’s Esophagus. Am. J. Gastroenterol. 2011, 106, 1961–1969.
- Yoon, H. New approaches to gastric cancer staging: Beyond endoscopic ultrasound, computed tomography and positron emission tomography. World J. Gastroenterol. 2014, 20, 13783–13790.
- Zhu, Z.; Gong, Y.; Xu, H. Clinical and pathological staging of gastric cancer: Current perspectives and implications. Eur. J. Surg. Oncol. (EJSO) 2020, 46, e14–e19.
- Papanikolaou, I.S.; Triantafyllou, M.; Triantafyllou, K.; Rösch, T. EUS in the management of gastric cancer. Ann. Gastroenterol. 2011, 24, 9–15.
- Valero, M.; Robles-Medranda, C. Endoscopic ultrasound in oncology: An update of clinical applications in the gastrointestinal tract. World J. Gastrointest. Endosc. 2017, 9, 243–254.
- Thakkar, S.; Kaul, V. Endoscopic Ultrasound Stagingof Esophageal Cancer. Gastroenterol. Hepatol. 2020, 16, 14–20.
- Thosani, N.; Singh, H.; Kapadia, A.; Ochi, N.; Lee, J.H.; Ajani, J.; Swisher, S.G.; Hofstetter, W.L.; Guha, S.; Bhutani, M.S. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: A systematic review and meta-analysis. Gastrointest. Endosc. 2012, 75, 242–253.
- Krill, T.; Baliss, M.; Roark, R.; Sydor, M.; Samuel, R.; Zaibaq, J.; Guturu, P.; Parupudi, S. Accuracy of endoscopic ultrasound in esophageal cancer staging. J. Thorac. Dis. 2019, 11, S1602–S1609.
- Pouw, R.E.; Heldoorn, N.; Herrero, L.A.; Kate, F.J.T.; Visser, M.; Busch, O.R.; Henegouwen, M.I.V.B.; Krishnadath, K.K.; Weusten, B.L.; Fockens, P.; et al. Do we still need EUS in the workup of patients with early esophageal neoplasia? A retrospective analysis of 131 cases. Gastrointest. Endosc. 2011, 73, 662–668.
- Young, P.E.; Gentry, A.B.; Acosta, R.D.; Greenwald, B.D.; Riddle, M. Endoscopic Ultrasound Does Not Accurately Stage Early Adenocarcinoma or High-Grade Dysplasia of the Esophagus. Clin. Gastroenterol. Hepatol. 2010, 8, 1037–1041.
- He, L.-J. Endoscopic ultrasonography for staging of T1a and T1b esophageal squamous cell carcinoma. World J. Gastroenterol. 2014, 20, 1340–1347.
- Prasad, G.A.; Wu, T.T.; Wigle, D.A.; Buttar, N.S.; Wongkeesong, L.; Dunagan, K.T.; Lutzke, L.S.; Borkenhagen, L.S.; Wang, K.K. Endoscopic and Surgical Treatment of Mucosal (T1a) Esophageal Adenocarcinoma in Barrett’s Esophagus. Gastroenterology 2009, 137, 815–823.
- Ngamruengphong, S.; Ferri, L.; Aihara, H.; Draganov, P.V.; Yang, D.J.; Perbtani, Y.B.; Jue, T.L.; Munroe, C.A.; Boparai, E.S.; Mehta, N.A.; et al. Efficacy of Endoscopic Submucosal Dissection for Superficial Gastric Neoplasia in a Large Cohort in North America. Clin. Gastroenterol. Hepatol. 2020, 19, 1611–1619.e1.
- Saftoiu, A.; Vilmann, P. Role of endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. J. Clin. Ultrasound 2009, 37, 1–17.
- Wang, B.-C.; Wang, K.K.; Paul, N.; Jayaraman, V.; Wang, Q.; Abboud, Y.; Jamil, L.H.; Gaddam, S.; Lo, S.K. Fluoroscopy-guided shaped endobiliary biopsy at endoscopic retrograde cholangiography can accurately diagnose biliary neoplasia: Results from a large cohort. Endosc. Int. Open 2021, 9, E1039–E1048.
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Prim. 2021, 7, 1–17.
- Duffy, A.; Capanu, M.; Abou-Alfa, G.; Huitzil, D.; Jarnagin, W.; Fong, Y.; D’Angelica, M.; DeMatteo, R.; Blumgart, L.; O’Reilly, E. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J. Surg. Oncol. 2008, 98, 485–489.
- Gurusamy, K.S.; Abu-Amara, M.; Farouk, M.; Davidson, B.R. Cholecystectomy for gallbladder polyp. Cochrane Database Syst. Rev. 2009, 2009, CD007052.
- Ito, H.; Ito, K.; D’Angelica, M.; Gonen, M.; Klimstra, D.; Allen, P.; DeMatteo, R.P.; Fong, Y.; Blumgart, L.H.; Jarnagin, W.R. Accurate Staging for Gallbladder Cancer: Implications for Surgical Therapy and Pathological Assessment. Ann. Surg. 2011, 254, 320–325.
- Azuma, T.; Yoshikawa, T.; Araida, T.; Takasaki, K. Differential diagnosis of polypoid lesions of the gallbladder by endoscopic ultrasonography. Am. J. Surg. 2001, 181, 65–70.
- Hijioka, S.; Hara, K.; Mizuno, N.; Imaoka, H.; Ogura, T.; Haba, S.; Mekky, M.A.; Bhatia, V.; Hosoda, W.; Yatabe, Y.; et al. Diagnostic yield of endoscopic retrograde cholangiography and of EUS-guided fine needle aspiration sampling in gallbladder carcinomas. J. Hepato-Biliary-Pancreatic Sci. 2011, 19, 650–655.
- Appel, B.L.; Tolat, P.; Evans, D.B.; Tsai, S. Current Staging Systems for Pancreatic Cancer. Cancer J. 2012, 18, 539–549.
- Noordzij, I.C.; Curvers, W.L.; Schoon, E.J. Endoscopic resection for early esophageal carcinoma. J. Thorac. Dis. 2019, 11, S713–S722.
- Shah, P.M.; Gerdes, H. Endoscopic options for early stage esophageal cancer. J. Gastrointest. Oncol. 2015, 6, 20–30.
- Park, C.H.; Yang, D.-H.; Kim, J.W.; Kim, J.-H.; Min, Y.W.; Lee, S.H.; Bae, J.H.; Chung, H.; Choi, K.D.; Park, J.C.; et al. Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer. Clin. Endosc. 2020, 53, 142–166.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21.
- Ahn, J.Y.; Park, H.J.; Park, Y.S.; Lee, J.H.; Choi, K.-S.; Jeong, K.W.; Kim, D.H.; Choi, K.D.; Song, H.J.; Lee, G.H.; et al. Endoscopic Resection for Undifferentiated-Type Early Gastric Cancer: Immediate Endoscopic Outcomes and Long-Term Survivals. Am. J. Dig. Dis. 2015, 61, 1158–1164.
- Ahmed, M. Gastrointestinal neuroendocrine tumors in 2020. World J. Gastrointest. Oncol. 2020, 12, 791–807.
- Sato, Y.; Hashimoto, S.; Mizuno, K.-I.; Takeuchi, M.; Terai, S. Management of gastric and duodenal neuroendocrine tumors. World J. Gastroenterol. 2016, 22, 6817–6828.
- Scherübl, H.; Cadiot, G.; Jensen, R.; Rösch, T.; Stölzel, U.; Klöppel, G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: Small tumors, small problems? Endoscopy 2010, 42, 664–671.
- Sivandzadeh, G.R.; Ejtehadi, F.; Shoaee, S.; Aminlari, L.; Niknam, R.; Taghavi, A.R.; Geramizadeh, B.; Hormati, A.; Safarpour, A.R.; Lankarani, K.B. Endoscopic mucosal resection: Still a reliable therapeutic option for gastrointestinal neuroendocrine tumors. BMC Gastroenterol. 2021, 21, 1–6.
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 839–868.
- Kwon, Y.H. Long-term follow up of endoscopic resection for type 3 gastric NET. World J. Gastroenterol. 2013, 19, 8703–8708.
- Wang, H.; Feng, X.; Ye, S.; Wang, J.; Liang, J.; Mai, S.; Lai, M.; Feng, H.; Wang, G.; Zhou, Y. A comparison of the efficacy and safety of endoscopic full-thickness resection and laparoscopic-assisted surgery for small gastrointestinal stromal tumors. Surg. Endosc. 2015, 30, 3357–3361.
- Andalib, I.; Yeoun, D.; Reddy, R.; Xie, S.; Iqbal, S. Endoscopic resection of gastric gastrointestinal stromal tumors originating from the muscularis propria layer in North America: Methods and feasibility data. Surg. Endosc. 2017, 32, 1787–1792.
- Wang, W.; Liu, C.-X.; Niu, Q.; Wang, A.-L.; Shi, N.; Ma, F.-Z.; Hu, Y.-B. OTSC assisted EFTR for the treatment of GIST: 40 cases analysis. Minim. Invasive Ther. Allied Technol. 2020, 31, 238–245.
- Hadjicostas, P.; Malakounides, N.; Varianos, C.; Kitiris, E.; Lerni, F.; Symeonides, P. Radiofrequency ablation in pancreatic cancer. Hpb 2006, 8, 61–64.
- Yousaf, M.N.; Ehsan, H.; Muneeb, A.; Wahab, A.; Sana, M.K.; Neupane, K.; Chaudhary, F.S. Role of Radiofrequency Ablation in the Management of Unresectable Pancreatic Cancer. Front. Med. 2021, 7, 1070.
- Park, D.H.; Choi, J.-H.; Oh, D.; Lee, S.S.; Seo, D.-W.; Lee, S.K.; Kim, M.-H. Endoscopic Ultrasonography-Guided Ethanol Ablation for Small Pancreatic Neuroendocrine Tumors: Results of a Pilot Study. Clin. Endosc. 2015, 48, 158–164.
- Oh, H.-C.; Seo, D.W.; Song, T.J.; Moon, S.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.; Kim, J. Endoscopic Ultrasonography-Guided Ethanol Lavage With Paclitaxel Injection Treats Patients With Pancreatic Cysts. Gastroenterology 2011, 140, 172–179.
- Song, T.J.; Seo, D.W.; Lakhtakia, S.; Reddy, N.; Oh, D.W.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.-H. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest. Endosc. 2015, 83, 440–443.
- Chathadi, K.V.; Khashab, M.A.; Acosta, R.D.; Chandrasekhara, V.; Eloubeidi, M.A.; Faulx, A.L.; Fonkalsrud, L.; Lightdale, J.R.; Saltzman, J.R.; Shaukat, A.; et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest. Endosc. 2015, 82, 773–781.
- Seewald, S.; Omar, S.; Soehendra, N. Endoscopic resection of tumors of the ampulla of Vater: How far up and how deep down can we go? Gastrointest. Endosc. 2006, 63, 789–791.
- Alali, A.; Espino, A.; Moris, M.; Martel, M.; Schwartz, I.; Cirocco, M.; Streutker, C.; Mosko, J.; Kortan, P.; Barkun, A.; et al. Endoscopic Resection of Ampullary Tumours: Long-term Outcomes and Adverse Events. J. Can. Assoc. Gastroenterol. 2019, 3, 17–25.
- Pérez-Cuadrado-Robles, E.; Piessevaux, H.; Moreels, T.G.; Yeung, R.; Aouattah, T.; Komuta, M.; Dano, H.; Jouret-Mourin, A.; Deprez, P.H. Combined excision and ablation of ampullary tumors with biliary or pancreatic intraductal extension is effective even in malignant neoplasms. United Eur. Gastroenterol. J. 2019, 7, 369–376.
- Sofi, A.A.; Khan, M.A.; Das, A.; Sachdev, M.; Khuder, S.; Nawras, A.; Lee, W. Radiofrequency ablation combined with biliary stent placement versus stent placement alone for malignant biliary strictures: A systematic review and meta-analysis. Gastrointest. Endosc. 2018, 87, 944–951.e1.
- Yang, J.; Wang, J.; Zhou, H.; Wang, Y.; Huang, H.; Jin, H.; Lou, Q.; Shah, R.J.; Zhang, X. Endoscopic radiofrequency ablation plus a novel oral 5-fluorouracil compound versus radiofrequency ablation alone for unresectable extrahepatic cholangiocarcinoma. Gastrointest. Endosc. 2020, 92, 1204–1212.e1.
