Grapevine crown gall (GCG), which is caused by tumorigenic Allorhizobium vitis (=Rhizobium vitis), is the most important bacterial disease in grapevine, and its economic impact on grapevine is very high. When young vines develop GCG, they often die, whereas older vines may show stress and poor growth depending on the severity of GCG, because GCG interferes with the vascular system of the grapevine trunk and prevents nutrient flow, leading to inferior growth and death. Viticultural practices and chemical control designed to inhibit GCG are only partially effective presently; thus, a biocontrol procedure could be a desirable and effective approach for GCG prevention.
- Allorhizobium vitis
- grapevine crown gall
- biocontrol
1. Introduction
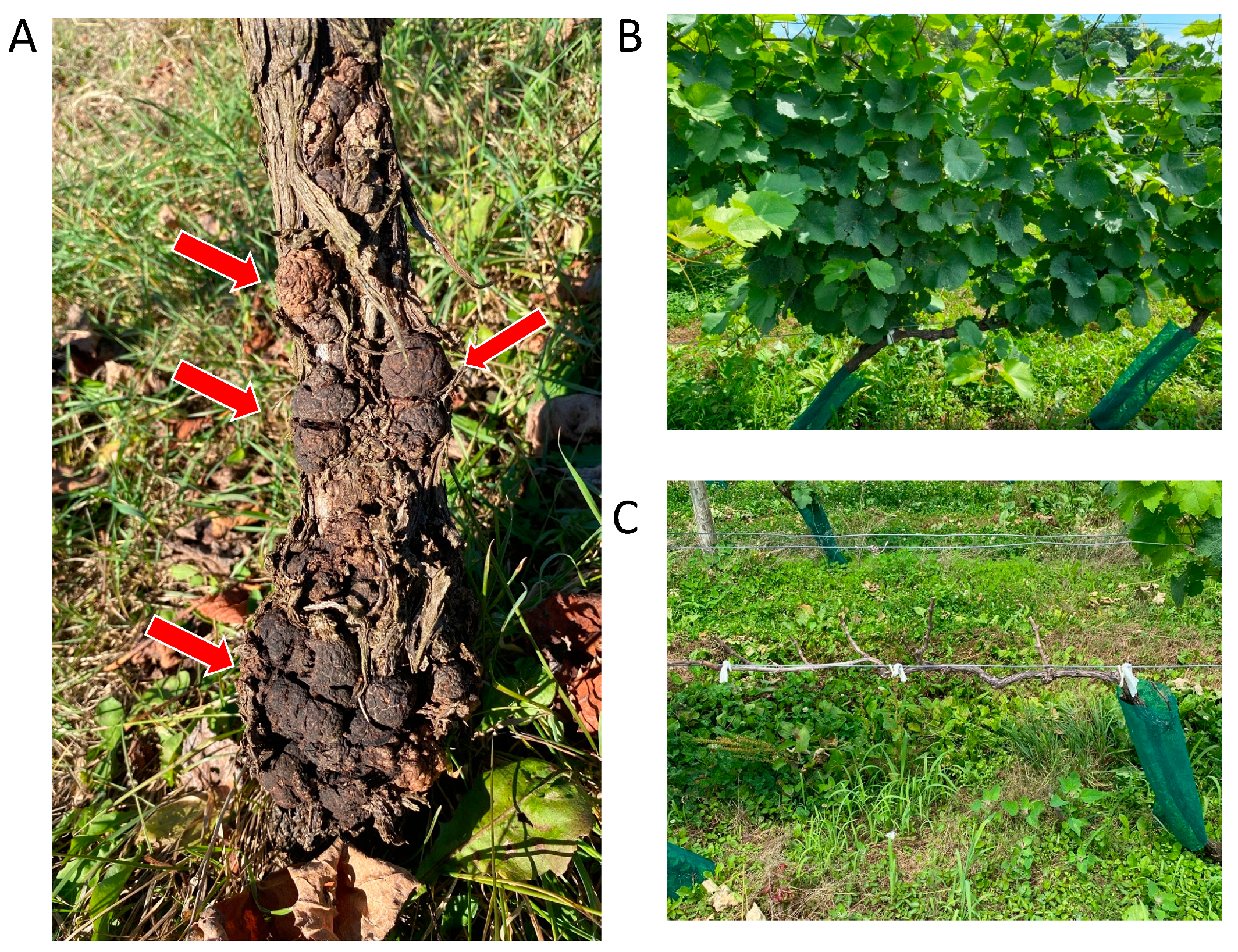
1.1. What Is Crown Gall?
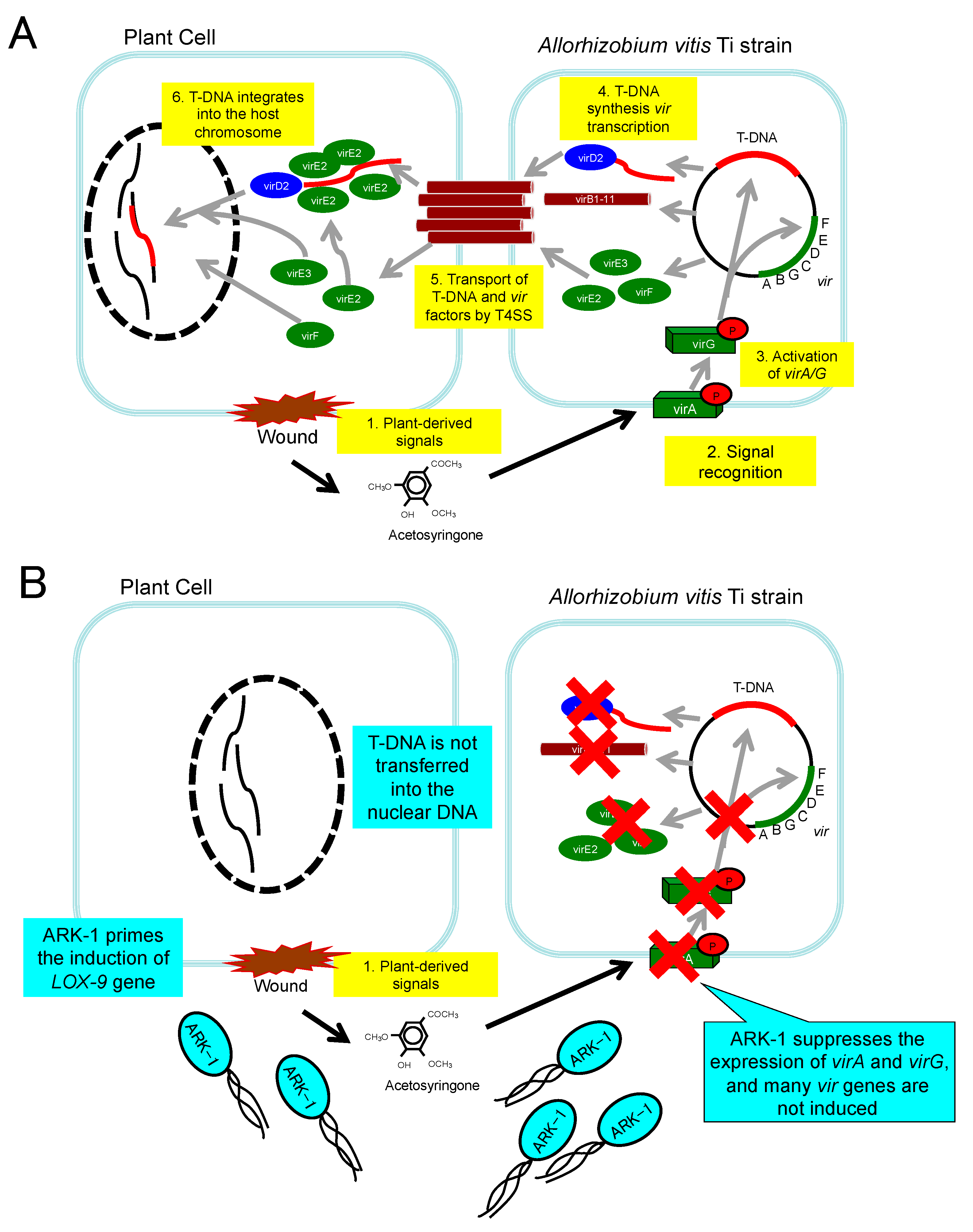
1.2. Mechanism of GCG Development
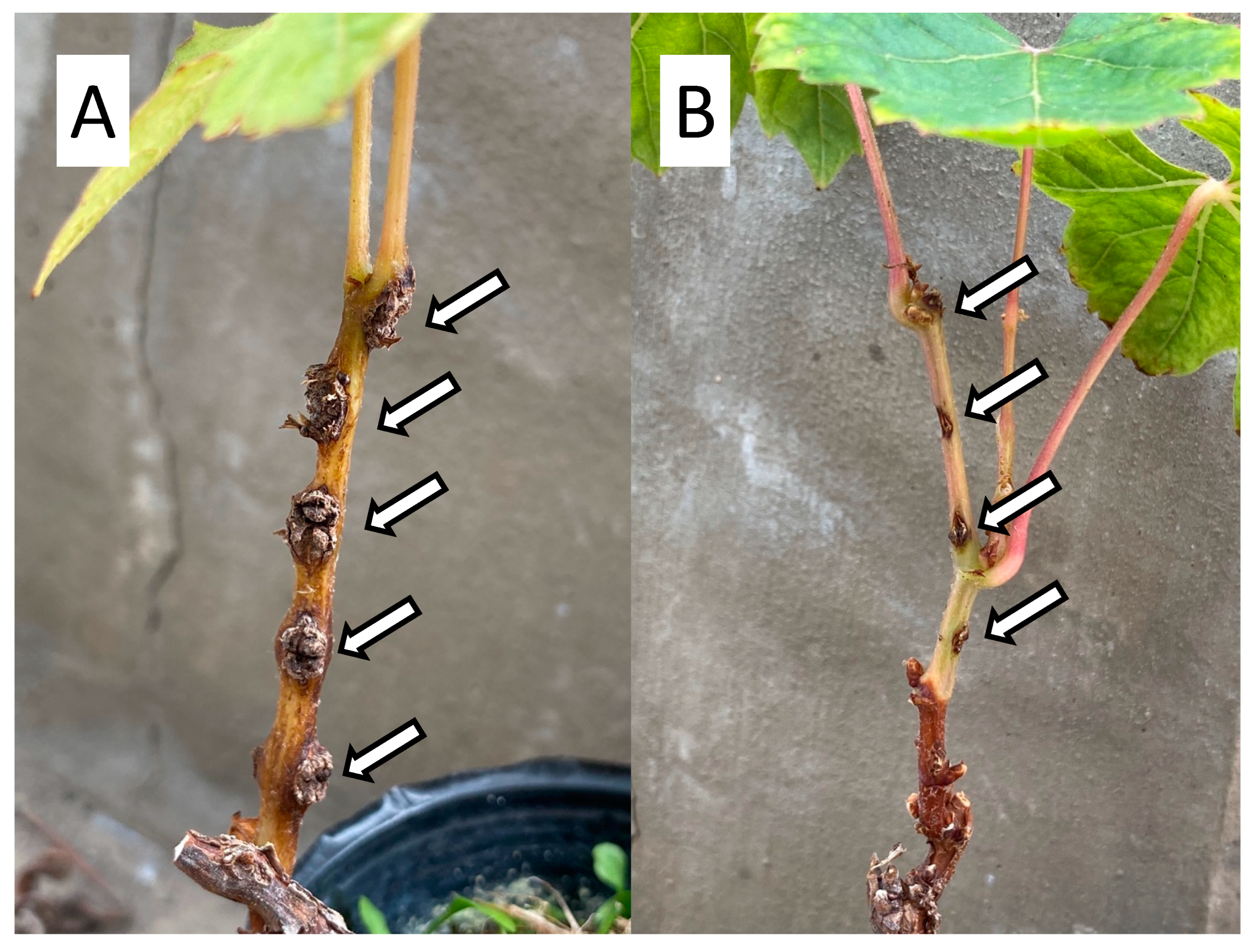
1.3. Necessity of GCG Management
| Bacterium | Strain | Origin | Reference |
|---|---|---|---|
| Allorhizobium vitis (nonpathogenic) | VAR03-1 | Grapevine, Japan | [2][4][33][34][36][37][42][43][44] |
| Allorhizobium vitis (nonpathogenic) | ARK-1 | Grapevine, Japan | [4][35][40][41][45][46] |
| Allorhizobium vitis (nonpathogenic) | ARK-2 | Grapevine, Japan | [4][35] |
| Allorhizobium vitis (nonpathogenic) | ARK-3 | Grapevine, Japan | [4][35] |
| Allorhizobium vitis (nonpathogenic) | F2/5 | Grapevine, South Africa | [20][21][22][23][24] |
| Rhizobium rhizogenes (tumorigenic) | J73 | Plum, South Africa | [25] |
| Allorhizobium vitis (nonpathogenic) | E26 | Grapevine, China | [27][28] |
| Agrobacterium radiobacter (nonpathogenic) | HLB-2 | Hop, China | [26] |
| Rahnella aquatilis | HX2 | Grapevine, China | [29] |
| Agrobacterium radiobacter (nonpathogenic) | MI15 | Grapevine, China | [30] |
2. Screening Tests for Biocontrol Agents
3. Root-Dipping Inoculation for Practical Use
3.1. Field Trials
3.2. Population Dynamics of ARK-1 in Roots of Grapevine
This entry is adapted from the peer-reviewed paper 10.3390/applmicrobiol2040075
References
- Kado, C.I. Crown gall. The Plant Health Instructor 2002.
- Kawaguchi, A. Biological control for grapevine crown gall. In Grapevines: Varieties, Cultivation and Management; Szabo, P.V., Shojania, J., Eds.; Nova Science Publishers: New York, NY, USA, 2012; pp. 153–167.
- Burr, T.J.; Bazzi, C.; Süle, S.; Otten, L. Crown gall of grape: Biology of Agrobacterium vitis and the development of disease control strategies. Plant Dis. 1998, 82, 1288–1297.
- Kawaguchi, A.; Inoue, K.; Tanina, K.; Nita, M. Biological control for grapevine crown gall using nonpathogenic Rhizobium vitis strain ARK-1. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 547–560.
- Kawaguchi, A.; Inoue, K. Grapevine crown gall caused by Rhizobium radiobacter (Ti) in Japan. J. Gen. Plant Pathol. 2009, 75, 205–212.
- Mousavi, S.A.; Willems, A.; Nesme, X.; de Lajudie, P.; Lindstrom, K. Revised phylogeny of Rhizobiaceae: Proposal of the delineation of Pararhizobium gen. nov., and 13 new species combinations. Syst. Appl. Microbiol. 2015, 38, 84–90.
- Kawaguchi, A. Risk assessment of inferior growth and death of grapevines due to crown gall. Euro. J. Plant Pathol. 2022, 162. in press.
- Chilton, M.D.; Drummond, M.H.; Merlo, D.J.; Sciaky, D.; Montoya, A.L.; Gordon, M.P.; Nester, E.W. Stable incorporation of plasmid DNA into higher plant cells: The moleculer basis of crown gall tumorigenesis. Cell 1977, 11, 263–271.
- Zupan, J.R.; Zambryski, P. The Agrobacterium DNA transfer complex. Crit. Rev. Plant Sci. 1997, 16, 279–295.
- Gelvin, S.B. Traversing the cell: Agrobacterium T-DNA’s journey to the host genome. Front. Plant Sci. 2012, 3, 52.
- Nester, E.W. Agrobacterium: Nature’s genetic engineer. Front. Plant Sci. 2015, 5, 730.
- New, P.B.; Kerr, A. Biological control of grown gall: Field measurements and glasshouse experiments. J. Appl. Bacteriol. 1972, 35, 279–287.
- Kerr, A.; Htay, K. Biological control of crown gall through bacteriocin production. Physiol. Plant Pathol. 1974, 4, 37–44.
- Moore, L.W.; Warren, G. Agrobacterium radiobacter strain 84 and biological control of crown gall. Ann. Rev. Phytopathol. 1979, 17, 163–179.
- Kerr, A. Biological control of crown gall through production of agrocin 84. Plant Dis. 1980, 64, 24–30.
- Kerr, A.; Bullard, G. Biocontrol of crown gall by Rhizobium rhizogenes: Challenges in biopesticide commercialization. Agronomy 2020, 20, 1126.
- Reader, J.S.; Ordoukhanian, P.T.; Kim, J.G.; de Crécy-Lagard, V.; Hwang, I.; Farrand, S.; Schimmel, P. Major biocontrol of plant tumors targets tRNA synthetase. Science 2005, 309, 1533.
- Jones, D.A.; Kerr, A. Agrobacterium radiobacter strain K1026, a genetically engineered derivative of strain K84, for biological control of crown gall. Plant Dis. 1989, 73, 15–18.
- Penyalver, R.; Vicedo, B.; López, M.M. Use of the genetically engineered Agrobacterium strain K1026 for biological control of crown gall. Euro. J. Plant Pathol. 2000, 106, 801–810.
- Staphorst, J.L.; van Zyl, F.G.H.; Strijdom, B.W.; Groenewold, Z.E. Agrocin-producing pathogenic and nonpathogenic biotype-3 strains of Agrobacterium tumefaciens active against biotype-3 pathogens. Curr. Microbiol. 1985, 12, 45–52.
- Burr, T.J.; Reid, C.L. Biological control of grape crown gall with nontumorigenic Agrobacterium vitis F2 ⁄ 5. Am. J. Enol. Viticul. 1994, 45, 213–219.
- Burr, T.J.; Reid, C.L.; Taglicti, E.; Bazzi, C.; Süle, S. Biological control of grape crown gall by strain F2/5 is not associated with agrocin production or competition for attachment site on grape cells. Phytopathology 1997, 87, 706–711.
- Burr, T.J.; Otten, L. Crown gall of grape: Biology and disease management. Annu. Rev. Phytopathol. 1999, 37, 53–80.
- Kaewnum, S.; Zheng, D.; Reid, C.L.; Johnson, K.L.; Gee, J.C.; Burr, T.J. A host-specific biological control of grape crown gall by Agrobacterium vitis strain F2/5; its regulation and population dynamics. Phytopathology 2013, 103, 427–435.
- Webster, J.; Thomson, J.A. Agrocin-producing Agrobacterium tumefaciens strain active against grapevine isolates. Appl. Environ. Microbiol. 1986, 52, 217–219.
- Chen, X.Y.; Xiang, W.N. A strain of Agrobacterium radiobacter inhibits growth and gall formation by biotype III strains of Agrobacterium tumefaciens from grapevine. Acta. Microbiol. Sin. 1986, 26, 193–199.
- Wang, H.M.; Wang, H.X.; Ng, T.B.; Li, J.Y. Purification and characterization of an antibacterial compound produced by Agrobacterium vitis strain E26 with activity against A. tumefaciens. Plant Pathol. 2003, 52, 134–139.
- Liang, Y.; Di, Y.; Zhao, J.; Ma, D. A biotype 3 strain of Agrobacterium radiobacter inhibits crown gall formation on grapevine. Acta Microbiol. Sin. 1990, 30, 165–171.
- Chen, F.; Guo, Y.B.; Wang, J.H.; Li, J.Y.; Wang, H.M. Biological control of grape crown gall by Rahnella aquatilis HX2. Plant Dis. 2007, 91, 957–963.
- Xie, X.M.; You, J.F.; Chen, P.M.; Guo, J.M. On a strain MI15 of Agrobacterium radiobacter for biological control of grapevine crown gall. Acta Phytopathol. Sin. 1993, 23, 137–141.
- Ferrigo, D.; Causin, R.; Raiola, A. Effect of potential biocontrol agents selected among grapevine endophytes on crown gall disease. Bio Control. 2017, 62, 821–833.
- Kawaguchi, A.; Sawada, H.; Inoue, K.; Nasu, H. Multiplex PCR for the identification of Agrobacterium biover 3 strains. J. Gen. Plant Pathol. 2005, 71, 54–59.
- Kawaguchi, A.; Inoue, K.; Nasu, H. Inhibition of crown gall formation by Agrobacterium radiobacter biovar 3 strains isolated from grapevine. J. Gen. Plant Pathol. 2005, 71, 422–430.
- Kawaguchi, A.; Inoue, K. New antagonistic strains of non-pathogenic Agrobacterium vitis to control grapevine crown gall. J. Phytopathol. 2012, 160, 509–518.
- Kawaguchi, A. Studies on the diagnosis and biological control of grapevine crown gall and phylogenetic analysis of tumorigenic Rhizobium vitis. J. Gen. Plant Pathol. 2009, 75, 462–463.
- Kawaguchi, A.; Sawada, H.; Ichinose, Y. Phylogenetic and serological analyses reveal genetic diversity of Agrobacterium vitis strains in Japan. Plant Pathol. 2008, 57, 747–753.
- Kawaguchi, A. Genetic diversity of Rhizobium vitis strains in Japan based on multilocus sequence analysis of pyrG, recA and rpoD. J. Gen. Plant Pathol. 2011, 77, 299–303.
- Kawaguchi, A.; Sone, T.; Ochi, S.; Matsushita, Y.; Noutoshi, Y.; Nita, M. Origin of pathogens of grapevine crown gall disease in Hokkaido in Japan as characterized by molecular epidemiology of Allorhizobium vitis strains. Life 2021, 11, 1265.
- Wong, A.T.; Kawaguchi, A.; Nita, M. Efficacy of a biological control agent Rhizobium vitis ARK-1 against Virginia R. vitis isolates, and relative relationship among Japanese and Virginia R. vitis isolates. Crop Prot. 2021, 146, 105685.
- Kawaguchi, A.; Noutoshi, Y. Migration of biological control agent Rhizobium vitis strain ARK-1 in grapevine stems and inhibition of galls caused by tumorigenic strain of R. vitis. J. Gen. Plant Pathol. 2022, 88, 63–68.
- Kawaguchi, A.; Inoue, K.; Nasu, H. Biological control of grapevine crown gall by nonpathogenic Agrobacterium vitis strain VAR03-1. J. Gen. Plant Pathol. 2007, 73, 133–138.
- Kawaguchi, A.; Inoue, K.; Ichinose, Y. Biological control of crown gall of grapevine, rose, and tomato by nonpathogenic Agrobacterium vitis strain VAR03-1. Phytopathology 2008, 98, 1218–1225.
- Kawaguchi, A.; Kondo, K.; Inoue, K. Biological control of apple crown gall by nonpathogenic Rhizobium vitis strain VAR03-1. J. Gen. Plant Pathol. 2012, 78, 287–293.
- Kawaguchi, A. Biological control of crown gall on grapevine and root colonization by nonpathogenic Rhizobium vitis strain ARK-1. Microbes Environ. 2013, 28, 306–311.
- Kawaguchi, A.; Inoue, K.; Tanina, K. Evaluation of the nonpathogenic Agrobacterium vitis strain ARK-1 for crown gall control in diverse plant species. Plant Dis. 2015, 99, 409–414.
- Gan, H.M.; Szegedi, E.; Fersi, R.; Chebil, S.; Kovács, L.; Kawaguchi, A.; Hudson, A.O.; Burr, T.J.; Michael, A.; Savka, M.A. Insight into the microbial co-occurrence and diversity of 73 grapevine (Vitis vinifera) crown galls collected across the northern hemisphere. Front. Microbiol. 2019, 10, 1896.
